Inhibition of BIRC2 Sensitizes α7-HPV-Related Cervical Squamous Cell Carcinoma to Chemotherapy
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics and Prognosis of HPV Genotype Groups
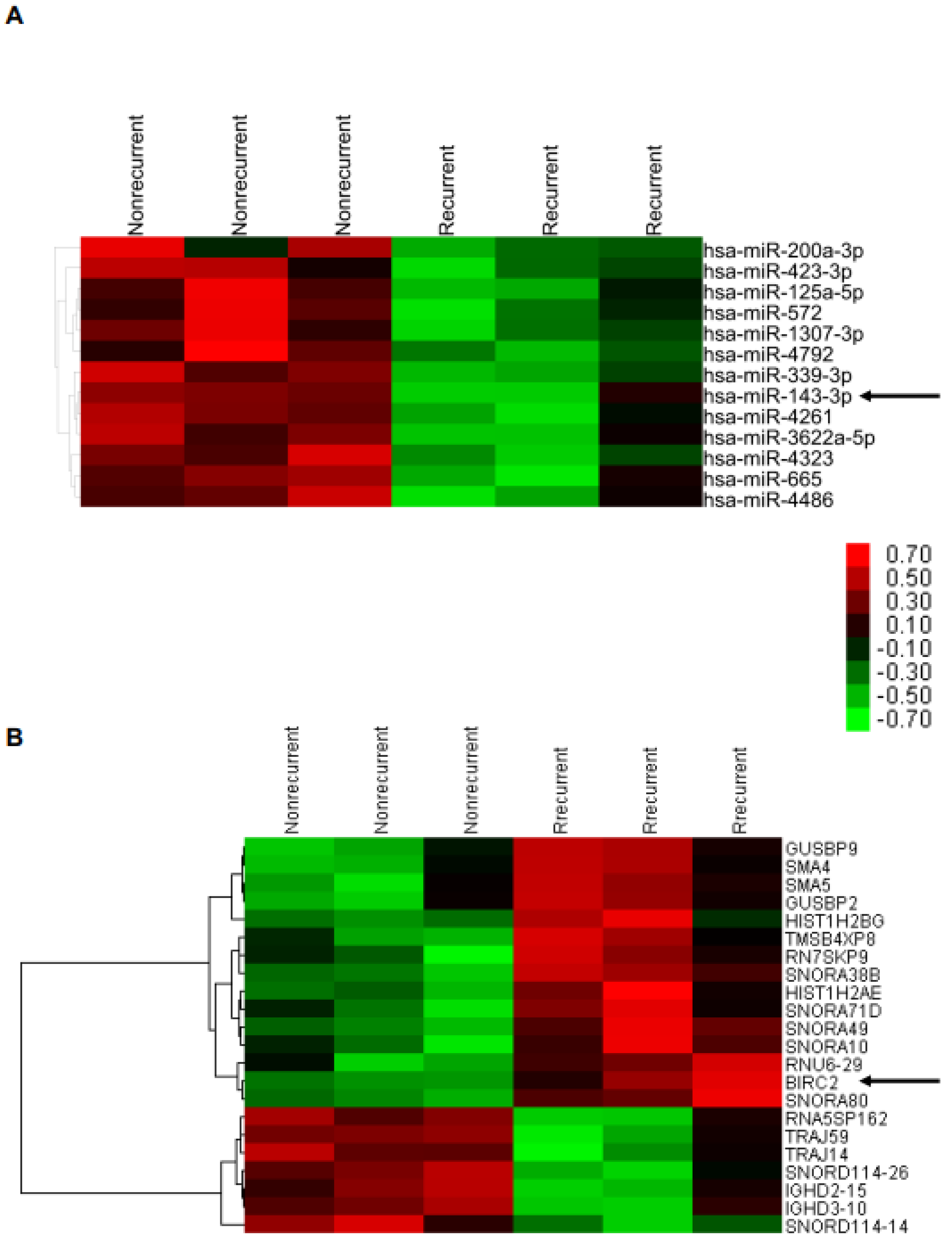
2.2. Lower miR-143-3p Expression in Cervical SCC with RT/CCRT Failure
2.3. Higher BIRC2 mRNA Expression in α7-HPV-Related Cervical SCC
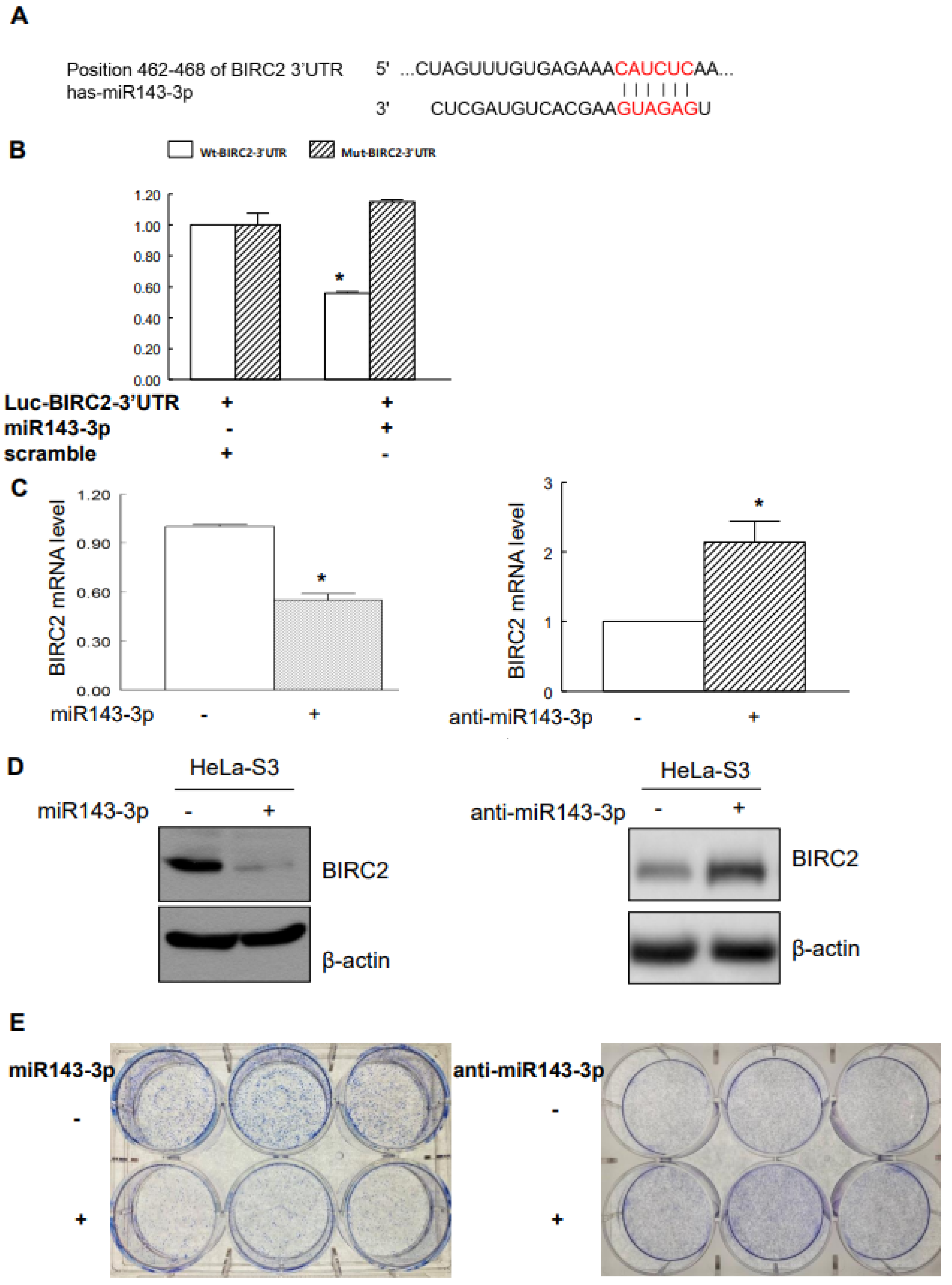
2.4. Suppression of BIRC2 by miR143-3p in Cervical Cancer Cells
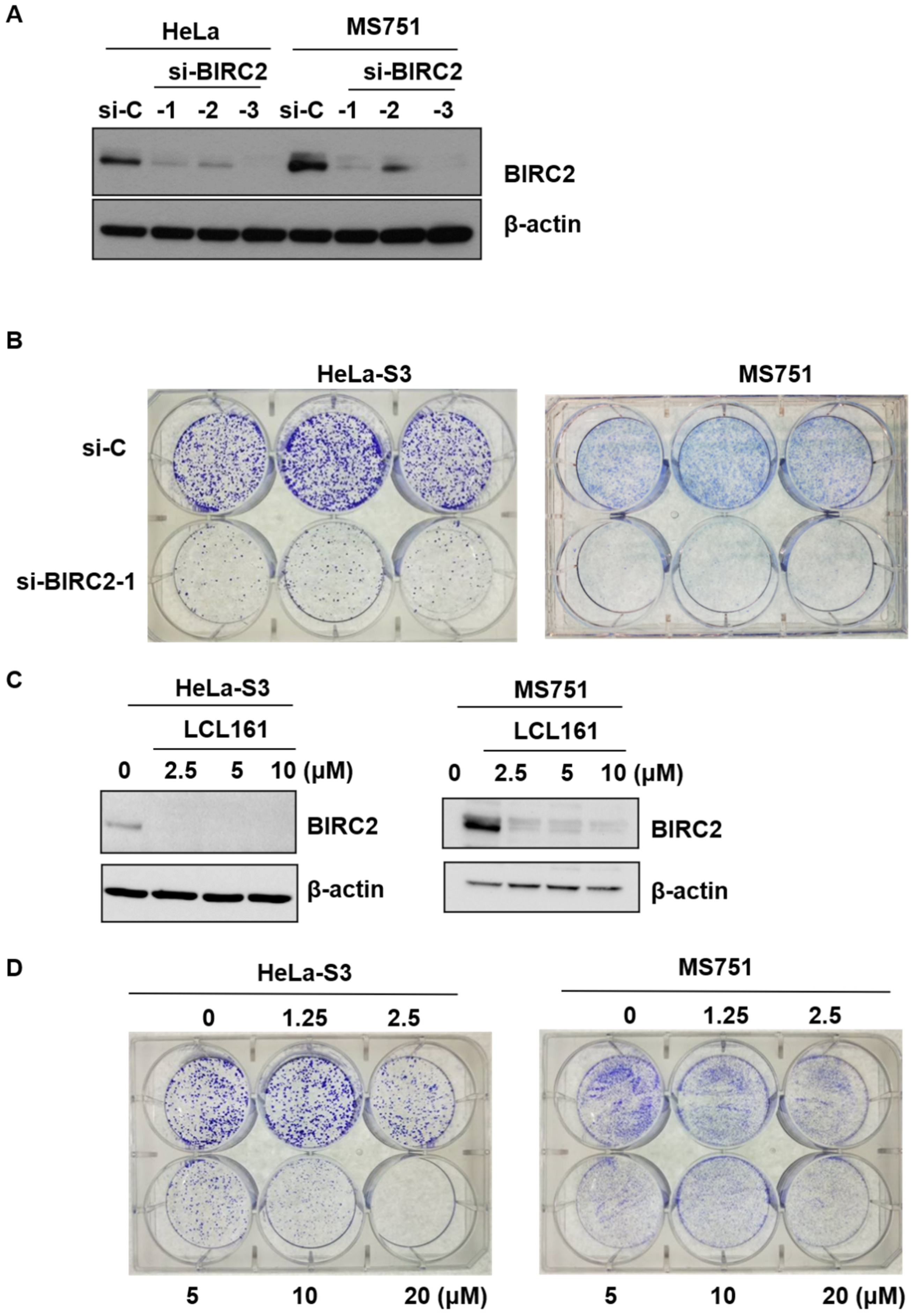
2.5. Role of LC161 in Colony Formation
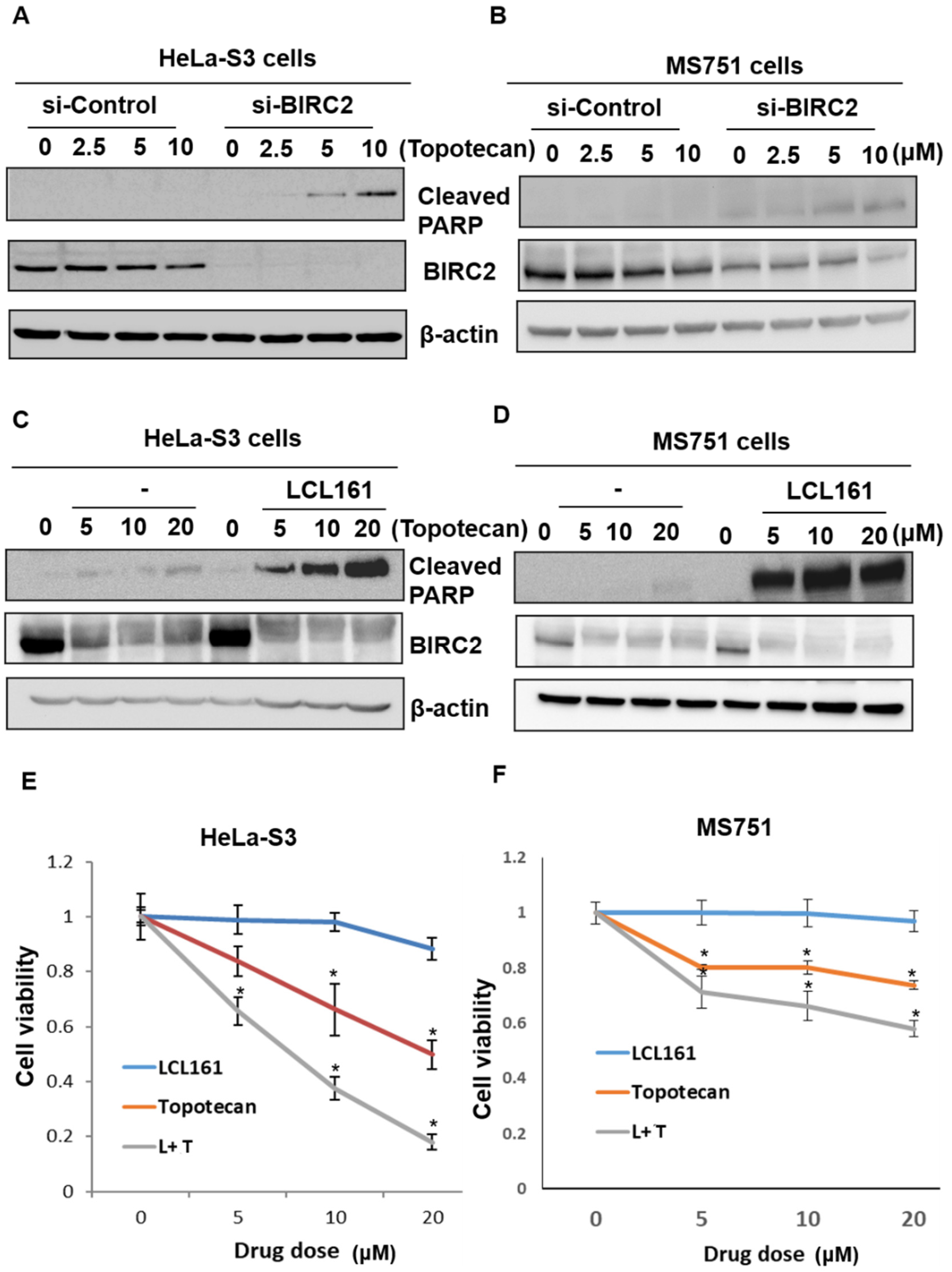
2.6. Inhibition of BIRC2 Sensitizes Cervical Cancer to Topotecan-Mediated Cell Death and Cell Viability
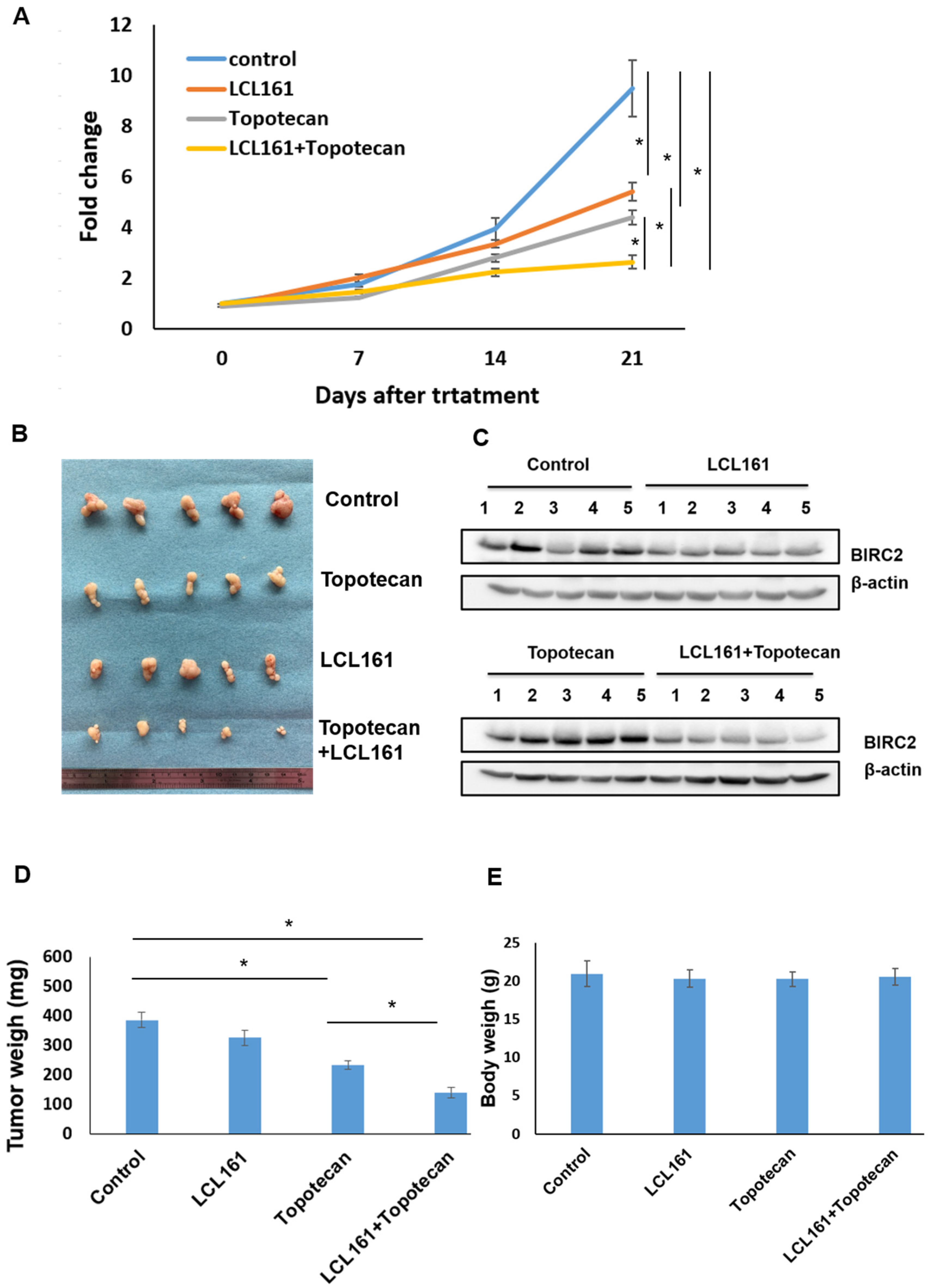
