Modulation of p75NTR on Mesenchymal Stem Cells Increases Their Vascular Protection in Retinal Ischemia-Reperfusion Mouse Model
Abstract
1. Introduction
2. Results
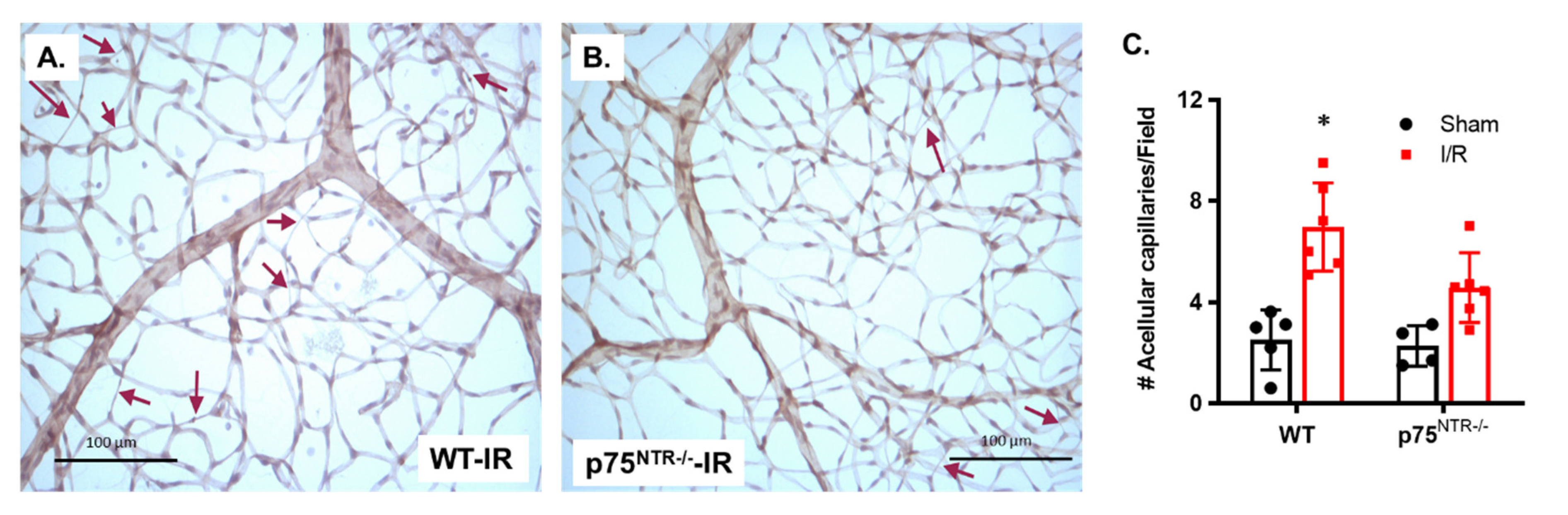
2.1. Deletion of p75NTR Prevented Retinal Capillary Degeneration in Ischemia/Reperfusion Model
2.2. Mesenchymal Stem Cells (MSCs) Are Engrafted to Retina Capillaries and Improve Vascular Protection in Both WT and p75NTR-/- Mice
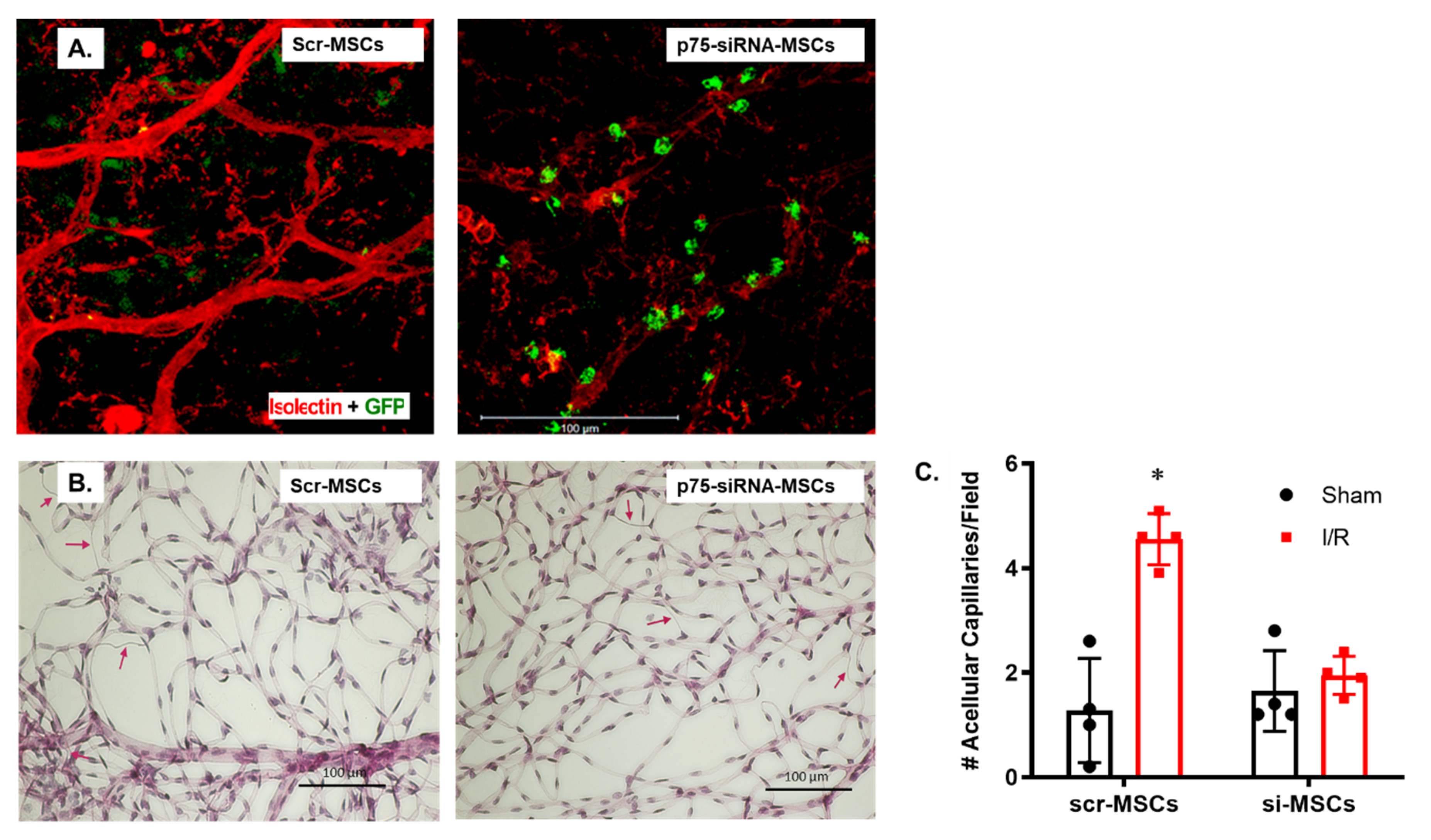
2.3. Silencing p75NTR Expression in MSCs Increased Their Homing and Vascular Protection in Ischemic Retinas
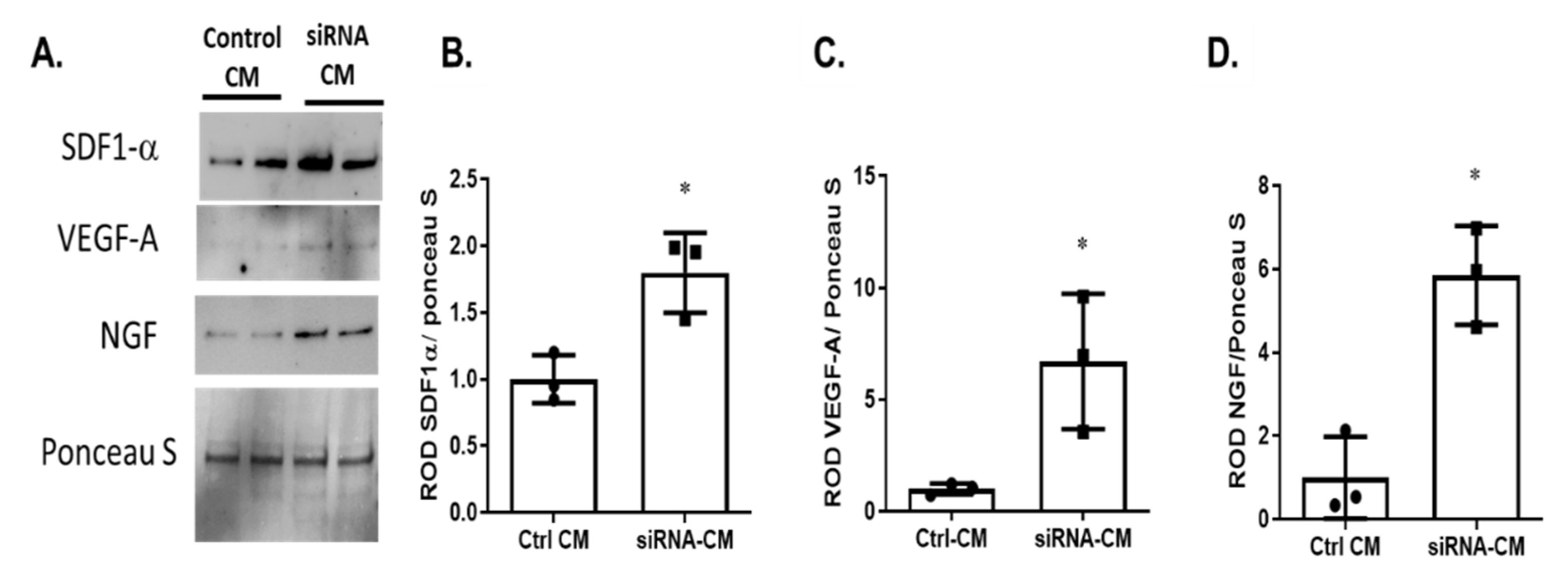
2.4. Silencing p75NTR Expression on MSCs Improves Their Secretome
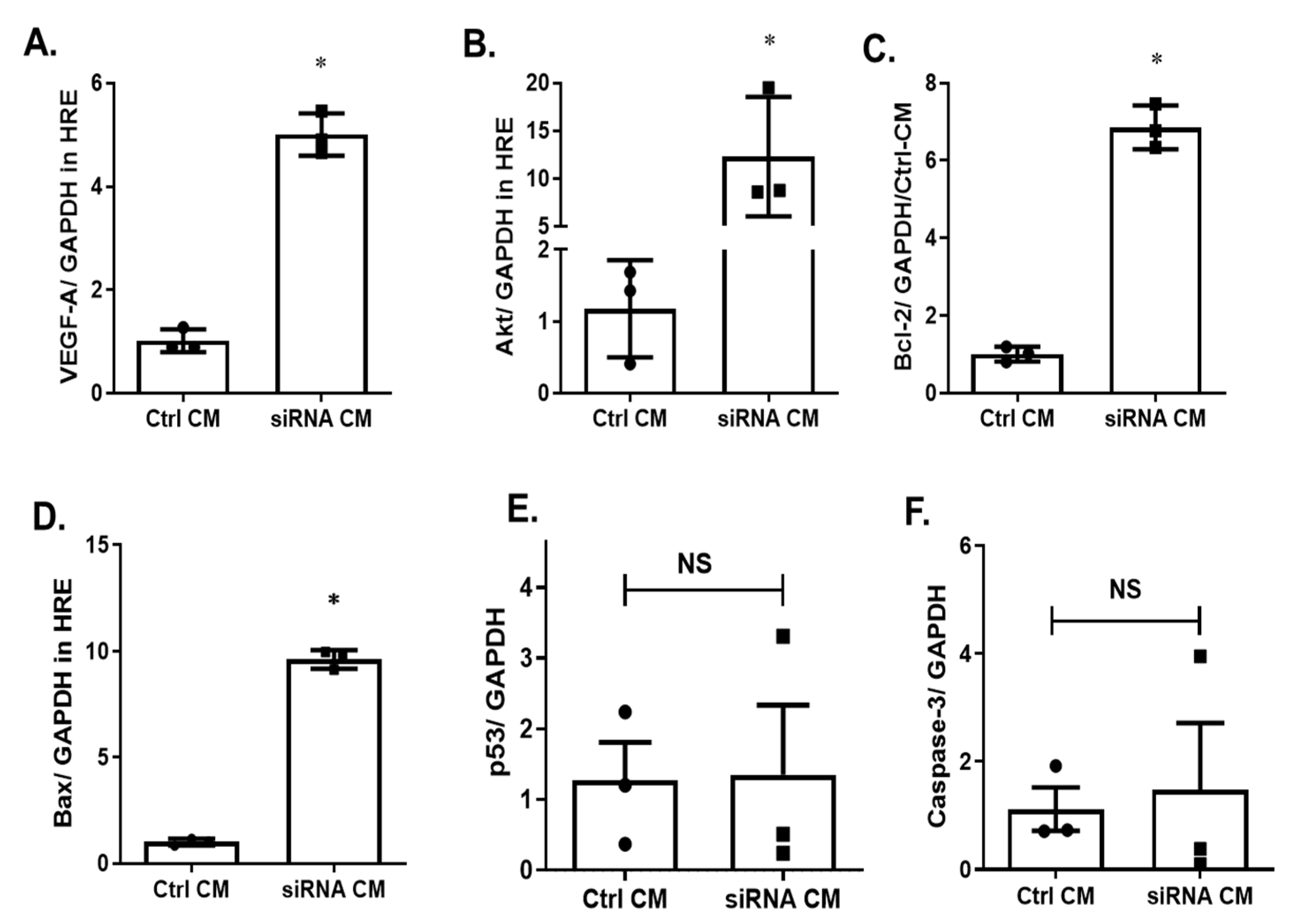
2.5. Silencing p75NTR Expression on MSCs Improves Paracrine Effect on HREs
2.6. Silencing p75NTR Expression on MSCs Enhanced Angiogenic Response in HREs
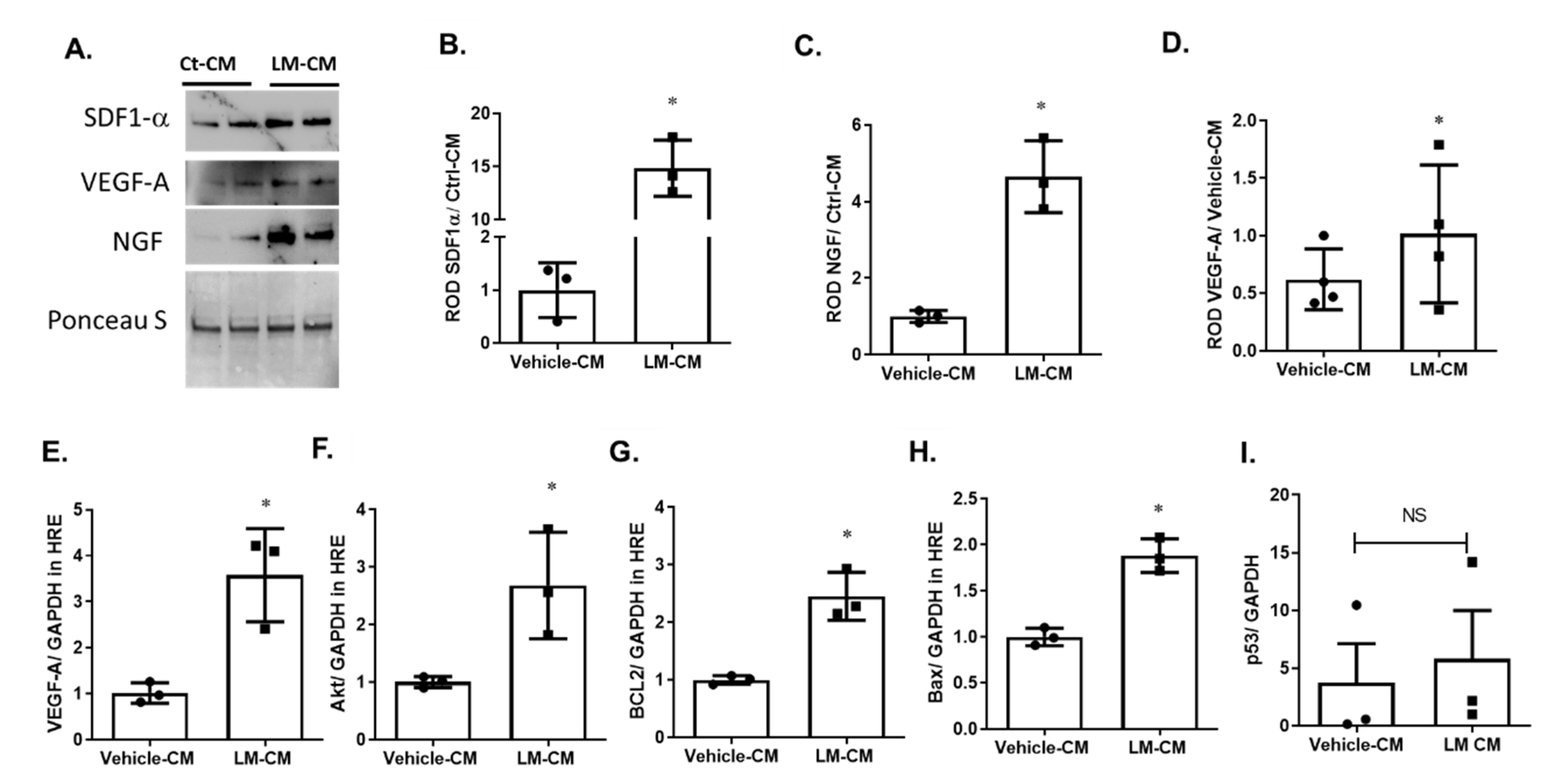
2.7. Modulating p75NTR on MSCs Using LM11A-31 Improved Their Secretome and Improved Paracrine Effect in HREs
2.8. Conditioned Medium of LM11A-31-Treated MSCs Enhanced Angiogenic Response in HREs
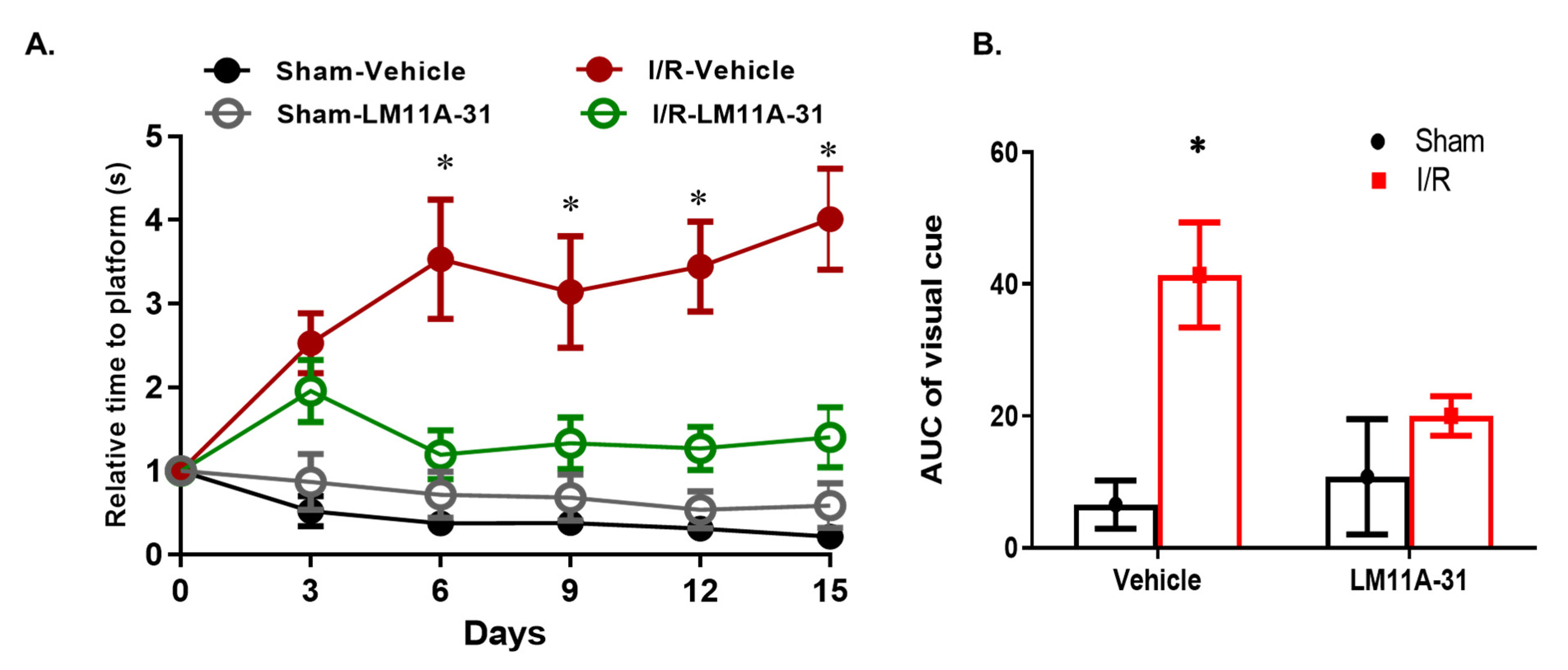
2.9. Modulating p75NTR Using LM11A-31 Improves Visual Acuity in WT Mice Post I/R Injury
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Retinal Ischemia/Reperfusion
4.3. Mesenchymal Stem Cells (MSCs) Culture
4.4. Intravitreal Injection of MSCs
4.5. Vascular Localization of MSCs
4.6. Isolation of Retinal Vasculature and Determination of Aacellular Capillaries
4.7. Endothelial Cell Cultures
4.8. Real-Time Quantitative PCR
4.9. Cell Migration Assay
4.10. Tube Formation Assay
4.11. Visual Acuity Test
4.12. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| Bax | Bcl-2-like protein 4 |
| Bcl-2 | B-cell lymphoma 2 |
| CM | Conditioned medium |
| CXCR-4 | Chemokine receptor type 4 |
| CXCR-7 | Chemokine receptor type 7 |
| HREs | Human retinal endothelial cells |
| I/R | Ischemia reperfusion |
| MSCs | Mesenchymal stem cells |
| NGF | Nerve growth factor |
| PASH | Periodic acid–Schiff and hematoxylin |
| P75NTR | p75 neurotrophin receptor |
| PDR | Proliferative diabetic retinopathy |
