Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations
Abstract
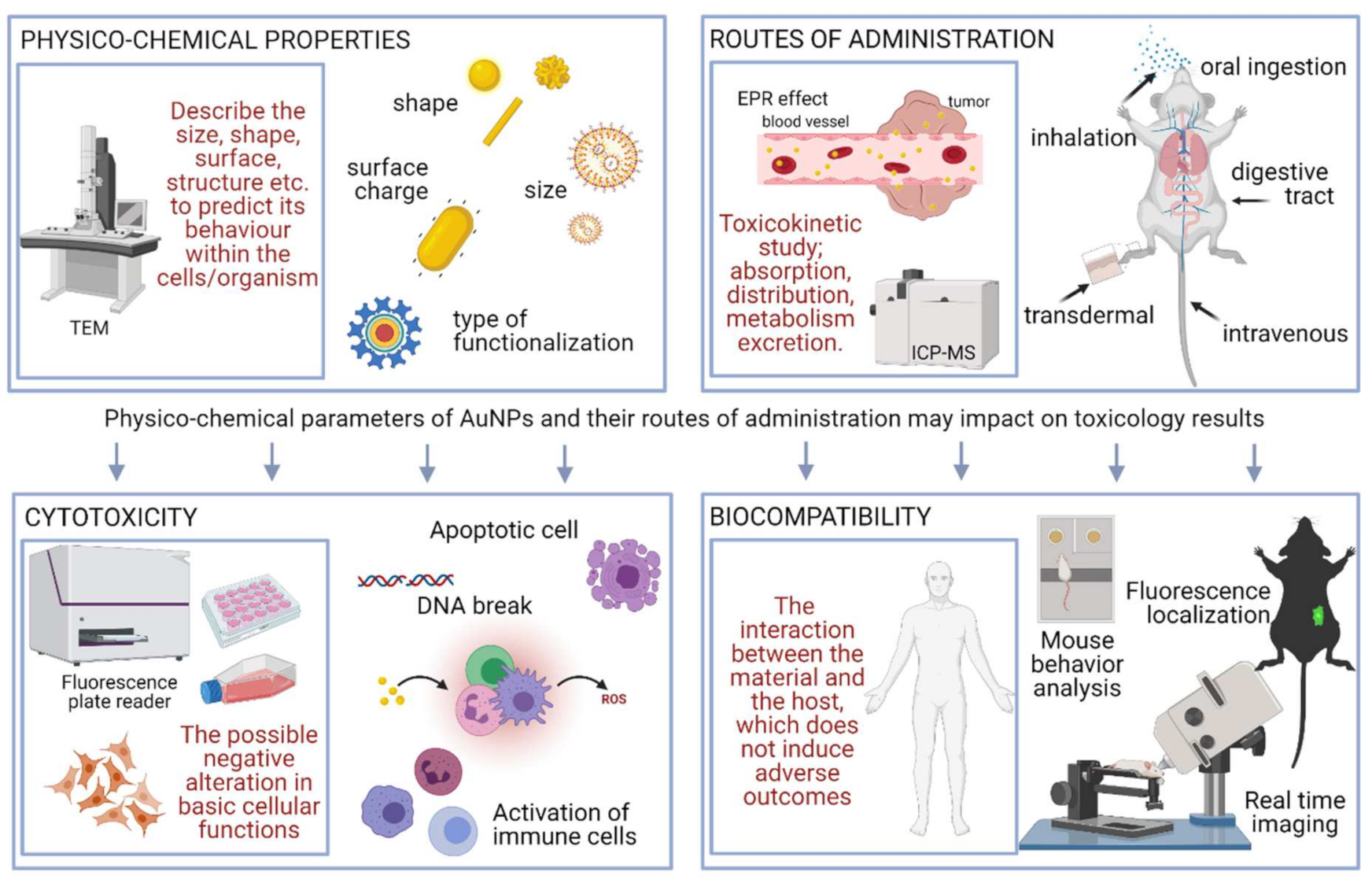
1. Introduction
2. Cytotoxicity and Biocompatibility: Definition and Criteria
Impact of NPs’ Size/Shape/Functionalisation on Their Biological Activity
3. Methods for Toxicity Evaluation
3.1. In Vitro Methodology
3.2. In Vivo Methodology
4. Safety Considerations
5. Regulation
5.1. Regulating Institutions
5.2. Standards and Certificates
6. Perspective, Recent Advances, and Conclusions
Funding
Conflicts of Interest
References
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Ramrakhiani, M. Nanostructures and their applications. Recent Res. Sci. Technol. 2012, 4, 14–19. [Google Scholar]
- Narayanan, R.; El-Sayed, M.A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Banerjee, K.; Das, S.; Choudhury, P.; Ghosh, S.; Baral, R.; Choudhuri, S.K. A Novel Approach of Synthesizing and Evaluating the Anticancer Potential of Silver Oxide Nanoparticles in vitro. Chemotherapy 2017, 62, 279–289. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of noncytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Stasyuk, N.Y.; Smutok, O.V.; Zakalskiy, A.E.; Zakalska, O.M.; Gonchar, M.V. Methylamine-sensitive amperometric biosensor based on (His)6-tagged Hansenula polymorpha methylamine oxidase immobilized on the gold nanoparticles. Biomed. Res. Int. 2014, 2014, 480498. [Google Scholar] [CrossRef] [PubMed]
- Olveira, S.; Forster, S.P.; Seeger, S. Nanocatalysis: Academic Discipline and Industrial Realities. J. Nanotechnol. 2014, 2014, 324089. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Yonzon, C.R.; Haes, A.J.; Duyne, R.P.V. Localized surface plasmon resonance biosensors. Nanomedicine 2006, 1, 219–228. [Google Scholar] [CrossRef]
- Bulavinets, T.; Kulpa-Greszta, M.; Tomaszewska, A.; Kus-Liśkiewicz, M.; Bielatowicz, G.; Yaremchuk, I.; Barylyak, A.; Bobitski, Y.; Pązik, R. Efficient NIR energy conversion of plasmonic silver nanostructures fabricated with the laser-assisted synthetic approach for endodontic applications. RSC Adv. 2020, 10, 38861–38872. [Google Scholar] [CrossRef]
- Murali, K.; Neelakandan, M.S.; Thomas, S. Biomedical applications of gold nanoparticles. JSM Nanotechnol. Nanomed. 2018, 6, 1064. [Google Scholar]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Cao-Milán, R.; Liz-Marzán, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Biocompatibility and drug delivery systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; Taylor & Francis: Milton Park, UK, 2005. [Google Scholar] [CrossRef]