2.7. Synergistic Effects of LCL161 and Topotecan on Tumorigenesis in a Xenograft Tumor Model
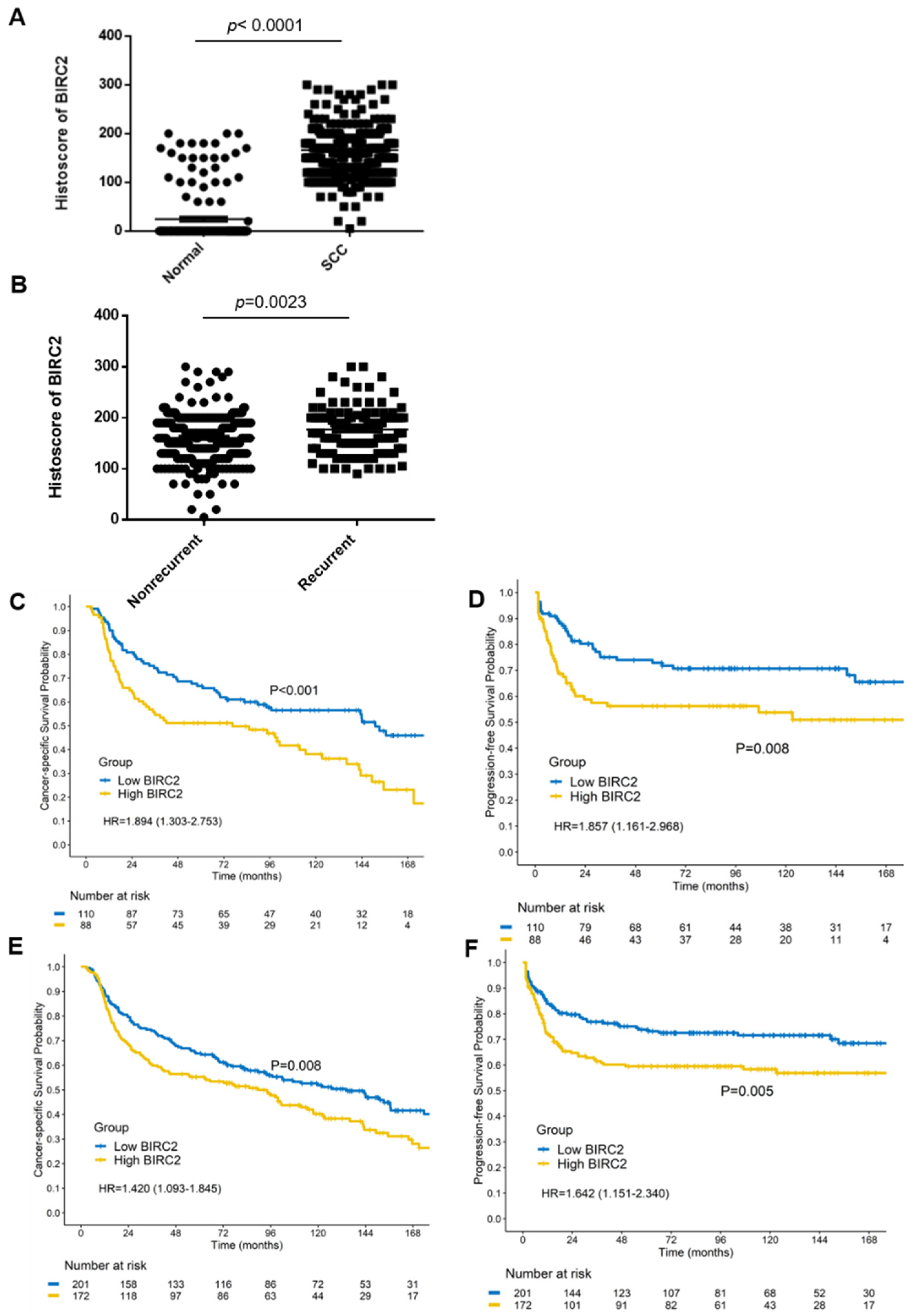
2.8. BIRC2 Protein Expression in α7-HPV-Related Cervical SCC (Including Mixed Infections)
2.9. Univariate and Multivariate Analyses of Prognostic Factors
3. Discussion
4. Materials and Methods
4.1. Patients and Immunohistochemistry and Clinical Tissue Specimens
4.2. HPV Genotyping
4.3. RNA Extraction from FFPE
4.4. miRNA 4.0 Array
4.5. HTA 2.0
4.6. Cell Lines and Culture
4.7. Antibodies and Reagents
4.8. Plasmids
4.9. DNA Transfection
4.10. Reporter Gene Assay
4.11. Western Blotting
4.12. RNA Extraction and Real-Time qPCR
4.13. Clonogenic Assays
4.14. Cell Viability Assay
