The Protective Function and Modification of Secondary Metabolite Accumulation in Response to Light Stress in Dracocephalum forrestii Shoots
Abstract
:1. Introduction
2. Results and Discussion
2.1. Light-Mediated Morphology and Growth
2.2. Bioactive Compound Production
2.3. Antioxidant Response
3. Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Light Conditions
4.3. Analysis of Photosynthetic Pigments
4.4. Extraction of Phenolic Acids
4.5. Determination of Phenolic Acids Content
4.6. Determination of Activities of Antioxidant Enzymes
4.7. Antioxidant Assays
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mera, I.F.G.; Falconí, D.E.G.; Córdova, V.M. Secondary metabolites in plants: Main classes, phytochemical analysis and pharmacological activities. Rev. Bionatura. 2019, 4, 1000–1009. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. App. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Weremczuk-Jeżyna, I.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. Effect of cytokinins on shoots proliferation and rosmarinic and salvianolic acid B production in shoot culture of Dracocephalum forrestti W.W. Smith. Acta Physiol. Plant. 2018, 40, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-M.; Yang, X.-W.; Li, Y.-L.; Shen, Y.-H.; Feng, L.; Wang, Y.-H.; Zeng, H.-W.; Liu, X.-H.; Zhang, C.-S.; Long, C.-L.; et al. Chemical constituents of Dracocephalum forrestii. Planta Med. 2009, 75, 1591–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weremczuk-Jeżyna, I.; Lisiecki, P.; Gonciarz, W.; Kuźma, Ł.; Szemraj, M.; Chmiela, M.; Grzegorczyk-Karolak, I. Transformed shoots of Dracocephalum forrestii W.W. Smith from different bioreactor systems as a rich source of natural phenolic compounds. J. Biotechnol. 