- Jia, H.Y.; Liu, Y.; Zhang, X.J.; Han, L.; Du, L.B.; Tian, Q.; Xu, Y.C. Potential Oxidative Stress of Gold Nanoparticles by Induced-NO Releasing in Serum. J. Am. Chem. Soc. 2009, 131, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss-Stein, S.; Bornemann, J.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wu, H.Y.; Wu, D.; Wang, Y.Y.; Chang, J.H.; Zhai, Z.B.; Meng, A.M.; Liu, P.X.; Zhang, L.A.; Fan, F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Cambre, M.; Lee, H.-J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Villiers, C.; Freitas, H.; Couderc, R.; Villiers, M.-B.; Marche, P. Analysis of the toxicity of gold nano particles on the immune system: Effect on dendritic cell functions. J. Nanopart. Res. 2010, 12, 55–60. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Ng, C.T.; Tang, F.M.A.; Li, J.J.e.; Ong, C.; Yung, L.L.Y.; Bay, B.H. Clathrin-Mediated Endocytosis of Gold Nanoparticles In Vitro. Anat. Rec. 2015, 298, 418–427. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz-Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Biazar, E.; Jafarpour, M.; Montazeri, M.; Majdi, A.; Aminifard, S.; Zafari, M.; Akbari, H.R.; Rad, H.G. Nanotoxicology and nanoparticle safety in biomedical designs. Int. J. Nanomed. 2011, 6, 1117–1127. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, D.; Shen, X.; Liu, P.-X.; Yang, N.; Zhao, B.; Zhang, H.; Sun, Y.-M.; Zhang, L.-A.; Fan, F.-Y. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int. J. Nanomed. 2011, 6, 2071–2081. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Di Bucchianico, S.; Fabbrizi, M.R.; Cirillo, S.; Uboldi, C.; Gilliland, D.; Valsami-Jones, E.; Migliore, L. Aneuploidogenic effects and DNA oxidation induced in vitro by differently sized gold nanoparticles. Int. J. Nanomed. 2014, 9, 2191–2204. [Google Scholar] [CrossRef]
- Vetten, M.A.; Tlotleng, N.; Tanner Rascher, D.; Skepu, A.; Keter, F.K.; Boodhia, K.; Koekemoer, L.-A.; Andraos, C.; Tshikhudo, R.; Gulumian, M. Label-free in vitro toxicity and uptake assessment of citrate stabilised gold nanoparticles in three cell lines. Part. Fibre Toxicol. 2013, 10, 50. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Shim, J.; Kang, M.-S.; Moon, S.-H. Optimized coverage of gold nanoparticles at tyrosinase electrode for measurement of a pesticide in various water samples. J. Hazard. Mater. 2008, 156, 141–147. [Google Scholar] [CrossRef]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in Understanding Size and Shape Dependent Toxicity of Gold Nanomaterials in Human Skin Keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Bhamidipati, M.; Fabris, L. Multiparametric Assessment of Gold Nanoparticle Cytotoxicity in Cancerous and Healthy Cells: The Role of Size, Shape, and Surface Chemistry. Bioconjugate Chem. 2017, 28, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Burtea, C.; Thirifays, C.; Häfeli, U.O.; Mahmoudi, M. Crucial ignored parameters on nanotoxicology: The importance of toxicity assay modifications and “cell vision”. PLoS ONE 2012, 7, e29997. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Laurent, S.; Shokrgozar, M.A.; Hosseinkhani, M. Toxicity Evaluations of Superparamagnetic Iron Oxide Nanoparticles: Cell “Vision” versus Physicochemical Properties of Nanoparticles. ACS Nano 2011, 5, 7263–7276. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconjugate Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.; Banerjee, S.K. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Yang, Y.; Matsubara, S.; Nogami, M.; Shi, J. Controlling the aggregation behavior of gold nanoparticles. Mater. Sci. Eng. B 2007, 140, 172–176. [Google Scholar] [CrossRef]