4.15. Animals and Treatment
4.16. Statistical Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.H.; Chang, C.J.; Huang, H.J.; Hsueh, S.; Chao, A.; Yang, J.E.; Lin, C.T.; Huang, S.L.; Hong, J.H.; Chou, H.H.; et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J. Clin. Oncol. 2007, 25, 3628–3634. [Google Scholar] [CrossRef]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000, 92, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, M.H.; Castle, P. Epidemiologic studies of a necessary causal risk factor: Human papillomavirus infection and cervical neoplasia. J. Natl. Cancer Inst. 2003, 95, E2. [Google Scholar] [CrossRef]
- Kang, W.D.; Kim, C.H.; Cho, M.K.; Kim, J.W.; Cho, H.Y.; Kim, Y.H.; Choi, H.S.; Kim, S.M. HPV-18 is a poor prognostic factor, unlike the HPV viral load, in patients with stage IB-IIA cervical cancer undergoing radical hysterectomy. Gynecol. Oncol. 2011, 121, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lai, C.H.; Huang, H.J.; Chao, A.; Chang, C.J.; Chang, T.C.; Chou, H.H.; Hong, J.H. Clinical effect of human papillomavirus genotypes in patients with cervical cancer undergoing primary radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lai, C.H.; Huang, Y.T.; Chao, A.; Chou, H.H.; Hong, J.H. HPV genotypes predict survival benefits from concurrent chemotherapy and radiation therapy in advanced squamous cell carcinoma of the cervix. Int. J. Radiat Oncol. Biol. Phys. 2012, 84, e499–e506. [Google