2019, 306, 125–133. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, C.G.; Jung, J.Y. Acacetin (5,7-dihydroxy-40-metoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-B/Akt signaling in prostate cancer cells. Int. J. Mol. Med. 2014, 33, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Escobar-Bravo, R.; Chen, G.; Kim, H.K.; Grosser, K.; Van Dam, N.M.; Leiss, K.A.; Klinkhamer, P.G.L. Ultraviolet radiation exposure time and intensity modulate tomato resistance to herbivory through activation of jasmonic acid signaling. J. Exp. Bot. 2019, 70, 315–327. [Google Scholar] [CrossRef]
- Naznin, T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with Red LEDs Enhance growth characteristics, pigment content and antioxidant capacity in lettuce, spinach, kale, basil and sweet pepper in a controlled environment. Plant 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. Hortscience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Bello-Bello, J.J.; Perez-Sato, J.A.; Cruz-Cruz, C.A.; Martinez-Estrada, E. Light-emitting diodes: Progress in plant micropropagation. InTech 2017, 6, 93–103. [Google Scholar]
- Silva, M.M.A.; de Oliveira, A.L.B.; Oliveira-Filho, R.A.; Gouveia-Neto, A.S.; Camara, T.J.R.; Willadino, L.G. Effect of blue/red LED light combination on growth and morphogenesis of Saccharum officinarum plantlets in vitro. In Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XII; Farkas, D.L., Nicolau, D.V., Leif, R.C., Eds.; SPIE: San Francisco, CA, USA, 2014; Volume 8947, p. 89471X. [Google Scholar]