| SDF-1 | Stromal cell-derived factor-1 |
| VEGF | Vascular endothelial growth factor |
References
- Das, A.; McGuire, P.G. Retinal and choroidal angiogenesis: Pathophysiology and strategies for inhibition. Prog. Retin. Eye Res. 2003, 22, 721–748. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarieh, M.; Grieshaber, M.C.; Flammer, J. Oxygen and blood flow: Players in the pathogenesis of glaucoma. Mol. Vis. 2008, 14, 224–233. [Google Scholar] [PubMed]
- Coucha, M.; Elshaer, S.L.; Eldahshan, W.S.; Mysona, B.A.; El-Remessy, A.B. Molecular mechanisms of diabetic retinopathy: Potential therapeutic targets. Middle East. Afr J. Ophthalmol. 2015, 22, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F.; Liu, T.Y.A. Pan-American Collaborative Retina Study, G. Intravitreal Bevacizumab in Diabetic Retinopathy. Recommendations from the Pan-American Collaborative Retina Study Group (PACORES): The 2016 Knobloch Lecture. Asia Pac. J. Ophthalmol. 2018, 7, 36–39. [Google Scholar] [CrossRef]
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringden, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials, G. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Meirelles Lda, S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Evans, W.; Pentecost, M.; Lenin, R.; Periasamy, R.; Jha, K.A.; Alli, S.; Gentry, J.; Thomas, S.M.; Sohl, N.; et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2(Akita) mouse. Stem Cell Res. Ther. 2018, 9, 322. [Google Scholar] [CrossRef]
- Mazo, M.; Cemborain, A.; Gavira, J.J.; Abizanda, G.; Arana, M.; Casado, M.; Soriano, M.; Hernandez, S.; Moreno, C.; Ecay, M.; et al. Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity. Cell Transplant. 2012, 21, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Kicic, A.; Shen, W.Y.; Wilson, A.S.; Constable, I.J.; Robertson, T.; Rakoczy, P.E. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J. Neurosci. 2003, 23, 7742–7749. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Di Lauro, S.; Garcia-Gutierrez, M.T.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp. Eye Res. 2019, 185, 107671. [Google Scholar] [CrossRef] [PubMed]