- Basu, S.; Ghosh, S.K.; Kundu, S.; Panigrahi, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, T. Biomolecule induced nanoparticle aggregation: Effect of particle size on interparticle coupling. J. Colloid Interface Sci. 2007, 313, 724–734. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Coradeghini, R.; Gioria, S.; García, C.P.; Nativo, P.; Franchini, F.; Gilliland, D.; Ponti, J.; Rossi, F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 2013, 217, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Wang, L.; Hou, X.; Liu, W.; Chen, C. Interaction of gold nanoparticles with proteins and cells. Sci. Technol. Adv. Mater. 2015, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef]
- Okoampah, E.; Mao, Y.; Yang, S.; Sun, S.; Zhou, C. Gold nanoparticles–biomembrane interactions: From fundamental to simulation. Colloids Surf. B: Biointerfaces 2020, 196, 111312. [Google Scholar] [CrossRef] [PubMed]
- Tedja, R.; Lim, M.; Amal, R.; Marquis, C. Effects of Serum Adsorption on Cellular Uptake Profile and Consequent Impact of Titanium Dioxide Nanoparticles on Human Lung Cell Lines. ACS Nano 2012, 6, 4083–4093. [Google Scholar] [CrossRef] [PubMed]
- Freese, C.; Unger, R.E.; Deller, R.C.; Gibson, M.I.; Brochhausen, C.; Klok, H.-A.; Kirkpatrick, C.J. Uptake of poly(2-hydroxypropylmethacrylamide)-coated gold nanoparticles in microvascular endothelial cells and transport across the blood–brain barrier. Biomater. Sci. 2013, 1, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Vanhecke, D.; Kuhn, D.A.; Jimenez de Aberasturi, D.; Balog, S.; Milosevic, A.; Urban, D.; Peckys, D.; de Jonge, N.; Parak, W.J.; Petri-Fink, A.; et al. Involvement of two uptake mechanisms of gold and iron oxide nanoparticles in a co-exposure scenario using mouse macrophages. Beilstein J. Nanotechnol. 2017, 8, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Powers, J.M. (Eds.) Chapter 6-Biocompatibility and Tissue Reaction to Biomaterials. In Craig’s Restorative Dental Materials, 13th ed.; Mosby: Saint Louis, MO, USA, 2012; pp. 109–133. [Google Scholar]
- Pavlovich, E.; Volkova, N.; Yakymchuk, E.; Perepelitsyna, O.; Sydorenko, M.; Goltsev, A. In Vitro Study of Influence of Au Nanoparticles on HT29 and SPEV Cell Lines. Nanoscale Res. Lett. 2017, 12, 494. [Google Scholar] [CrossRef][Green Version]
- Ben Tahar, I.; Fickers, P.; Dziedzic, A.; Płoch, D.; Skóra, B.; Kus-Liśkiewicz, M. Green pyomelanin-mediated synthesis of gold nanoparticles: Modelling and design, physico-chemical and biological characteristics. Microb. Cell Fact. 2019, 18, 210. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://sites.broadinstitute.org/ccle (accessed on 8 August 2021).