- Bello-Bello, J.J.; Iglesias-Andreu, L.G.; Avilés-Viñas, S.A.; Gómez-Uc, E.; Canto-Flick, A.; Santana-Buzzy, N. Somaclonal variation in Habanero Pepper (Capsicum chinense Jacq.) as assessed ISSR molecular markers. Hortsciences 2014, 49, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Nhut, D.; Takamura, N.T.; Watanabe, H.; Tanaka, M. Light emitting diodes (LEDs) as a radiation source for micropropagation of strawberry. In Transplant Production in the 21st Century; Kubota, C., Chun, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 114–118. [Google Scholar]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [Green Version]
- Sabzalian, M.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Moe, R.; Morgan, L.; Grindal, G. Growth and plant morphology of Cucumis sativus and Fuchsia x hybrid are influenced by light quality during the photoperiod and by diurnal temperature alternations. Acta Hortic. 2002, 580, 229–234. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Moon, H.K.; Park, S.Y.; Kim, Y.W.; Kim, C.S. Growth of Tsuru-rindo (Tripterospermum japonicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Gam, D.T.; Khoi, P.H.; Ngoc, P.B.; Linh, L.H.; Hung, L.H.; Anh, P.T.L.; Thu, N.T.; Hien, N.T.T.; Khan, D.T.; Ha, C.H. LED light promote growth and flavonoid accumulation Anolectophilus roxburghii and are linked to the enhance of several related genes. Plants 2020, 9, 1344. [Google Scholar] [CrossRef]
- Zhang, W.J.; Su, J.; Tan, M.Y.; Liu, G.L.; Pang, Y.J.; Shen, H.G.; Qi, J.L.; Yang, Y. Expression analysis of shikonin-biosynthetic genes in response to M9 medium and light in Lithospermum erythrorhizon cell cultures. Plant Cell Tissue Organ Cult. 2010, 101, 135–142. [Google Scholar] [CrossRef]