- Borkowska-Kuczkowska, A.; Slugocka, D.; Swiatkowska-Flis, B.; Boruczkowski, D. The use of mesenchymal stem cells for the treatment of progressive retinal diseases: A review. Regen. Med. 2019, 14, 321–329. [Google Scholar] [CrossRef]
- Elshaer, S.L.; El-Remessy, A.B. Implication of the neurotrophin receptor p75(NTR) in vascular diseases: Beyond the eye. Expert Rev. Ophthalmol. 2017, 12, 149–158. [Google Scholar] [CrossRef]
- Tomellini, E.; Lagadec, C.; Polakowska, R.; Le Bourhis, X. Role of p75 neurotrophin receptor in stem cell biology: More than just a marker. Cell Mol. Life Sci. 2014, 71, 2467–2481. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, Z.; Gu, Y.; Li, J.; Xu, L.; Xu, W.; Lu, J.; Xu, J. Adult rat mesenchymal stem cells differentiate into neuronal-like phenotype and express a variety of neuro-regulatory molecules in vitro. Neurosci. Res. 2010, 66, 46–52. [Google Scholar] [CrossRef]
- Mikami, Y.; Ishii, Y.; Watanabe, N.; Shirakawa, T.; Suzuki, S.; Irie, S.; Isokawa, K.; Honda, M.J. CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev. 2011, 20, 901–913. [Google Scholar] [CrossRef]
- Mysona, B.A.; Al-Gayyar, M.M.; Matragoon, S.; Abdelsaid, M.A.; El-Azab, M.F.; Saragovi, H.U.; El-Remessy, A.B. Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats. Diabetologia 2013, 56, 2329–2339. [Google Scholar] [CrossRef]
- Shanab, A.Y.; Mysona, B.A.; Matragoon, S.; El-Remessy, A.B. Silencing p75(NTR) prevents proNGF-induced endothelial cell death and development of acellular capillaries in rat retina. Mol. Ther Methods Clin. Dev. 2015, 2, 15013. [Google Scholar] [CrossRef]
- Mohamed, R.; Shanab, A.Y.; El Remessy, A.B. Deletion of the Neurotrophin Receptor p75(NTR) Prevents Diabetes-Induced Retinal Acellular Capillaries in Streptozotocin-Induced Mouse Diabetic Model. J. Diabetes Metab. Disord. Control 2017, 4. [Google Scholar] [CrossRef]
- Elshaer, S.L.; El-Remessy, A.B. Deletion of p75(NTR) prevents vaso-obliteration and retinal neovascularization via activation of Trk- A receptor in ischemic retinopathy model. Sci. Rep. 2018, 8, 12490. [Google Scholar] [CrossRef]
