Linalool Nanoemulsion Preparation, Characterization and Antimicrobial Activity against Aeromonas hydrophila
Abstract
:1. Introduction
2. Results
2.1. The Diameter of LN with Different MCT Concentrations
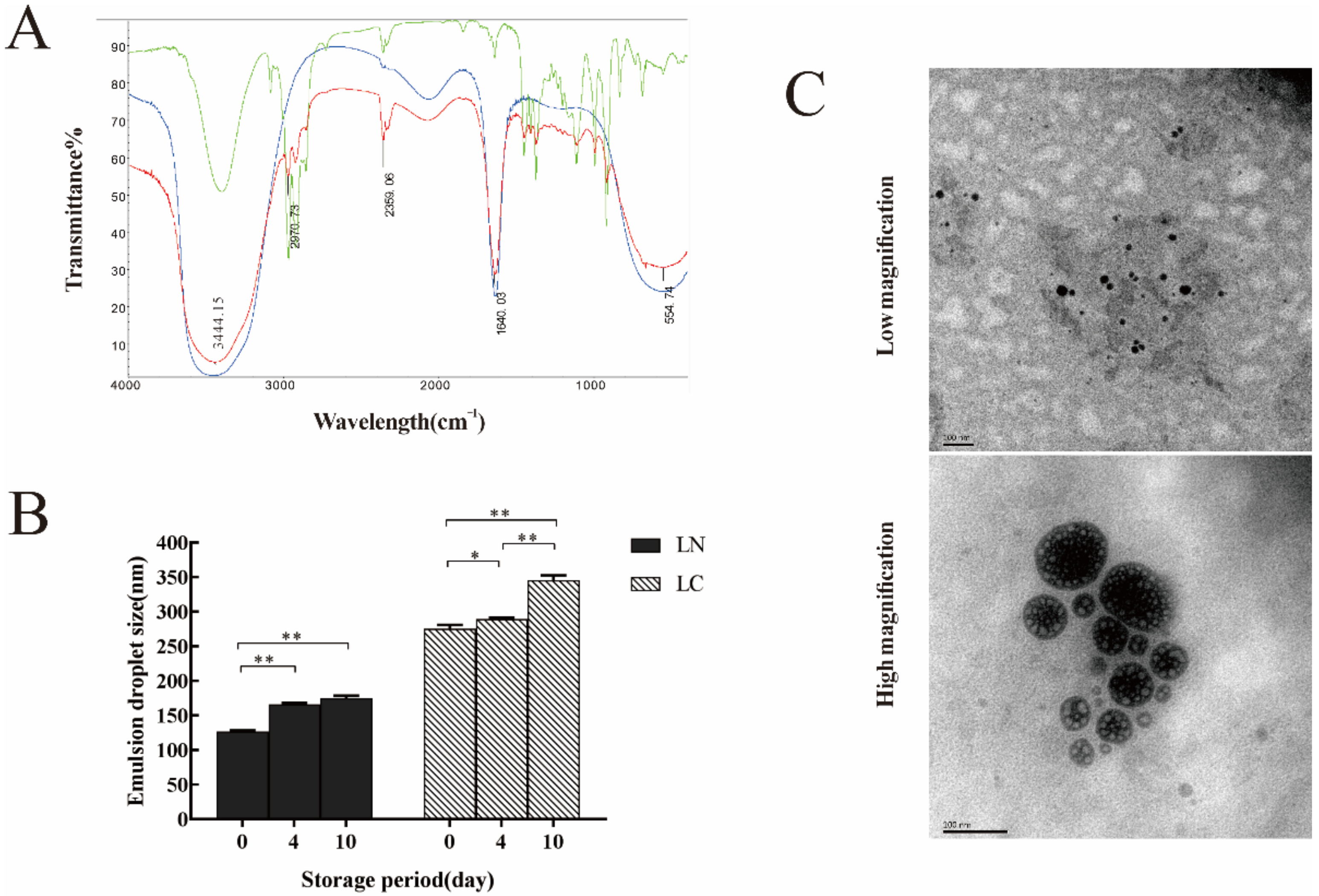
2.2. Structural, Thermal Stability and Morphological Observations of LN
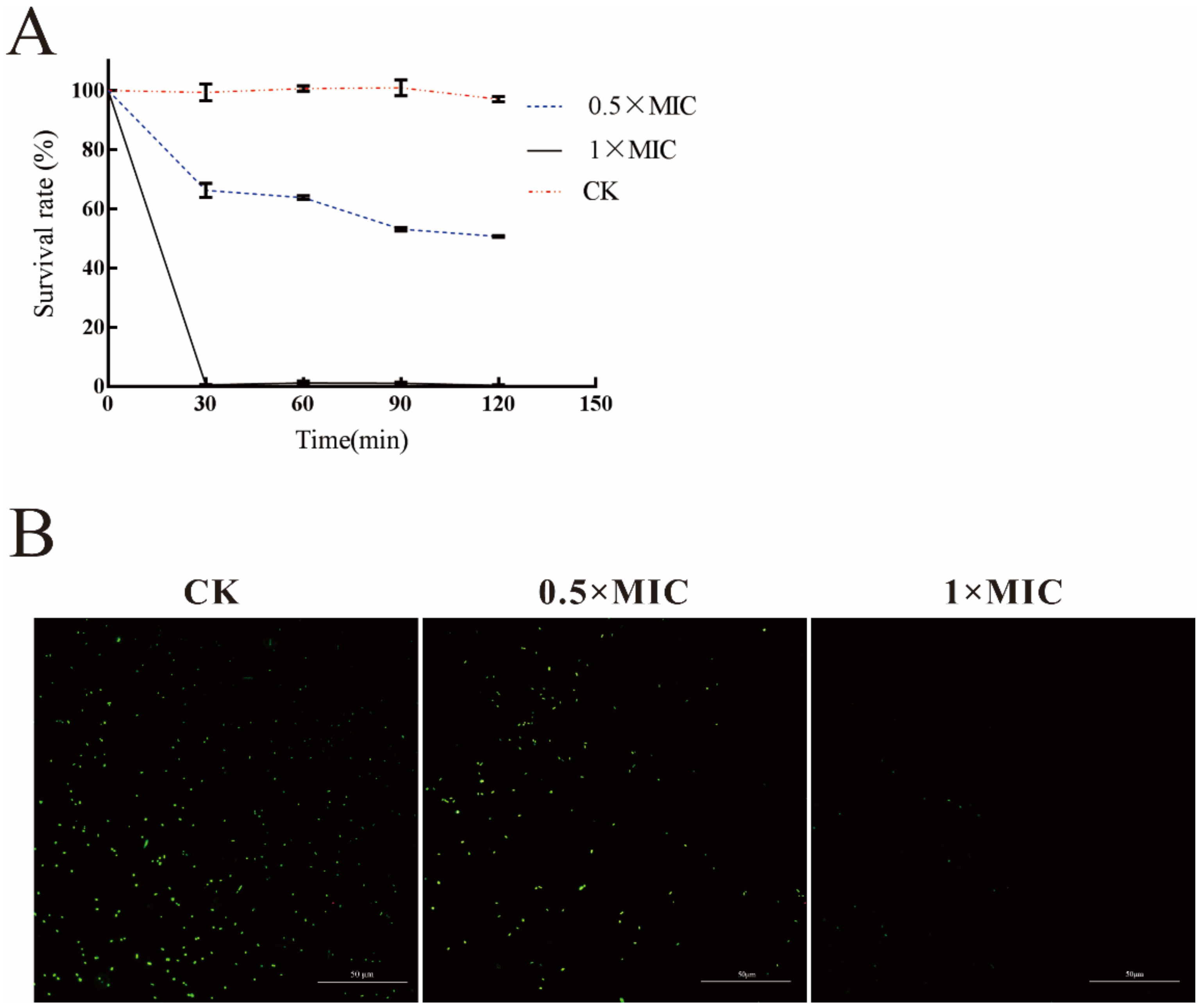
2.3. Analysis of the Antibacterial Activity of LN against A. hydrophila
2.4. SEM Observation
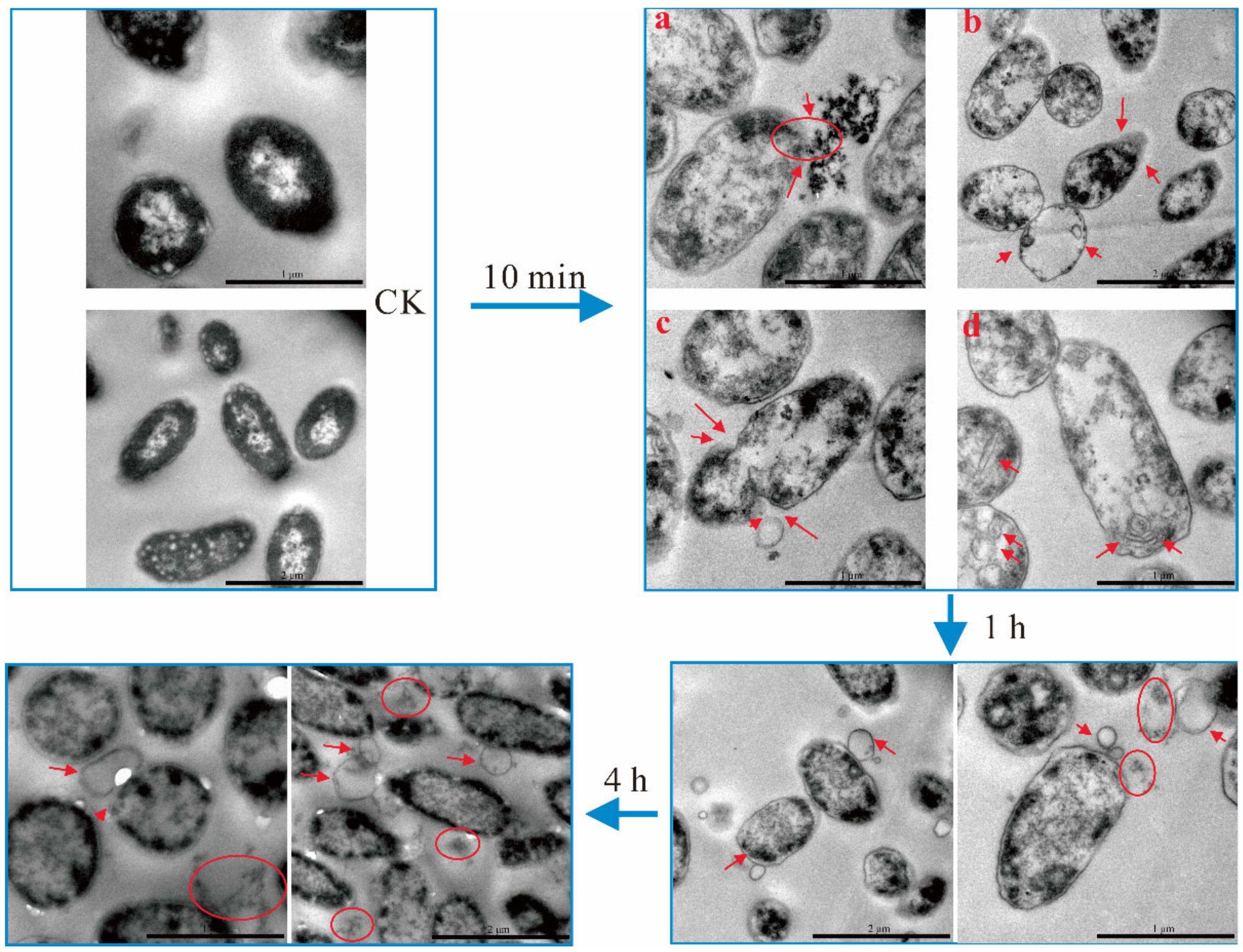
2.5. TEM Observation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Nanoemulsion
4.2.1. The Measurement of Droplet Size
4.2.2. The Measurement of the Stability of Nanoemulsion and Colostrum
4.2.3. Fourier Transform Infrared (FTIR) Assay
4.2.4. Observation of TEM
4.3. Antibacterial Activity Assay
4.3.1. Determination of MIC and MBC
4.3.2. Fuorescence Microscope Assay
4.3.3. Cell Activity Assay
4.3.4. SEM Assay
4.3.5. TEM Assay
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelhamed, H.; Banes, M.; Karsi, A.; Lawrence, M.L. Recombinant ATPase of Virulent Aeromonas hydrophila Protects Channel Catfish Against Motile Aeromonas Septicemia. Front. Immunol. 2019, 10, 1641. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, W.; Zhang, Y.; Zhou, Y.; Xu, J.; Gao, X.; Zhang, S.; Zhang, X. Impact of Aeromonas hydrophila and infectious spleen and kidney necrosis virus infections on susceptibility and host immune response in Chinese perch (Siniperca chuatsi). Fish. Shellfish Immunol. 2020, 105, 117–125. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Xu, W.; Fang, R.; Wu, Q.; He, H.; Xu, C.; Zhou, C.; Cao, J.; Chen, L.; et al. Taxonomy, virulence determinants and antimicrobial susceptibility of Aeromonas spp. isolated from bacteremia in southeastern China. Antimicrob. Resist. Infect. Control. 2021, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Teunis, P.; Figueras, M.J. Reassessment of the Enteropathogenicity of Mesophilic Aeromonas Species. Front. Microbiol. 2016, 7, 1395. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Bravo, A.; Kilgore, P.B.; Andersson, J.A.; Blears, E.; Figueras, M.J.; Hasan, N.A.; Colwell, R.R.; Sha, J.; Chopra, A.K. T6SS and ExoA of flesh-eating Aeromonas hydrophila in peritonitis and necrotizing fasciitis during mono-and polymicrobial infections. Proc. Natl. Acad. Sci. USA 2019, 116, 24084–24092. [Google Scholar] [CrossRef]
- Stratev, D.; Odeyemi, O.A. Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: A mini-review. J. Infect. Public Health 2016, 9, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou YB chang against Listeria monocytogenes. Food Control. 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crop. Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Trong Le, N.; Viet Ho, D.; Quoc Doan, T.; Tuan Le, A.; Raal, A.; Usai, D.; Madeddu, S.; Marchetti, M.; Usai, M.; Rappelli, P. In vitro antimicrobial activity of essential oil extracted from leaves of Leoheo domatiophorus Chaowasku, DT Ngo and HT Le in Vietnam. Plants 