- Aly, U.I.; El-Shabrawi, H.M.; Hanafy, M. Impact of culture conditions on alkaloid production from undifferentiated cell suspension cultures of Egyptian henbane. Aust. J. Basic Appl. Sci. 2010, 4, 4717–4725. [Google Scholar]
- Cioć, M.; Szewczyk, A.; Żupnik, M.; Kalisz, A.; Pawłowska, B. LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tissue Organ Cult. 2018, 132, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xiong, X.; Feng, B. Cardiovascular effects of salvianolic acid B. Evid. Based Complement. Altern. Med. 2013, 2013, 247948. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic acids: Potential source of natural drugs for the treatment of fibrosis disease and cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.J.; Lee, M.S.; Lin, M.K.; Ko, C.Y.; Chang, W.T. Blue light decreases tanshinone IIA content in Salvia miltiorrhiza hairy roots via genes regulation. J. Photochem. Photobiol. B Biol. 2018, 183, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Agrawal, D.C.; Lee, M.R.; Lee, R.J.; Kuo, C.L.; Wu, C.R.; Chang, H.C. Influence of LED light spectra on in vitro somatic embryogenesis and LC–MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: A medicinal herb. Bot. Stud. 2016, 57, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szopa, A.; Ekiert, H.; Szewczyk, A.; Fugas, E. Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. under different light conditions. Plant Cell Tissue Organ Cult. 2012, 110, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Zahir, A.; Hano, C. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J. Photochem. Photobiol. B Biol. 2019, 190, 172–178. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kochan, E.; Szymczyk, P.; Lisiecki, P.; Kuźma, Ł.; Grzegorczyk-Karolak, I. The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii W.W. Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochem. Lett. 2019, 30, 254–260. [Google Scholar] [CrossRef]
- Szopa, A.; Starzec, A.; Ekiert, H. The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J. Photochem. Photobiol. B Biol. 2018, 179, 91–97. [Google Scholar] [CrossRef]
- Mawphlang, O.I.L.; Kharshiing, E.V. Photoreceptor mediated plant growth responses: Implications for photoreceptor engineering toward improved performance in crops. Front. Plant Sci. 2017, 8, 1181. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, Ł.; Pawłowska, B.; Ekiert, H. The influence of light quality on the production of bioactive metabolites–verbascoside, isoverbascoside and phenolic acids and the content of photosynthetic pigments in biomass of Verbena officinalis L. cultured in vitro. J. Photochem. Photobiol. B Biol. 2020, 203, 111768. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria L. (American skullcap) grown in vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hort. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.H.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Liu, C.S.; Cheng, Y.; Hu, J.; Zhang, W.; Chen, N.; Zhang, J. Comparison of antioxidant activities between salvianolic acid B and Ginkgo biloba extract (EGb 761)1. Acta Pharmacol. Sin. 2006, 27, 1137–1145. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hua, L.; Yuan, Y.N.; Dai, H.Q.; Yang, B. Antioxidant activity and components of traditional chinense medicine formula consisting of Crataegus pinnatifida and Salvia milttiorrhiza. BMC Complement. Altern. Med. 2013, 13, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Hano, C.; Abbasi, B.H. Monochromatic lights-induced trends in antioxidant and antidiabetic polyphenol accumulation in in vitro callus cultures of Lepidium sativum L. J. Photochem. Photobiol. B Biol. 2019, 196, 111505. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Mahadevappa, P.; Giridhar, P. Influence of photoperiod on growth, bioactive compounds and antioxidant activity in callus cultures of Basella rubra L. J. Photochem. Photobiol. B Biol. 2020, 209, 111937. [Google Scholar] [CrossRef]
- Khan, T.; Ullah, M.A.; Garros, L.; Hano, C.; Abbasi, B.H. Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J. Photochem. Photobiol. B Biol. 2019, 190, 163–171. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Oren, R.; Werk, K.S.; Buchmann, N.; Zimmermann, R. Chlorophyll-nutrient relationships identify nutritionally caused decline in Picea abies stands. Can. J. For. Res. 1993, 23, 1187–1195. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectra determination of chlorophylls a and b as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie Weinhem: New York, NY, USA, 1983; pp. 273–286. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Abassi, N.A.; Kushad, M.M.; Endress, A.G. Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hort. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpine. Plant Cell Tissue Organ Cult. 2015, 122, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Weremczuk-Jeżyna, I.; Grzegorczyk-Karolak, I.; Frydrych, B.; Królicka, A.; Wysokińska, H. Hairy roots of Dracocephalum moldavica rosmarinic acid content and antioxidant potential. Acta Physiol. Plant. 2013, 35, 2095–2103. [Google Scholar] [CrossRef] [Green Version]