- Barcelona, P.F.; Sitaras, N.; Galan, A.; Esquiva, G.; Jmaeff, S.; Jian, Y.; Sarunic, M.V.; Cuenca, N.; Sapieha, P.; Saragovi, H.U. p75NTR and Its Ligand ProNGF Activate Paracrine Mechanisms Etiological to the Vascular, Inflammatory, and Neurodegenerative Pathologies of Diabetic Retinopathy. J. Neurosci. 2016, 36, 8826–8841. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, N.; Lu, Q.; Zhang, N.; Zheng, D.; Li, J. Enhanced protein expressions of sortilin and p75NTR in retina of rat following elevated intraocular pressure-induced retinal ischemia. Neurosci. Lett. 2007, 429, 169–174. [Google Scholar] [CrossRef]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef]
- Nakamura, T.; Endo, K.; Kinoshita, S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells 2007, 25, 628–638. [Google Scholar] [CrossRef]
- Gentry, J.J.; Casaccia-Bonnefil, P.; Carter, B.D. Nerve growth factor activation of nuclear factor kappaB through its p75 receptor is an anti-apoptotic signal in RN22 schwannoma cells. J. Biol. Chem. 2000, 275, 7558–7565. [Google Scholar] [CrossRef]
- Coulson, E.J.; Reid, K.; Shipham, K.M.; Morley, S.; Kilpatrick, T.J.; Bartlett, P.F. The role of neurotransmission and the Chopper domain in p75 neurotrophin receptor death signaling. Prog. Brain Res. 2004, 146, 41–62. [Google Scholar] [CrossRef]
- Johnston, A.L.; Lun, X.; Rahn, J.J.; Liacini, A.; Wang, L.; Hamilton, M.G.; Parney, I.F.; Hempstead, B.L.; Robbins, S.M.; Forsyth, P.A.; et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 2007, 5, e212. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Alwhaibi, A.; Mohamed, R.; Lemtalsi, T.; Coucha, M.; Longo, F.M.; El-Remessy, A.B. Modulation of the p75 neurotrophin receptor using LM11A-31 prevents diabetes-induced retinal vascular permeability in mice via inhibition of inflammation and the RhoA kinase pathway. Diabetologia 2019, 62, 1488–1500. [Google Scholar] [CrossRef]
- Stitt, A.W.; O’Neill, C.L.; O’Doherty, M.T.; Archer, D.B.; Gardiner, T.A.; Medina, R.J. Vascular stem cells and ischaemic retinopathies. Prog. Retin. Eye Res. 2011, 30, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Quirici, N.; Soligo, D.; Bossolasco, P.; Servida, F.; Lumini, C.; Deliliers, G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002, 30, 783–791. [Google Scholar] [CrossRef]