Scholar] [CrossRef]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Lin, C.Y.; Lee, Y.S.; Tsai, C.L.; Wei, P.C.; Hsueh, S.; Wu, T.I.; Tsai, C.N.; Wang, C.J.; Chao, A.S.; et al. Regulation of ovarian cancer progression by microRNA-187 through targeting Disabled homolog-2. Oncogene 2012, 31, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Soutto, M.; Wang, W.; Piazuelo, M.B.; Zhu, S.; Guo, Y.; Maturana, M.J.; Corvalan, A.H.; Chen, X.; et al. Integrated Analysis of Mouse and Human Gastric Neoplasms Identifies Conserved microRNA Networks in Gastric Carcinogenesis. Gastroenterology 2019, 156, 1127–1139.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Jia, J.; Wang, X.; Liu, Y.; Wang, C.; Fan, R. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol. Ther. 2018, 19, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Infante, J.R.; Dees, E.C.; Olszanski, A.J.; Dhuria, S.V.; Sen, S.; Cameron, S.; Cohen, R.B. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Runckel, K.; Barth, M.J.; Mavis, C.; Gu, J.J.; Hernandez-Ilizaliturri, F.J. The SMAC mimetic LCL-161 displays antitumor activity in preclinical models of rituximab-resistant B-cell lymphoma. Blood Adv. 2018, 2, 3516–3525. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN National Clinical Practice Guidelines in Oncology (NCCN Guidelines): Cervical Cancer, Version: 1.2021. Available online: https://www.nccn.org/professionals/ (accessed on 22 June 2021).