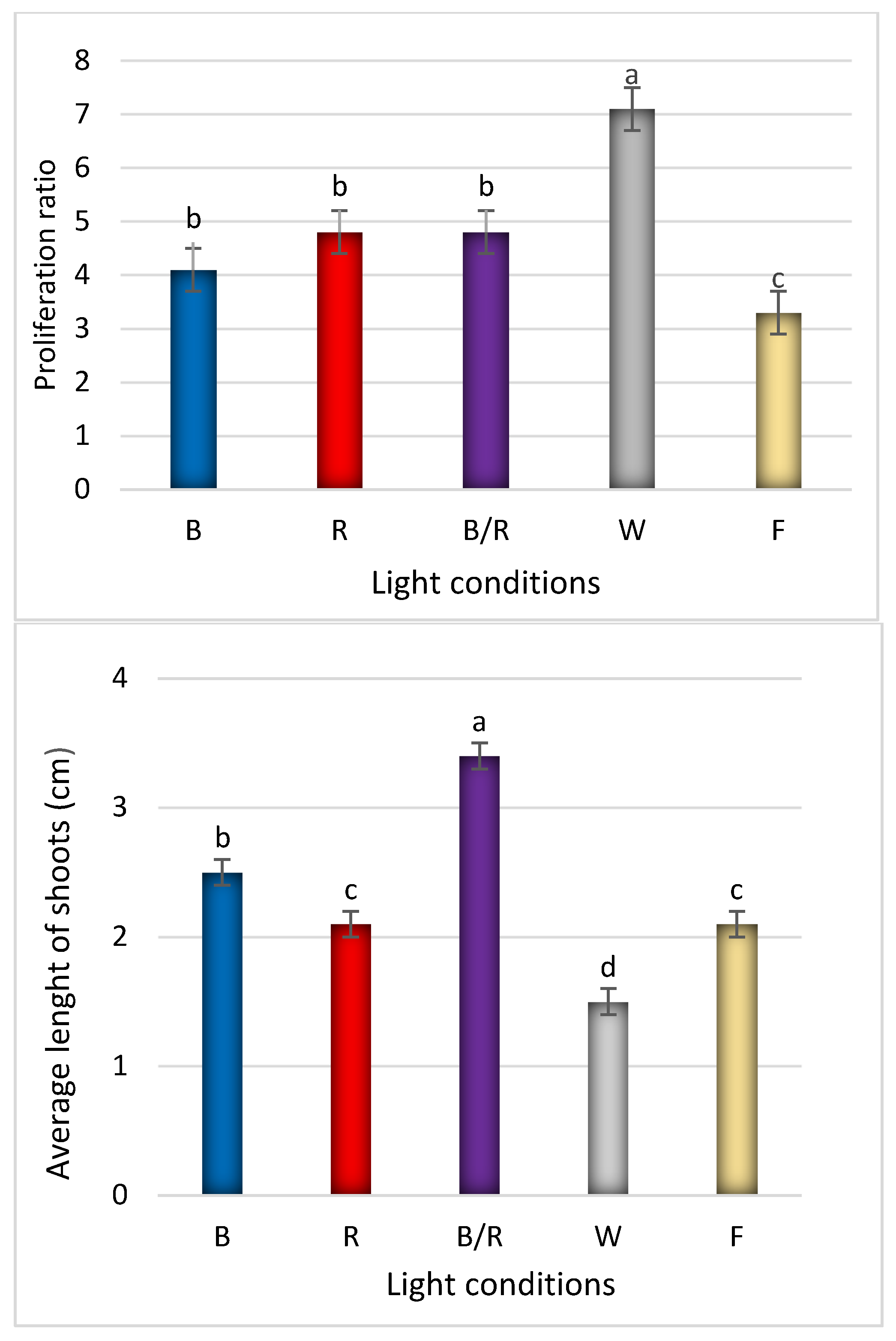
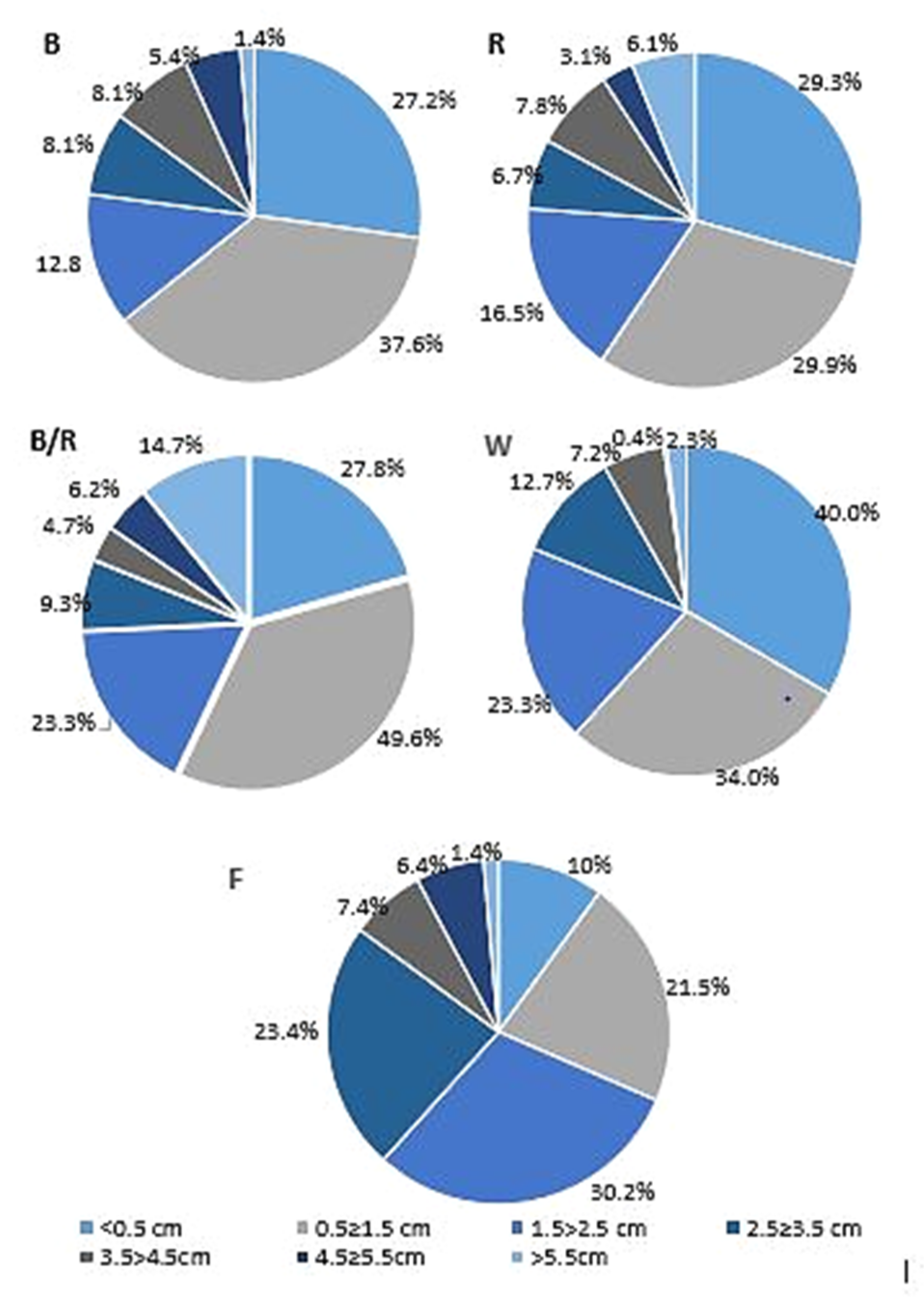

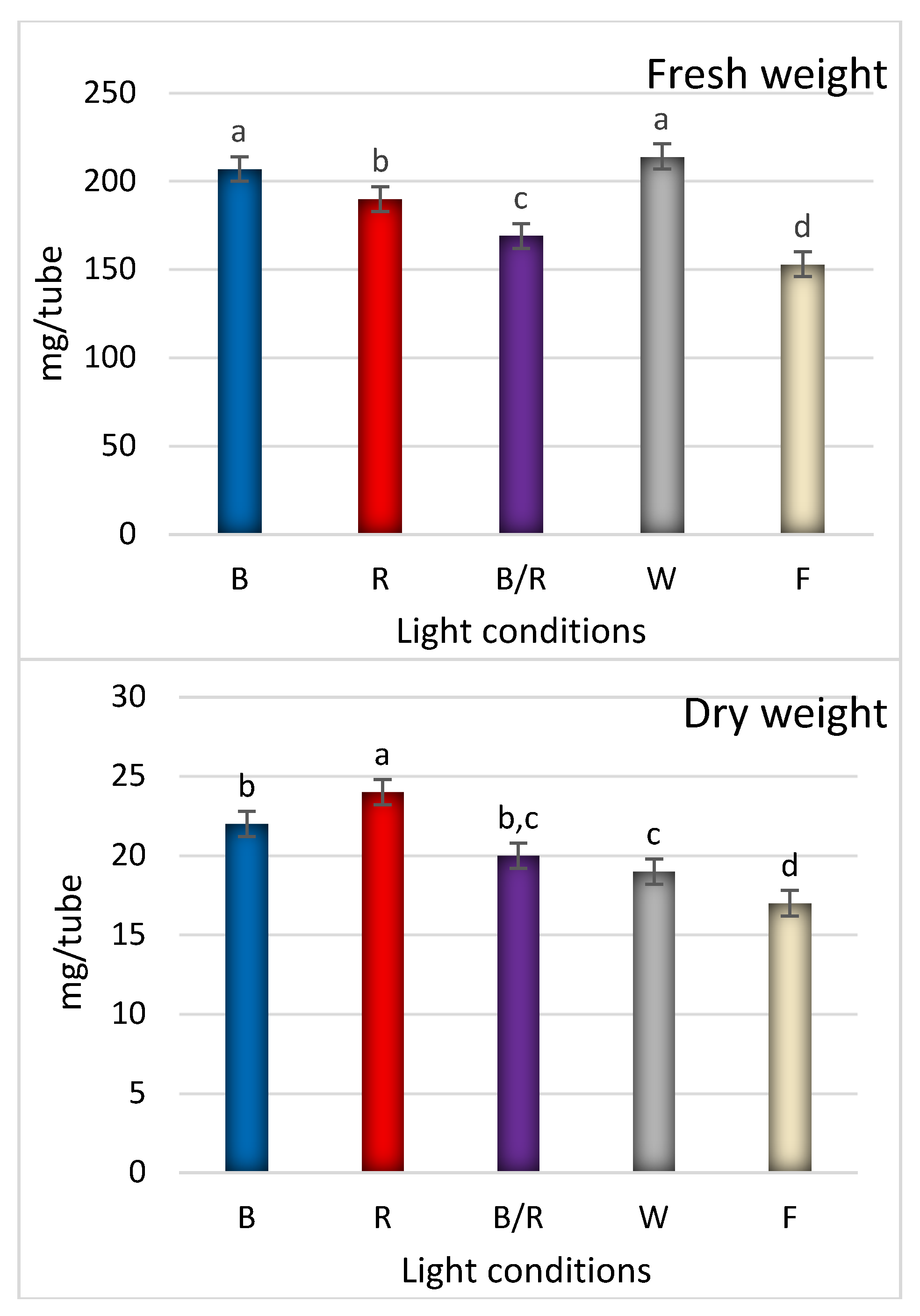
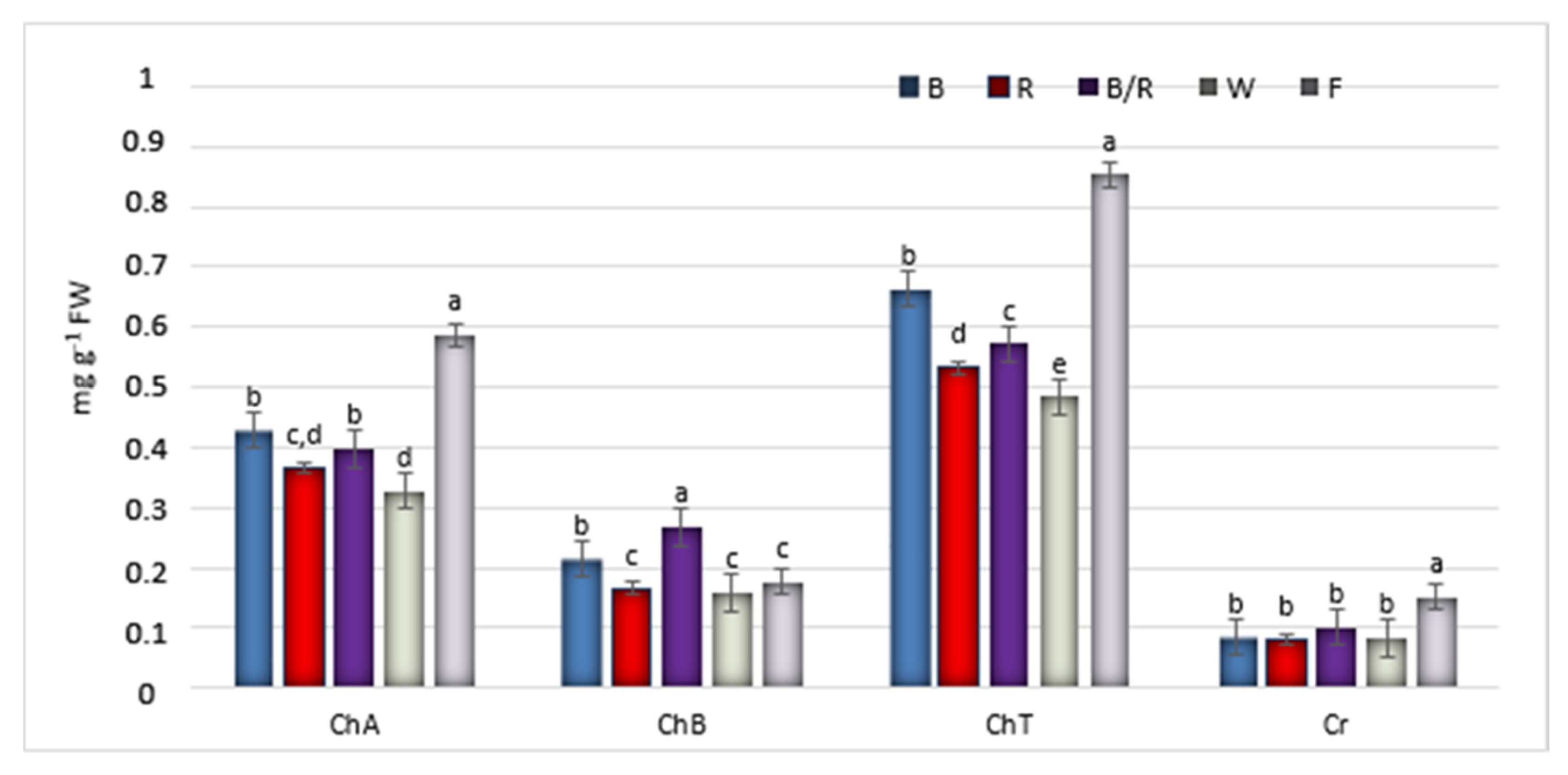
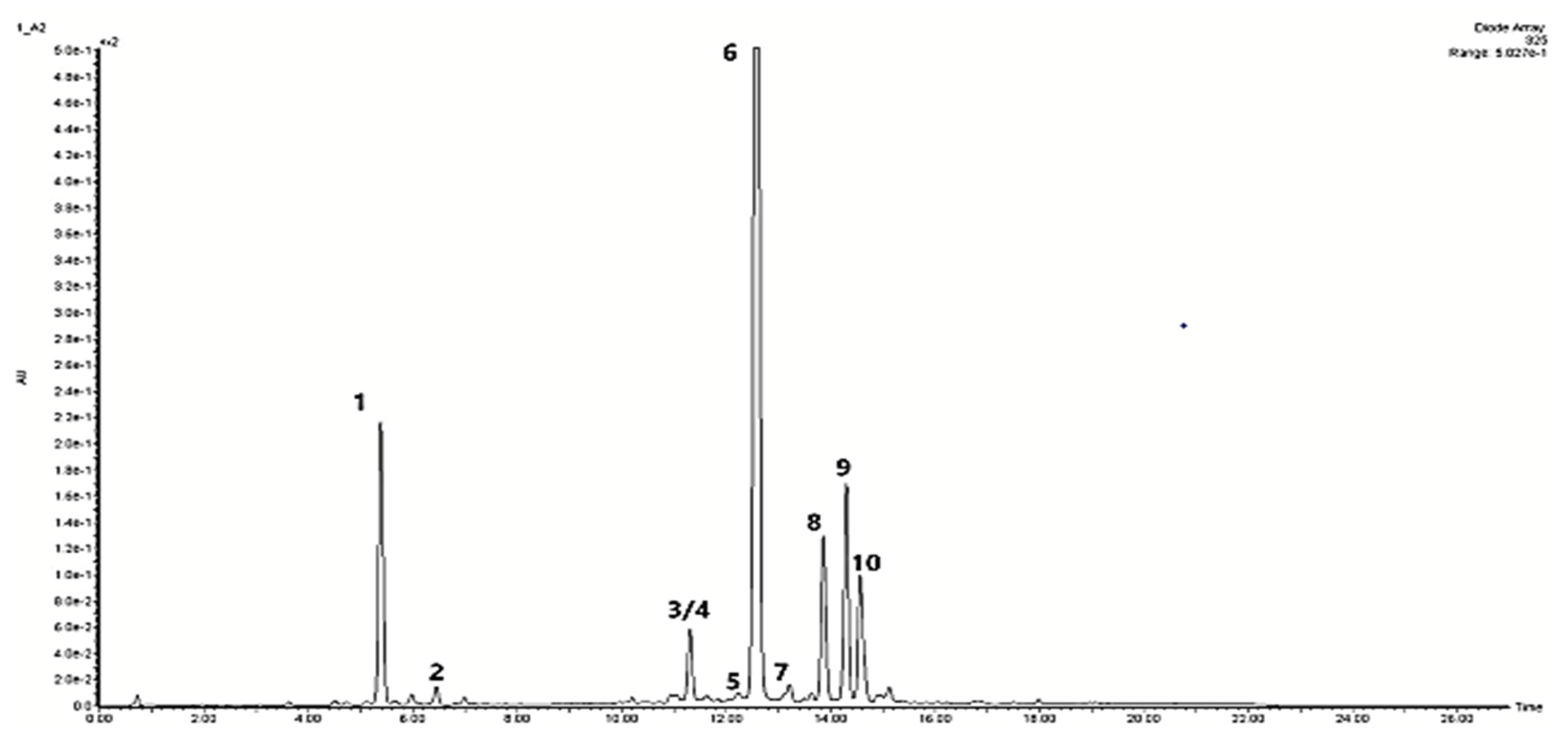
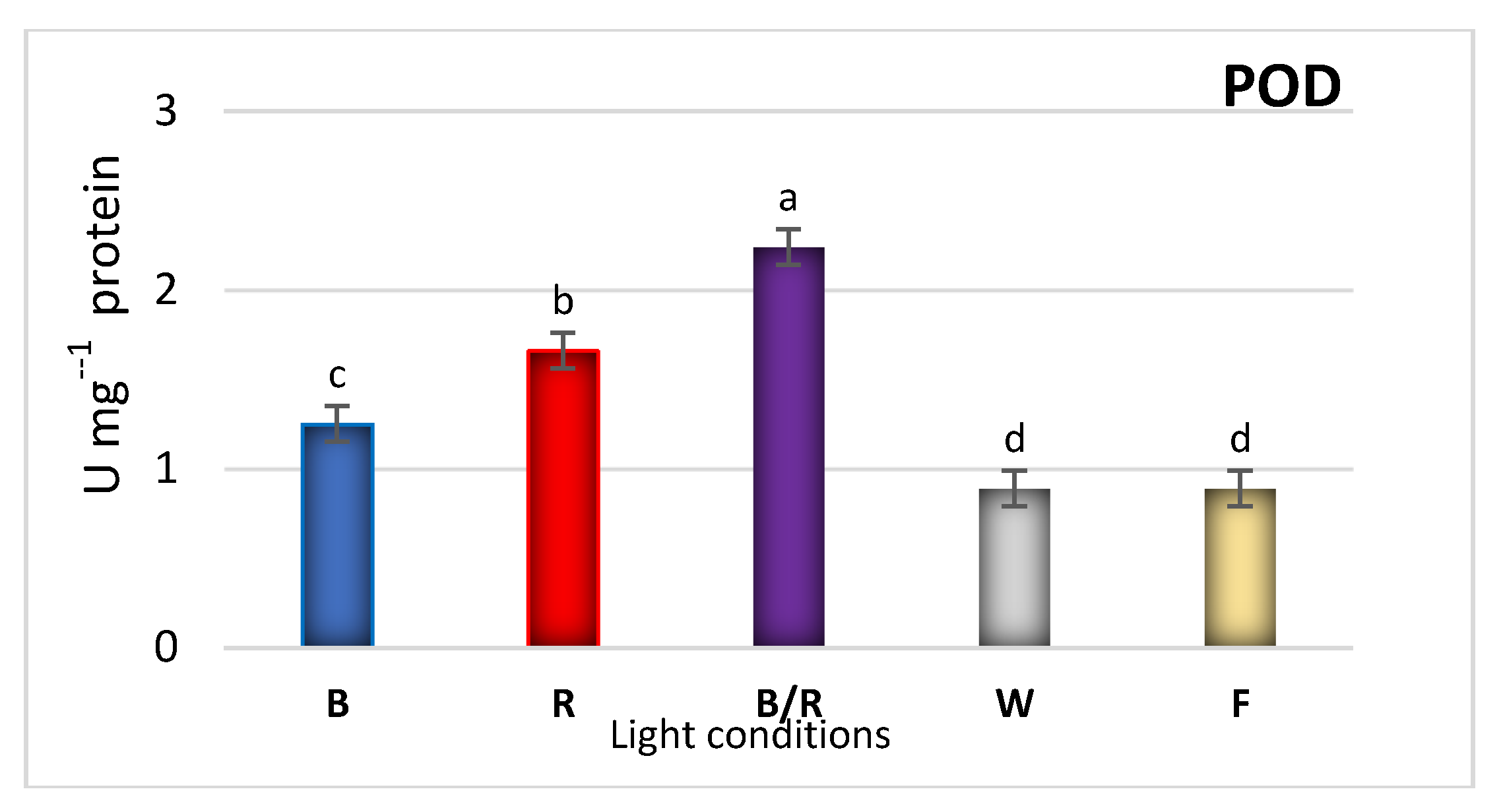
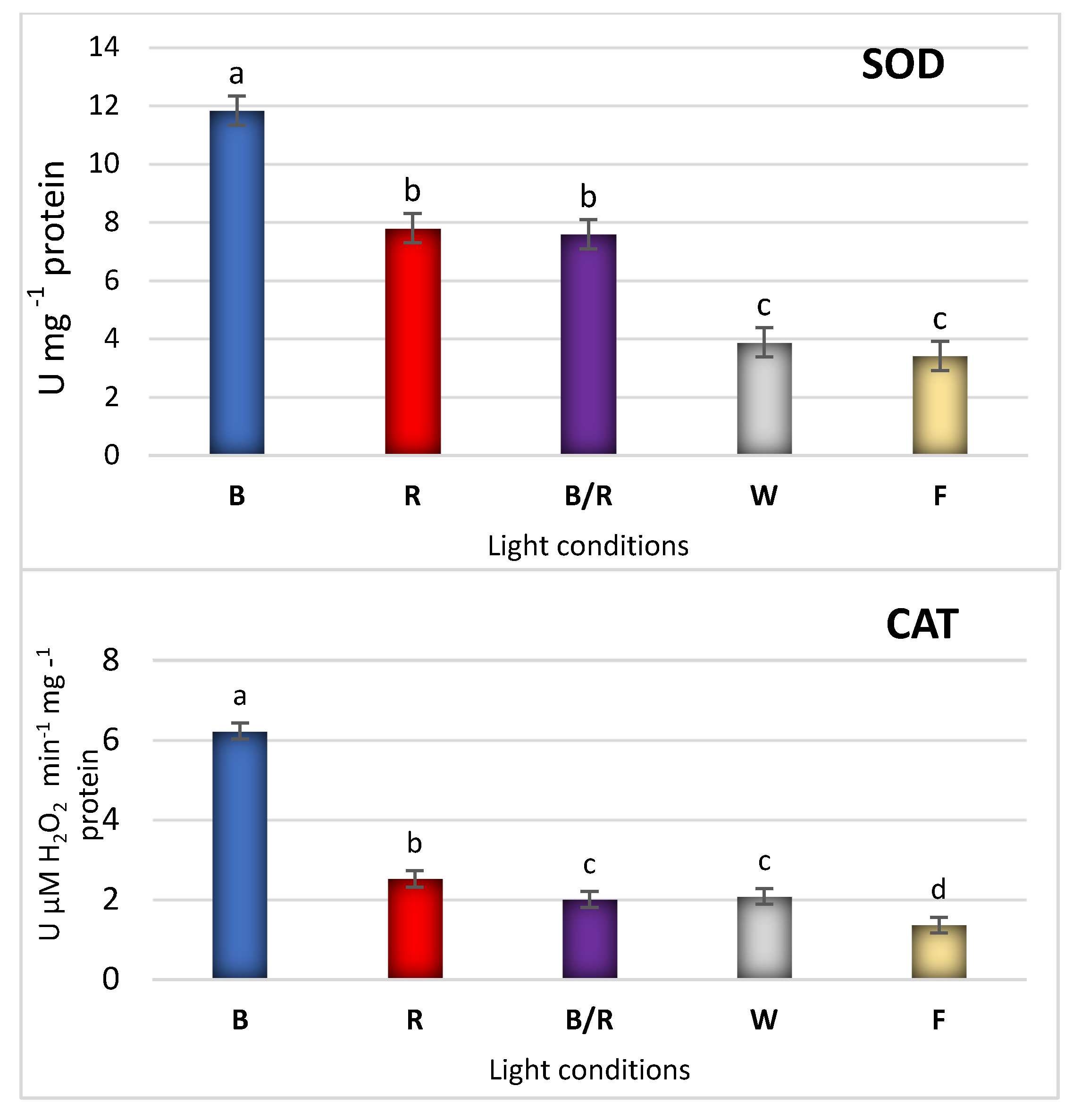
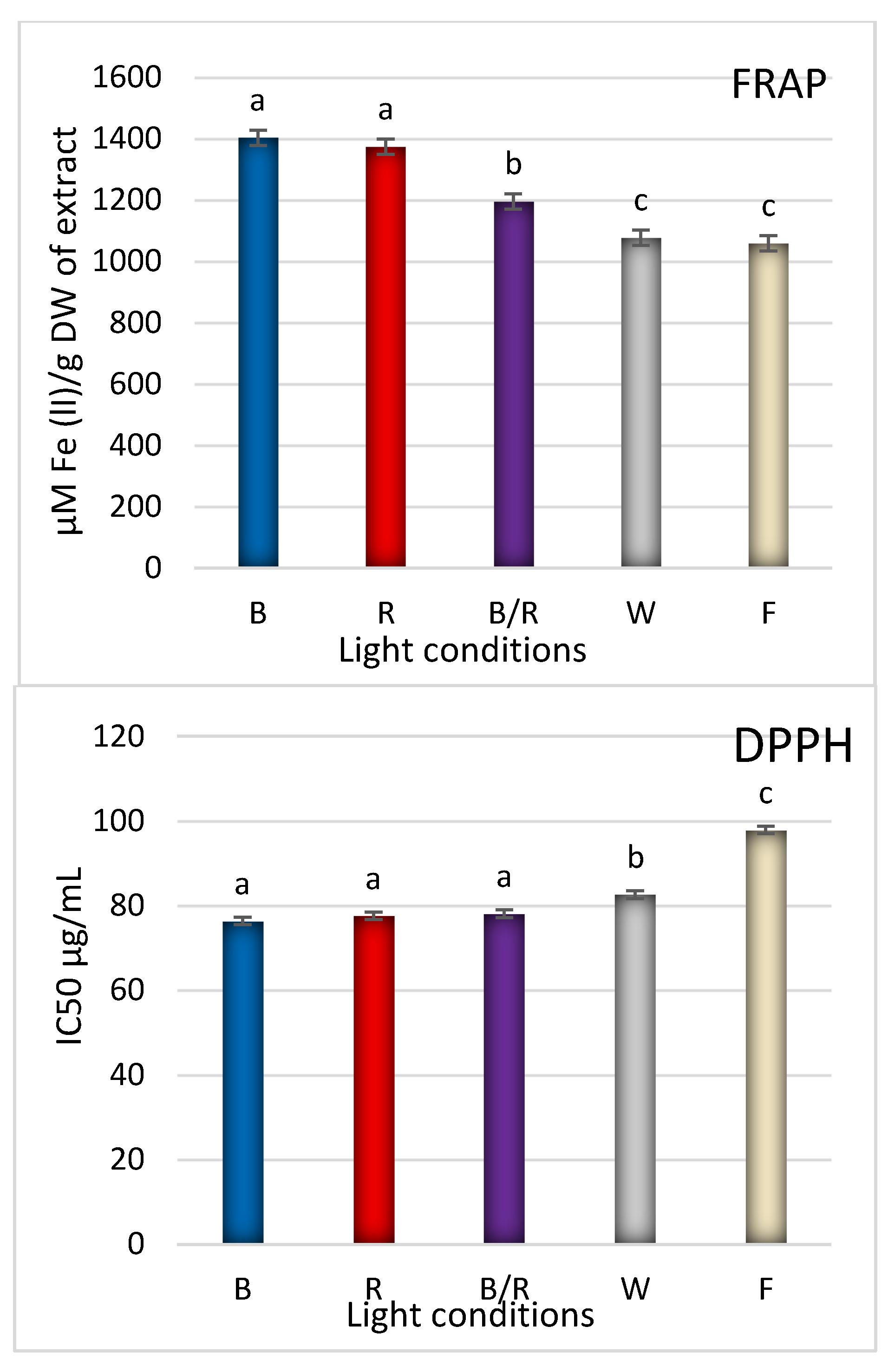
| Peak No. | Compound | Compound Content [mg/g DW] under Different Light Treatments | ||||
|---|---|---|---|---|---|---|
| Blue | Red | Red/Blue | White | Control | ||
| 1 | Chlorogenic acid | 2.001 ± 0.065 a | 1.021 ± 0.125 c | 2.043 ± 0.045 a | 1.839 ± 0.143 b | 1.086 ± 0.101 c |
| 2 | Caffeic acid | 0.096 ± 0.002 b | 0.040 ± 0.003 d | 0.049 ± 0.005 cd | 0.054 ± 0.025 c | 0.133 ± 0.011 a |
| ¾ | Salvianolic acid I/H Salvianolic acid E | 1.364 ± 0.050 c | 1.196 ± 0.135 c | 1.921 ± 0.093 a | 1.513 ± 0.151 b | 0.777 ± 0.066 d |
| 5 | Dicaffeoylquinic acid | 0.049 ± 0.003 b | 0.043 ± 0.004 b | 0.043 ± 0.003 b | 0.06 ± 0.006 a | 0.053 ± 0.007 a,b |
| 6 | Rosmarinic acid | 9.187 ± 0.320 b | 10.896 ± 0.810 a | 6.833 ± 0.591 c | 4.942 ± 0.314 d | 11.461 ± 0.759 a |
| 7 | Lithospermic acid | 0.492 ± 0.016 b | 0.151 ± 0.004 d | 0.552 ± 0.016 a | 0.546 ± 0.045 a,b | 0.414 ± 0.028 c |
| 8 | Salvianolic acid B | 3.285 ± 0.253 a,b | 3.608 ± 0.144 a | 2.842 ± 0.251 b | 2.884 ± 0.278 b | 0.289 ± 0.020 c |
| 9 | Apigenin p-coumarylrhamnoside | 2.448 ± 0.053 a | 1.171 ± 0.084 c | 1.632 ± 0.110 b | 1.180 ± 0.140 c | 1.305 ± 0.061 c |
| 10 | Metyl rosmarinate | 1.096 ± 0.028 a | 1.035 ± 0.013 b | 1.137 ± 0.045 a | 0.806 ± 0.063 c | 0.551 ± 0.028 d |