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Wang, Y.; Liu, H. Gold Nanoparticles Promote Proliferation of Human Periodontal Ligament Stem Cells and Have Limited Effects on Cells Differentiation. J. Nanomater. 2016, 2016, 1431836. [Google Scholar] [CrossRef]
- Tedesco, S.; Doyle, H.; Blasco, J.; Redmond, G.; Sheehan, D. Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat. Toxicol. 2010, 100, 178–186. [Google Scholar] [CrossRef]
- Mechanisms of Cell Death in Oxidative Stress. Antioxid. Redox Signal. 2007, 9, 49–89. [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jia, J.; Jiang, C.; Zhai, S. Gold Nanoparticle-Induced Cell Death and Potential Applications in Nanomedicine. Int. J. Mol. Sci. 2018, 19, 754. [Google Scholar] [CrossRef] [PubMed]
- de Alteriis, E.; Falanga, A.; Galdiero, S.; Guida, M.; Maselli, V.; Galdiero, E. Genotoxicity of gold nanoparticles functionalized with indolicidin towards Saccharomyces cerevisiae. J. Environ. Sci. 2018, 66, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Li, H.; Liu, Y.; Zhang, S.; Feng, Q.; Xiao, K. The effect of particle size on the genotoxicity of gold nanoparticles. J. Biomed. Mater. Res. Part A 2017, 105, 710–719. [Google Scholar] [CrossRef]
- Devanabanda, M.; Latheef, S.A.; Madduri, R. Immunotoxic effects of gold and silver nanoparticles: Inhibition of mitogen-induced proliferative responses and viability of human and murine lymphocytes in vitro. J. Immunotoxicol. 2016, 13, 897–902. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef]
- Aslantürk, Ö. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. In Biotechnology Annual Review; 2005; Volume 11, pp. 127–152. [Google Scholar] [CrossRef]
- Guarnieri, D.; Sabella, S.; Muscetti, O.; Belli, V.; Malvindi, M.A.; Fusco, S.; Luca, E.; Pompa, P.; Netti, P. Transport across the cell-membrane dictates nanoparticle fate and toxicity: A new paradigm in nanotoxicology. Nanoscale 2014, 6, 10264–10273. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ganesan, S. In Vitro Cytotoxicity Assay on Gold Nanoparticles with Different Stabilizing Agents. J. Nanomater. 2012, 2012, 734398. [Google Scholar] [CrossRef]
- Almutary, A.; Sanderson, B.J.S. The MTT and Crystal Violet Assays: Potential Confounders in Nanoparticle Toxicity Testing. Int. J. Toxicol. 2016, 35, 454–462. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Tee, J.K.; Ong, C.N.; Bay, B.H.; Ho, H.K.; Leong, D.T. Oxidative stress by inorganic nanoparticles. WIREs Nanomed. Nanobiotechnol. 2016, 8, 414–438. [Google Scholar] [CrossRef]
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; Peixoto de Almeida, M.; Araújo, A.M.; Bastos, M.d.L.; Carmo, H. Gold Nanoparticles Induce Oxidative Stress and Apoptosis in Human Kidney Cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Au-Aguilar Diaz De Leon, J.; Au-Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, e61122. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox. Biol. 2013, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta Int. J. Clin. Chem. 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Li, J.J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef]
- Berninghausen, O.; Leippe, M. Necrosis versus apoptosis as the mechanism of target cell death induced by Entamoeba histolytica. Infect. Immun. 1997, 65, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Koç, E.; Çelik-Uzuner, S.; Uzuner, U.; Çakmak, R. The Detailed Comparison of Cell Death Detected by Annexin V-PI Counterstain Using Fluorescence Microscope, Flow Cytometry and Automated Cell Counter in Mammalian and Microalgae Cells. J. Fluoresc. 2018, 28, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Vanoirbeek, J.A.J.; Hoet, P.H.M. Interactions of nanomaterials with the immune system. WIREs Nanomed. Nanobiotechnol. 2012, 4, 169–183. [Google Scholar] [CrossRef]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://database.ich.org/sites/default/files/S8_Guideline_0.pdf (accessed on 8 August 2021).