- Lin, C.Y.; Chao, A.; Wu, R.C.; Lee, L.Y.; Ueng, S.H.; Tsai, C.L.; Lee, Y.S.; Peng, M.T.; Yang, L.Y.; Huang, H.J.; et al. Synergistic effects of pazopanib and hyperthermia against uterine leiomyosarcoma growth mediated by downregulation of histone acetyltransferase 1. J. Mol. Med. 2020, 98, 1175–1188. [Google Scholar] [CrossRef]
- Deng, Y.W.; Hao, W.J.; Li, Y.W.; Li, Y.X.; Zhao, B.C.; Lu, D. Hsa-miRNA-143-3p Reverses Multidrug Resistance of Triple-Negative Breast Cancer by Inhibiting the Expression of Its Target Protein Cytokine-Induced Apoptosis Inhibitor 1 In Vivo. J. Breast Cancer 2018, 21, 251–258. [Google Scholar] [CrossRef]
- Zhuang, M.; Shi, Q.; Zhang, X.; Ding, Y.; Shan, L.; Shan, X.; Qian, J.; Zhou, X.; Huang, Z.; Zhu, W.; et al. Involvement of miR-143 in cisplatin resistance of gastric cancer cells via targeting IGF1R and BCL2. Tumour. Biol. 2015, 36, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, X.; Guo, X.; Tian, Z.; Su, M.; Long, Y.; Huang, C.; Zhou, F.; Liu, M.; Wu, X.; et al. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol. Med. Rep. 2012, 5, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Inhibitor of Apoptosis (IAP) proteins as therapeutic targets for radiosensitization of human cancers. Cancer Treat. Rev. 2012, 38, 760–766. [Google Scholar] [CrossRef]
- Samanta, D.; Huang, T.Y.; Shah, R.; Yang, Y.; Pan, F.; Semenza, G.L. BIRC2 Expression Impairs Anti-Cancer Immunity and Immunotherapy Efficacy. Cell Rep. 2020, 32, 108073. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Cheung, C.H.A. An Updated Review of Smac Mimetics, LCL161, Birinapant, and GDC-0152 in Cancer Treatment. Appl. Sci. 2021, 11, 335. [Google Scholar] [CrossRef]
- Chao, A.; Lin, C.T.; Lai, C.H. Updates in systemic treatment for metastatic cervical cancer. Curr. Treat. Options Oncol. 2014, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Molecular pathways: Targeting inhibitor of apoptosis proteins in cancer--from molecular mechanism to therapeutic application. Clin. Cancer Res. 2014, 20, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Wang, H.; Zhang, B.; Chen, Y.; Zhang, Y.; Sun, X.; Xiao, G.; Nan, K.; Ren, H.; Qin, S. LCL161 increases paclitaxel-induced apoptosis by degrading cIAP1 and cIAP2 in NSCLC. J. Exp. Clin. Cancer Res. 2016, 35, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.M.; Lin, T.K.; Tseng, Y.Y.; Tu, C.H.; Lui, T.N.; Huang, S.F.; Hsieh, L.L.; Li, Y.Y. Targeting inhibitors of apoptosis proteins suppresses medulloblastoma cell proliferation via G2/M phase arrest and attenuated neddylation of p21. Cancer Med. 2018, 7, 3988–4003. [Google Scholar] [CrossRef] [PubMed]
- Chesi, M.; Mirza, N.N.; Garbitt, V.M.; Sharik, M.E.; Dueck, A.C.; Asmann, Y.W.; Akhmetzyanova, I.; Kosiorek, H.E.; Calcinotto, A.; Riggs, D.L.; et al. IAP antagonists induce anti-tumor immunity in multiple myeloma. Nat. Med. 2016, 22, 1411–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagaku, M.; Nagasawa, T.; Sato, C.; Fukigawa, Y.; Kawamura, H.; Tomabechi, H.; Takemoto, S.; Baba, T. Immunotherapy for uterine cervical cancer using checkpoint inhibitors: Future directions. Int. J. Molecul. Sci. 2020, 21, 2335. [Google Scholar]
- Merck Announces Phase 3 KEYNOTE-826 Trial Met Dual Primary Endpoints of Overall Survival and Progression-Free Survival in Patients with Persistent, Recurrent or Metastatic Cervical Ancer. News Release. Merck. Available online: https://bit.ly/3zI1iG531 (accessed on 22 June 2021).