| Total phenol content | 20.018 ± 0.801 a | 19.161 ± 1.360 a,b | 17.052 ± 1.170 b,c | 13.824 ± 1.181 d | 16.069 ± 1.090 c,d | |
| r | FRAP | DPPH | POD | SOD | CAT |
|---|---|---|---|---|---|
| Total phenol content | 0.91 | −0.67 | 0.40 | 0.88 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weremczuk-Jeżyna, I.; Hnatuszko-Konka, K.; Lebelt, L.; Grzegorczyk-Karolak, I. The Protective Function and Modification of Secondary Metabolite Accumulation in Response to Light Stress in Dracocephalum forrestii Shoots. Int. J. Mol. Sci. 2021, 22, 7965. https://doi.org/10.3390/ijms22157965
Weremczuk-Jeżyna I, Hnatuszko-Konka K, Lebelt L, Grzegorczyk-Karolak I. The Protective Function and Modification of Secondary Metabolite Accumulation in Response to Light Stress in Dracocephalum forrestii Shoots. International Journal of Molecular Sciences. 2021; 22(15):7965. https://doi.org/10.3390/ijms22157965
Chicago/Turabian StyleWeremczuk-Jeżyna, Izabela, Katarzyna Hnatuszko-Konka, Liwia Lebelt, and Izabela Grzegorczyk-Karolak. 2021. "The Protective Function and Modification of Secondary Metabolite Accumulation in Response to Light Stress in Dracocephalum forrestii Shoots" International Journal of Molecular Sciences 22, no. 15: 7965. https://doi.org/10.3390/ijms22157965
APA StyleWeremczuk-Jeżyna, I., Hnatuszko-Konka, K., Lebelt, L., & Grzegorczyk-Karolak, I. (2021). The Protective Function and Modification of Secondary Metabolite Accumulation in Response to Light Stress in Dracocephalum forrestii Shoots. International Journal of Molecular Sciences, 22(15), 7965. https://doi.org/10.3390/ijms22157965