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Elespuru, R.; Pfuhler, S.; Aardema, M.J.; Chen, T.; Doak, S.H.; Doherty, A.; Farabaugh, C.S.; Kenny, J.; Manjanatha, M.; Mahadevan, B.; et al. Genotoxicity Assessment of Nanomaterials: Recommendations on Best Practices, Assays, and Methods. Toxicol. Sci. 2018, 164, 391–416. [Google Scholar] [CrossRef]
- Doak, S.H.; Dusinska, M. NanoGenotoxicology: Present and the future. Mutagenesis 2016, 32, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Donner, E.M. Rationale of genotoxicity testing of nanomaterials: Regulatory requirements and appropriateness of available OECD test guidelines. Nanotoxicology 2010, 4, 409–413. [Google Scholar] [CrossRef]
- In Vitro Mammalian Cell Gene Mutation Thymidine Kinase. Available online: https://www.oecd.org/env/ehs/testing/.pdf (accessed on 18 June 2021).
- Wang, Y.; Zhang, H.; Shi, L.; Xu, J.; Duan, G.; Yang, H. A focus on the genotoxicity of gold nanoparticles. Nanomedicine 2020, 15, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; de Melo, T.C.; Mendes, T.B.; de Sá Júnior, P.L.; Nozima, B.H.N.; Ito, E.T.; de Carvalho, R.F.; de Souza, E.B.; de Cassia Stocco, R. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed. Pharmacother. 2015, 72, 74–82. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A.R. The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef]
- Wong, B.S.E.; Hu, Q.; Baeg, G.H. Epigenetic modulations in nanoparticle-mediated toxicity. Food Chem. Toxicol. 2017, 109, 746–752. [Google Scholar] [CrossRef]
- Chappell, G.; Pogribny, I.P.; Guyton, K.Z.; Rusyn, I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat. Res. Rev. Mutat. Res. 2016, 768, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Atyah, M.; Chen, W.-Y.; Zhou, C.-H. The various aspects of genetic and epigenetic toxicology: Testing methods and clinical applications. J. Transl. Med. 2017, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, J.; Zhou, X. A review: MicroRNA detection methods. Org. Biomol. Chem. 2015, 13, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of nanoparticles for therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. J. Control Release 2015, 220, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Juillerat-Jeanneret, L.; Dusinska, M.; Fjellsbø, L.M.; Collins, A.R.; Handy, R.D.; Riediker, M. Biological impact assessment of nanomaterial used in nanomedicine. Introduction to the NanoTEST project. Nanotoxicology 2015, 9, 5–12. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Available online: https://dda.creative-bioarray.com/immunotoxicology.html (accessed on 18 June 2021).
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef]
- Committee, E.S. Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011, 9, 2140. [Google Scholar] [CrossRef]
- Chou, L.Y.T.; Chan, W.C.W. Fluorescence-Tagged Gold Nanoparticles for Rapidly Characterizing the Size-Dependent Biodistribution in Tumor Models. Adv. Healthc. Mater. 2012, 1, 714–721. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef]
- Frère, A.; Evrard, B.; Mottet, D.; Piel, G. 18-Polymeric Nanoparticles as siRNA Drug Delivery System for Cancer Therapy: The Long Road to Therapeutic Efficiency. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 503–540. [Google Scholar]
- Toutain, P.L.; Bousquet-Mélou, A. Plasma clearance. J. Vet. Pharmacol. Ther. 2004, 27, 415–425. [Google Scholar] [CrossRef]
- Haute, D.V.; Berlin, J.M. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: Lessons from gold nanoparticles. Ther. Deliv. 2017, 8, 763–774. [Google Scholar] [CrossRef]
- Chen, F.; Goel, S.; Hernandez, R.; Graves, S.A.; Shi, S.; Nickles, R.J.; Cai, W. Dynamic Positron Emission Tomography Imaging of Renal Clearable Gold Nanoparticles. Small 2016, 12, 2775–2782. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, Z.; Zhong, H.; Ji, Y. Monitoring the penetration and accumulation of gold nanoparticles in rat skin ex vivo using surface-enhanced Raman scattering spectroscopy. J. Innov. Opt. Health Sci. 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Imani, M.; Halimi, M.; Khara, H. Effects of silver nanoparticles (AgNPs) on hematological parameters of rainbow trout, Oncorhynchus mykiss. Comp. Clin. Pathol. 2015, 24, 491–495. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Van Der Ven, L.T.M.; Sleijffers, A.; Park, M.V.D.Z.; Jansen, E.H.J.M.; Van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [PubMed]