2020, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Zhong, W.; Chen, K.; Tang, P.; Guo, J. Chemical composition and anti-biofilm activity of essential oil from Citrus medica L. var. sarcodactylis Swingle against Listeria monocytogenes. Ind. Crop. Prod. 2020, 144, 112036. [Google Scholar] [CrossRef]
- Guo, J.-j.; Gao, Z.-p.; Xia, J.-l.; Ritenour, M.A.; Li, G.-y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-listeria Activities of Linalool and Its Mechanism Revealed by Comparative Transcriptome Analysis. Front. Microbiol. 2019, 10, 2947. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of Linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef] [PubMed]
- Kalily, E.; Hollander, A.; Korin, B.; Cymerman, I.; Yaron, S. Mechanisms of resistance to Linalool in Salmonella Senftenberg and their role in survival on basil. Environ. Microbiol. 2016, 18, 3673–3688. [Google Scholar] [CrossRef]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef] [PubMed]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property. Polymer 2019, 11, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish. Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Tinh, T.H.; Elayaraja, S.; Mabrok, M.; Gallantiswara, P.C.D.; Vuddhakul, V.; Rodkhum, C. Antibacterial spectrum of synthetic herbal-based polyphenols against Vibrio parahaemolyticus isolated from diseased Pacific whiteleg shrimp (Penaeus vannamei) in Thailand. Aquaculture 2021, 533, 736070. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Topuz, O.K.; Ozvural, E.B.; Zhao, Q.; Huang, Q.; Chikindas, M.; Golukcu, M. Physical and antimicrobial properties of anise oil loaded nanoemulsions on the survival of foodborne pathogens. Food Chem. 2016, 203, 117–123. [Google Scholar] [CrossRef]