- Lin, C.Y.; Chao, A.; Wang, T.H.; Lee, L.Y.; Yang, L.Y.; Tsai, C.L.; Wang, H.S.; Lai, C.H. Nucleophosmin/B23 is a negative regulator of estrogen receptor alpha expression via AP2gamma in endometrial cancer cells. Oncotarget 2016, 7, 60038–60052. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Ma, T.; Zhang, X.; Lv, M.; Chen, L.; Tang, J.; Zhao, J. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in vinorelbine-resistant breast cancer cells. Gene 2015, 556, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Balasubramanian, M.N.; Donelan, W.; Fu, L.; Hayner, J.; Lopez, M.C.; Baker, H.V.; Kilberg, M.S. A mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)-dependent transcriptional program controls activation of the early growth response 1 (EGR1) gene during amino acid limitation. J. Biol. Chem. 2014, 289, 24665–24679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Lee, L.Y.; Wang, T.H.; Hsu, C.L.; Tsai, C.L.; Chao, A.; Lai, C.H. Palbociclib Promotes Dephosphorylation of NPM/B23 at Threonine 199 and Inhibits Endometrial Cancer Cell Growth. Cancers 2019, 11, 1025. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Lin, C.Y.; Wu, R.C.; Lee, Y.S.; Lee, L.Y.; Tsai, C.L.; Yang, L.Y.; Liu, H.; Chen, S.J.; Wang, T.H.; et al. The combination of everolimus and terameprocol exerts synergistic antiproliferative effects in endometrial cancer: Molecular role of insulin-like growth factor binding protein 2. J. Mol. Med. 2018, 96, 1251–1266. [Google Scholar] [CrossRef]

| Univariate | Multivariate (Stepwise) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N | HR | 95% C.I. | p-Value | HR | 95% C.I. | p-Value | ||
| Age | 1837 | 1.02 | 1.02–1.03 | <0.001 | 1.02 | 1.02–1.03 | <0.001 | ||
| FIGO Stage | |||||||||
| 1 | 380 | 1(ref) | 1(ref) | ||||||
| 2 | 888 | 1.32 | 1.11–1.58 | 0.002 | 1.35 | 1.13–1.62 | 0.001 | ||
| 3 | 448 | 2.31 | 1.91–2.79 | <0.001 | 2.28 | 1.88–2.75 | <0.001 | ||
| 4 | 121 | 4.68 | 3.64–6.02 | <0.001 | 4.86 | 3.77–6.26 | <0.001 | ||
| Differentiation | |||||||||
| 1 | 62 | 1(ref) | |||||||
| 2 | 775 | 1.18 | 0.83–1.69 | 0.361 | |||||
| 3 | 731 | 1.29 | 0.90–1.84 | 0.168 | |||||
| Unknown | 263 | 1.01 | 0.69–1.49 | 0.952 | |||||
| BIRC2 (cut-off 175) | |||||||||
| Low BIRC2 | 220 | 1(ref) | 1(ref) | ||||||
| High BIRC2 | 229 | 1.35 | 1.06–1.72 | 0.016 | 1.37 | 1.07–1.76 | 0.012 | ||
| Unknown | 1388 | 1.09 | 0.90–1.32 | 0.368 | 1.40 | 1.03–1.90 | 0.032 | ||
| HPV type | |||||||||
| NON-α7 | 1412 | 1(ref) | 1(ref) | ||||||
| α7+α7-mix * | 425 | 1.16 | 1.01–1.34 | 0.034 | 1.40 | 1.08–1.82 | 0.012 | ||
| HPV type | |||||||||
| NON-α7 | 1412 | 1(ref) | |||||||
| α7 | 224 | 1.18 | 0.98–1.41 | 0.083 | |||||
| α7-mix * | 201 | 1.15 | 0.95–1.39 | 0.144 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Wang, C.-C.; Wu, R.-C.; Yang, L.-Y.; Chang, C.-B.; Pan, Y.-B.; Chao, A.; Lai, C.-H. Inhibition of BIRC2 Sensitizes α7-HPV-Related Cervical Squamous Cell Carcinoma to Chemotherapy. Int. J. Mol. Sci. 2021, 22, 11020. https://doi.org/10.3390/ijms222011020
Lin C-Y, Wang C-C, Wu R-C, Yang L-Y, Chang C-B, Pan Y-B, Chao A, Lai C-H. Inhibition of BIRC2 Sensitizes α7-HPV-Related Cervical Squamous Cell Carcinoma to Chemotherapy. International Journal of Molecular Sciences. 2021; 22(20):11020. https://doi.org/10.3390/ijms222011020
Chicago/Turabian StyleLin, Chiao-Yun, Chun-Chieh Wang, Ren-Chin Wu, Lan-Yan Yang, Chen-Bin Chang, Yu-Bin Pan, Angel Chao, and Chyong-Huey Lai. 2021. "Inhibition of BIRC2 Sensitizes α7-HPV-Related Cervical Squamous Cell Carcinoma to Chemotherapy" International Journal of Molecular Sciences 22, no. 20: 11020. https://doi.org/10.3390/ijms222011020
APA StyleLin, C.-Y., Wang, C.-C., Wu, R.-C., Yang, L.-Y., Chang, C.-B., Pan, Y.-B., Chao, A., & Lai, C.-H. (2021). Inhibition of BIRC2 Sensitizes α7-HPV-Related Cervical Squamous Cell Carcinoma to Chemotherapy. International Journal of Molecular Sciences, 22(20), 11020. https://doi.org/10.3390/ijms222011020