- El-Drieny, E.A.E.A.; Sarhan, N.I.; Bayomy, N.A.; Elmajied Elsherbeni, S.A.; Momtaz, R.; Mohamed, H.E.-D. Histological and immunohistochemical study of the effect of gold nanoparticles on the brain of adult male albino rat. J. Microsc. Ultrastruct. 2015, 3, 181–190. [Google Scholar] [CrossRef]
- Khan, H.A.; Ibrahim, K.E.; Alrashood, S.T.; Alamery, S.; Alrokayan, S.H.; Al-Harbi, N.; Al-Mutary, M.G.; Sobki, S.H.; Khan, I. Immunohistochemistry of IL-1β, IL-6 and TNF-α in spleens of mice treated with gold nanoparticles. Saudi J. Biol. Sci. 2020, 27, 1163–1168. [Google Scholar] [CrossRef]
- Khan, H.A.; Ibrahim, K.E.; Khan, A.; Alrokayan, S.H.; Alhomida, A.S.; Lee, Y.-k. Comparative evaluation of immunohistochemistry and real-time PCR for measuring proinflammatory cytokines gene expression in livers of rats treated with gold nanoparticles. Exp. Toxicol. Pathol. 2016, 68, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules 2018, 23, 1848. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Xiao, S.; Liu, X.; Chen, X.; Xia, Q.; Lei, S.; Li, H.; Zhong, Z.; Xiao, K. The Effects of Gold Nanoparticles on Leydig Cells and Male Reproductive Function in Mice. Int. J. Nanomed. 2020, 15, 9499–9514. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-s2-r1-genotoxicity-testing-data-interpretation-pharmaceuticals-intended-human-use-step_en.pdf (accessed on 14 June 2021).
- Schulz, M.; Ma-Hock, L.; Brill, S.; Strauss, V.; Treumann, S.; Gröters, S.; van Ravenzwaay, B.; Landsiedel, R. Investigation on the genotoxicity of different sizes of gold nanoparticles administered to the lungs of rats. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2012, 745, 51–57. [Google Scholar] [CrossRef]
- Available online: https://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oral-toxicity-study-in-rodents_9789264070684-en (accessed on 14 June 2021).
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gao, Y.; Mu, L.; Zhou, Q. Knowledge gaps between nanotoxicological research and nanomaterial safety. Environ. Int. 2016, 94, 8–23. [Google Scholar] [CrossRef]
- Dusinska, M.; Dusinska, M.; Fjellsbø, L.; Magdolenova, Z.; Rinna, A.; Pran, E.R.; Bartonova, A.; Heimstad, E.; Harju, M.; Tran, L.; et al. Testing strategies for the safety of nanoparticles used in medical applications. Nanomedicine 2009, 4, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.G.; Proykova, A.; Santos, G.M.L. Dealing with nanosafety around the globe—Regulation vs. innovation. Int. J. Pharm. 2016, 509, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/media/115075/download (accessed on 14 June 2021).
- Sättler, D.; Schnöder, F.; Aust, N.; Ahrens, A.; Bögi, C.; Traas, T.; Tolls, J. Specific environmental release categories—A tool for improving chemical safety assessment in the EC—report of a multi-stakeholder workshop. Integr. Environ. Assess. Manag. 2012, 8, 580–585. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for Review of the Federal Strategy to Address Environmental, Health, and Safety Research Needs for Engineered Nanoscale Materials. Review of the Federal Strategy for Nanotechnology-Related Environmental, Health, and Safety Research; The National Research Council: Washington, DC, USA, 2009. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Rauscher, H.; Roebben, G.; Mech, A.; Gibson, P.; Kestens, V.; Linsinger, T.; Riego Sintes, J. An Overview of Concepts and Terms Used in the European Commission’s Definition of Nanomaterial, EUR 29647 EN, European Commission; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-79-99660-3. [Google Scholar]
- European Chemicals Agency. Human Health and Environmental Exposure Assessment and Risk Characterisation of Nanomaterials-Best Practice for Reach Registrants; European Chemicals Agency: Helsinki, Finland, 2014. [Google Scholar]
- Available online: https://ec.europa.eu/health/scientific_committees/emerging (accessed on 8 August 2021).