- Guerrero, S.; Inostroza-Riquelme, M.; Contreras-Orellana, P.; Diaz-Garcia, V.; Lara, P.; Vivanco-Palma, A.; Cardenas, A.; Miranda, V.; Robert, P.; Leyton, L.; et al. Curcumin-loaded nanoemulsion: A new safe and effective formulation to prevent tumor reincidence and metastasis. Nanoscale 2018, 10, 22612–22622. [Google Scholar] [CrossRef] [PubMed]
- González-González, C.R.; Labo-Popoola, O.; Delgado-Pando, G.; Theodoridou, K.; Doran, O.; Stratakos, A.C. The effect of cold atmospheric plasma and Linalool nanoemulsions against Escherichia coli O157: H7 and Salmonella on ready-to-eat chicken meat. LWT 2021, 149, 111898. [Google Scholar] [CrossRef]
- Taghavi, E.; Mirhosseini, H.; Rukayadi, Y.; Radu, S.; Biabanikhankahdani, R. Effect of microfluidization condition on physicochemical properties and inhibitory activity of nanoemulsion loaded with natural antibacterial mixture. Food Bioprocess. Technol. 2018, 11, 645–659. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion-and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Nouri, M.; Baghaee-Ravari, S.; Emadzadeh, B. Nano-emulsified savory and thyme formulation show limited efficacy to suppress Pectobacterium carotovorum subsp. carotovorum compared with pure oil. Ind. Crop. Prod. 2021, 161, 113216. [Google Scholar] [CrossRef]