- Arnhold, S.; Absenger, Y.; Klein, H.; Addicks, K.; Schraermeyer, U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.R.; Yuan, J.Q. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, N.; Koster, C.; Mouloud, Y.; Borger, V.; Felderhoff-Muser, U.; Bendix, I.; Giebel, B.; Herz, J. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Reduce Neuroinflammation, Promote Neural Cell Proliferation and Improve Oligodendrocyte Maturation in Neonatal Hypoxic-Ischemic Brain Injury. Front. Cell Neurosci. 2020, 14, 601176. [Google Scholar] [CrossRef]
- Huber, B.; Volz, A.C.; Kluger, P.J. How do culture media influence in vitro perivascular cell behavior? Cell Biol. Int. 2015, 39, 1395–1407. [Google Scholar] [CrossRef]
- Xu, J.; Gong, T.; Heng, B.C.; Zhang, C.F. A systematic review: Differentiation of stem cells into functional pericytes. FASEB J. 2017, 31, 1775–1786. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Luo, Z.; Wu, Q.; Fan, W.; Yao, X.; Li, Q.; Yan, H.; Wang, J. Abeta inhibits mesenchymal stem cell-pericyte transition through MAPK pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2018, 50, 776–781. [Google Scholar] [CrossRef]
- Urbich, C.; Aicher, A.; Heeschen, C.; Dernbach, E.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell Cardiol. 2005, 39, 733–742. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Yang, J.; Kong, X.; Zheng, F.; Guo, L.; Zhang, L.; Huang, Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur. J. Cardiothorac Surg. 2009, 36, 644–650. [Google Scholar] [CrossRef]
- Reiter, J.; Drummond, S.; Sammour, I.; Huang, J.; Florea, V.; Dornas, P.; Hare, J.M.; Rodrigues, C.O.; Young, K.C. Stromal derived factor-1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir. Res. 2017, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed. Res. Int. 2013, 2013, 561098. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.; Chen, W.; Han, N.; Li, K.; Guo, R.; Liu, Z.; Xiao, Y. Enhanced recruitment and hematopoietic reconstitution of bone marrow-derived mesenchymal stem cells in bone marrow failure by the SDF-1/CXCR4. J. Tissue Eng. Regen Med. 2020, 14, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.T.; Huang, Y.C.; Hsieh, M.L.; Lin, Y.J.; Shyu, W.C.; Chen, H.C.; Hsieh, C.H. Atypical chemokine receptor ACKR3/CXCR7 controls postnatal vasculogenesis and arterial specification by mesenchymal stem cells via Notch signaling. Cell Death Dis. 2020, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, W.; Zhang, S.; He, X.; Liu, Z.; Gao, D.; Xu, T. CXCR-7 receptor promotes SDF-1alpha-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res. 2014, 1575, 78–86. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. 2018, 17, 1667–1675. [Google Scholar] [CrossRef]
- Mead, B.; Chamling, X.; Zack, D.J.; Ahmed, Z.; Tomarev, S. TNFalpha-Mediated Priming of Mesenchymal Stem Cells Enhances Their Neuroprotective Effect on Retinal Ganglion Cells. Investig. Ophthalmol Vis. Sci. 2020, 61, 6. [Google Scholar] [CrossRef]
- Speidell, A.; Asuni, G.P.; Avdoshina, V.; Scognamiglio, S.; Forcelli, P.; Mocchetti, I. Reversal of Cognitive Impairment in gp120 Transgenic Mice by the Removal of the p75 Neurotrophin Receptor. Front. Cell Neurosci. 2019, 13, 398. [Google Scholar] [CrossRef]
- Wang, W.X.; Hu, X.Y.; Xie, X.J.; Liu, X.B.; Wu, R.R.; Wang, Y.P.; Gao, F.; Wang, J.A. Nerve growth factor induces cord formation of mesenchymal stem cell by promoting proliferation and activating the PI3K/Akt signaling pathway. Acta Pharmacol. Sin. 2011, 32, 1483–1490. [Google Scholar] [CrossRef][Green Version]
- Jadhao, C.S.; Bhatwadekar, A.D.; Jiang, Y.; Boulton, M.E.; Steinle, J.J.; Grant, M.B. Nerve growth factor promotes endothelial progenitor cell-mediated angiogenic responses. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Molostvov, G.; Morris, A.; Rose, P.; Basu, S. Modulation of Bcl-2 family proteins in primary endothelial cells during apoptosis. Pathophysiol. Haemost. Thromb. 2002, 32, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tep, C.; Lim, T.H.; Ko, P.O.; Getahun, S.; Ryu, J.C.; Goettl, V.M.; Massa, S.M.; Basso, M.; Longo, F.M.; Yoon, S.O. Oral administration of a small molecule targeted to block proNGF binding to p75 promotes myelin sparing and functional recovery after spinal cord injury. J. Neurosci. 