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Final Opinion on the Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices; European Comission: Brussels, Belgium, 2015. [Google Scholar]
- Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety (accessed on 14 June 2021).
- SCCS Members. The SCCS guidance on the safety assessment of nanomaterials in cosmetics. Regul. Toxicol. Pharmacol. 2020, 112, 104611. [Google Scholar] [CrossRef]
- Available online: https://www.efsa.europa.eu/en (accessed on 14 June 2021).
- Schoonjans, R.; Eryasa, F.B.; EFSA (European Food Safety Authority). Annual Report of the EFSA Scientific Network of Risk Assessment of Nanotechnologies in Food and Feed for 2018; EFSA Supporting Publication: Parma, Italy, 2019; 11p. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en (accessed on 14 June 2021).
- Committee for Medicinal Products for Human Use (CHMP). Reflection Paper on Surface Coatings: General Issues for Consideration Regarding Parenteral Administration of Coated Nanomedicine Products; European Medicines Agency, Committee for Medicinal Products for Human Use: London, UK, 2013. [Google Scholar]
- Available online: https://www.fda.gov/ (accessed on 14 June 2021).
- Food and Drug Administration. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology; Food and Drug Administration: Silver Spring, MD, USA.
- Available online: https://www.aqsiq.net/ (accessed on 23 August 2021).
- Available online: http://english.nanoctr.cas.cn/ (accessed on 23 August 2021).
- Available online: http://www.sac.gov.cn/ (accessed on 23 August 2021).
- Available online: https://www.gov.br/anvisa/pt-br (accessed on 23 August 2021).
- Available online: https://www.nanowerk.com/spotlight/spotid=5736.php (accessed on 14 June 2021).
- Available online: https://www.iso.org/standards.html (accessed on 14 June 2021).
- Lipsky, M.S.; Sharp, L.K. From idea to market: The drug approval process. J. Am. Board Fam. Pract. 2001, 14, 362–367. [Google Scholar]
- Eifler, A.C.; Thaxton, C.S. Nanoparticle Therapeutics: FDA Approval, Clinical Trials, Regulatory Pathways, and Case Study. In Biomedical Nanotechnology: Methods and Protocols; Hurst, S.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 325–338. [Google Scholar]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupanǒiǒ, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Myer, L.; Haynes, R.; Gannon, W.; Walker, M.; Seidel, G.; Byrnes, A.; Yuldasheva, N.; Tamarkin, L. Results of a completed phase I clinical trial of CYT-6091: A pegylated colloidal gold-TNF nanomedicine. J. Clin. Oncol. 2009, 27, 3586. [Google Scholar] [CrossRef]
- Available online: https://adisinsight.springer.com/drugs/800025369 (accessed on 14 June 2021).
| Type of Study | Principle of the Method and Application |
|---|---|
| Metabolic activity | Used to measure cellular metabolism by assessment of metabolically active cells [61]. |
| Cell proliferation and viability | Measures the balance between cell divisions and cell death in a response to NMs stimuli or assessment of the ratio of the live to dead cells [62]. |
| Oxidative stress | Measures the imbalance in free radical formation within a cell caused after exposure to NMs [63]. |
| Apoptosis assays | Analyses whether the cells can trigger their own death in response to extracellular signals such as MNs [64]. |
| Necrosis assays | Used to evaluate membrane integrity and determine the viability of cells [65,66]. |
| Genotoxicity | Identification of the damages of genetic information within a cell causing mutations after NM treatment [67,68]. |
| Immunotoxicity | Measures the immunomodulatory potential of the NMs; may be exhibited as either suppression or enhancement of the immune response [69,70]. |
| Type of Study | Principle of the Method and Application |
|---|---|
| Behavioural analysis and body weight | Investigation of changes in animal behaviour and body weight after exposure to AuNPs. |
| Biodistribution | Shows the localisation of AuNPs in tissues and organs; can be detected in live or killed animals. |
| Biodegradation and clearance | The examination of excretion and metabolism of nanoparticles at various time points after exposure. |
| Pharmacokinetic, haematology, and serum chemistry | Analysis of the components of the blood; estimation of the blood half-life of NMs. |
| Immunology | Evaluates the potential side effects of NMs, and includes inhibition or enhancement of the immune response; histopathology of lymphoid organs. |
| Histopathology | Examination of tissues exposed to NMs, with their localisation and identification of pathological changes in the structure of tissues. |
| Acute and repeated-dose toxicity | Describes the adverse effects of a substance that result either from a single exposure or from multiple exposures in a short period of time. |