- Daood, U.; Matinlinna, J.P.; Pichika, M.R.; Mak, K.K.; Nagendrababu, V.; Fawzy, A.S. A quaternary ammonium silane antimicrobial triggers bacterial membrane and biofilm destruction. Sci. Rep. 2020, 10, 10970. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, M.Y.; Yurtov, E.V. Nanoemulsions: The properties, methods of preparation and promising applications. Russ. Chem. Rev. 2012, 81, 21. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Tang, P.; Liu, T.; Zhao, T.; Guo, J.; Gao, Z. Linalool Nanoemulsion Preparation, Characterization and Antimicrobial Activity against Aeromonas hydrophila. Int. J. Mol. Sci. 2021, 22, 11003. https://doi.org/10.3390/ijms222011003
Zhong W, Tang P, Liu T, Zhao T, Guo J, Gao Z. Linalool Nanoemulsion Preparation, Characterization and Antimicrobial Activity against Aeromonas hydrophila. International Journal of Molecular Sciences. 2021; 22(20):11003. https://doi.org/10.3390/ijms222011003
Chicago/Turabian StyleZhong, Weiming, Puyu Tang, Ting Liu, Tianyu Zhao, Jiajing Guo, and Zhipeng Gao. 2021. "Linalool Nanoemulsion Preparation, Characterization and Antimicrobial Activity against Aeromonas hydrophila" International Journal of Molecular Sciences 22, no. 20: 11003. https://doi.org/10.3390/ijms222011003
APA StyleZhong, W., Tang, P., Liu, T., Zhao, T., Guo, J., & Gao, Z. (2021). Linalool Nanoemulsion Preparation, Characterization and Antimicrobial Activity against Aeromonas hydrophila. International Journal of Molecular Sciences, 22(20), 11003. https://doi.org/10.3390/ijms222011003