2013, 33, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.M.; Xie, Y.; Yang, T.; Harrington, A.W.; Kim, M.L.; Yoon, S.O.; Kraemer, R.; Moore, L.A.; Hempstead, B.L.; Longo, F.M. Small, nonpeptide p75NTR ligands induce survival signaling and inhibit proNGF-induced death. J. Neurosci. 2006, 26, 5288–5300. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.K.; Simmons, D.A.; Nguyen, T.V.; Vander Griend, L.; Xie, Y.; Zhang, H.; Yang, T.; Pollak, J.; Chang, T.; Arancio, O.; et al. Small molecule p75NTR ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol. Aging 2013, 34, 2052–2063. [Google Scholar] [CrossRef]
- Coucha, M.; Shanab, A.Y.; Sayed, M.; Vazdarjanova, A.; El-Remessy, A.B. Modulating Expression of Thioredoxin Interacting Protein (TXNIP) Prevents Secondary Damage and Preserves Visual Function in a Mouse Model of Ischemia/Reperfusion. Int. J. Mol. Sci. 2019, 20, 3969. [Google Scholar] [CrossRef]
- Herberg, S.; Kondrikova, G.; Hussein, K.A.; Periyasamy-Thandavan, S.; Johnson, M.H.; Elsalanty, M.E.; Shi, X.; Hamrick, M.W.; Isales, C.M.; Hill, W.D. Total body irradiation is permissive for mesenchymal stem cell-mediated new bone formation following local transplantation. Tissue Eng. Part A 2014, 20, 3212–3227. [Google Scholar] [CrossRef]
- Mohamed, R.; Coucha, M.; Elshaer, S.L.; Artham, S.; Lemtalsi, T.; El-Remessy, A.B. Inducible overexpression of endothelial proNGF as a mouse model to study microvascular dysfunction. Biochim. Biophys. Acta 2018, 1864, 746–757. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Abdelsaid, M.A.; Al-Azayzih, A.; Kumar, P.; Matragoon, S.; Nussbaum, J.J.; El-Remessy, A.B. Pronerve growth factor induces angiogenesis via activation of TrkA: Possible role in proliferative diabetic retinopathy. J. Diabetes Res. 2013, 2013, 432659. [Google Scholar] [CrossRef]
- Park, H.S.; Ashour, D.; Elsharoud, A.; Chugh, R.M.; Ismail, N.; El Andaloussi, A.; Al-Hendy, A. Towards Cell free Therapy of Premature Ovarian Insufficiency: Human Bone Marrow Mesenchymal Stem Cells Secretome Enhances Angiogenesis in Human Ovarian Microvascular Endothelial Cells. HSOA J. Stem Cells Res. Dev. Ther. 2019, 5. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef] [PubMed]