| Reproductive and developmental toxicity | Defined as adverse effects of a chemical substance on sexual function and fertility in adult males and females, as well as developmental toxicity in offspring. |
| Genotoxicity and mutagenicity | Impact of NMs on genetic materials and evaluation of the induction of permanent transmissible changes in the amount or structure of the genetic material of cells or organisms. |
| Name of the Authority | Regulations, Procedures, Standardisation, and References |
|---|---|
| Joint Research Centre (JRC) | Aims to provide evidence-based scientific support in the European policymaking process [140]. |
| European Chemicals Agency (ECHA) | Manages the implementation of all the regulations on registration, evaluation, authorisation, and restriction of chemicals (REACH) [141]. |
| Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) | Provides opinions on emerging or newly identified health and environmental risks; and on broad, complex, or multidisciplinary issues requiring a comprehensive assessment of risks to consumer safety or public health and related issues [142,143]. |
| Scientific Committee on Consumer Safety (SCCS) | Provides opinions on health and safety risks (chemical, biological, mechanical, and other physical risks) of nonfood consumer products (e.g., cosmetic products and their ingredients, toys, textiles, clothing, and personal care and household products) and services [144,145]. |
| European Food Safety Authority (EFSA) | Publishes guidelines on the evaluation of nanosafety in food products, with recommendations for analytical technologies [146,147]. |
| European Medicines Agency (EMA) | Fosters scientific excellence in the evaluation and supervision of medicines, for the benefit of public and animal health in the European Union (EU) [148,149]. |
| U.S. Food and Drug Administration (FDA) | Responsible for protecting the public health by ensuring the safety, efficacy, and security of drugs, biological products, medical devices, food supply, and cosmetics [150,151]. |
| Quality Supervision, Inspection, and Quarantine (AQSIQ) | Responsible for commodity inspection, food safety, certification, accreditation, and standardisation [152]. |
| Chinese National Nanotechnology Standardization Technical Committee (NSTC) | Reviews the standards for nanomaterials [153]. |
| Standardization Administration of the People’s Republic of China (SAC) | Setting up standards for nanomaterials and nanomaterial characterisation [154]. |
| Brazilian Health Surveillance Agency (ANVISA) | Promotes regulations on research, production, waste disposal, and the use of nanotechnologies [155]. |
| Name of the Organisation | Example of Standards, Guidance Documents, and References |
|---|---|
| ISO Technical Committee on Nanotechnologies (TC 229) | ISO 19007:2018; In vitro MTS assay for measuring the cytotoxic effect of nanoparticles. |
| European Committee for Standardisation (CEN) | CEN/TC 352:WI = 00352043; Guidance on the determination of aggregation and agglomeration state of nano-objects. |
| American Society for Testing and Materials (ASTM), Committee E56 on Nanotechnology | ASTM E2524-08(2013); Standard test method for analysis of hemolytic properties of nanoparticles. |
| Canadian Standards Association (CSA) | CSA Z12885; Exposure control program for engineered nanomaterials in occupational settings. |
| Organization for Economic Cooperation and Development (OECD), Working Party on Manufactured Nanomaterials (WPNM) | No. 85; Evaluation of in vitro methods for human hazard assessment applied in the OECD testing program for the safety of manufactured nanomaterials. |
| NanoTEST, EU Seventh Framework Programme (NanoTestFP7) | Developing alternative testing strategies and high-throughput toxicity testing protocols using in vitro and in silico methods essential for the risk assessment of nanoparticles used in medical diagnostics. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. https://doi.org/10.3390/ijms222010952
Kus-Liśkiewicz M, Fickers P, Ben Tahar I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. International Journal of Molecular Sciences. 2021; 22(20):10952. https://doi.org/10.3390/ijms222010952
Chicago/Turabian StyleKus-Liśkiewicz, Małgorzata, Patrick Fickers, and Imen Ben Tahar. 2021. "Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations" International Journal of Molecular Sciences 22, no. 20: 10952. https://doi.org/10.3390/ijms222010952
APA StyleKus-Liśkiewicz, M., Fickers, P., & Ben Tahar, I. (2021). Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. International Journal of Molecular Sciences, 22(20), 10952. https://doi.org/10.3390/ijms222010952