| Primer | Sequence (5′–3’) |
|---|---|
| Mouse p75NTR F | CCTGCCTGGACAGTGTTACG |
| Mouse p75NTR R | CACACAGGGAGCGGACATAC |
| Mouse VEGFA F | GTACCTCCACCATGCCAAGT |
| Mouse VEGFA R | GCATTCACATCTGCTGTGCT |
| Mouse Akt1 F | ATGAACGACGTAGCCATTGTG |
| Mouse Akt1 R | TTGTAGCCAATAAAGGTGCCAT |
| Mouse Bcl-2 F | GTGGTGGAGGAACTCTTCA |
| Mouse Bcl-2 R | GTTCCACAAAGGCATCCCAG |
| Mouse Bax F | AGCAAACTGGTGCTCAAGGC |
| Mouse Bax R | CCACAAAGATGGTCACTGTC |
| Mouse Caspase-3 F | CCTCAGAGAGACATTCATGG |
| Mouse Caspase-3 R | GCAGTAGTCGCCTCTGAAGA |
| Mouse P53 F | GGAAATTTGTATCCCGAGTATCTG |
| Mouse P53 R | GTCTTCCAGTGTGATGATGGTAA |
| Mouse GAPDH F | CACATTGGGGGTAGGAACAC |
| Mouse GAPDH R | AACTTTGGGCATTGTGGAAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshaer, S.L.; Park, H.-s.; Pearson, L.; Hill, W.D.; Longo, F.M.; El-Remessy, A.B. Modulation of p75NTR on Mesenchymal Stem Cells Increases Their Vascular Protection in Retinal Ischemia-Reperfusion Mouse Model. Int. J. Mol. Sci. 2021, 22, 829. https://doi.org/10.3390/ijms22020829
Elshaer SL, Park H-s, Pearson L, Hill WD, Longo FM, El-Remessy AB. Modulation of p75NTR on Mesenchymal Stem Cells Increases Their Vascular Protection in Retinal Ischemia-Reperfusion Mouse Model. International Journal of Molecular Sciences. 2021; 22(2):829. https://doi.org/10.3390/ijms22020829
Chicago/Turabian StyleElshaer, Sally L., Hang-soo Park, Laura Pearson, William D. Hill, Frank M. Longo, and Azza B. El-Remessy. 2021. "Modulation of p75NTR on Mesenchymal Stem Cells Increases Their Vascular Protection in Retinal Ischemia-Reperfusion Mouse Model" International Journal of Molecular Sciences 22, no. 2: 829. https://doi.org/10.3390/ijms22020829
APA StyleElshaer, S. L., Park, H.-s., Pearson, L., Hill, W. D., Longo, F. M., & El-Remessy, A. B. (2021). Modulation of p75NTR on Mesenchymal Stem Cells Increases Their Vascular Protection in Retinal Ischemia-Reperfusion Mouse Model. International Journal of Molecular Sciences, 22(2), 829. https://doi.org/10.3390/ijms22020829







