Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications
Abstract
1. Introduction
2. Pulsed Electromagnetic Fields
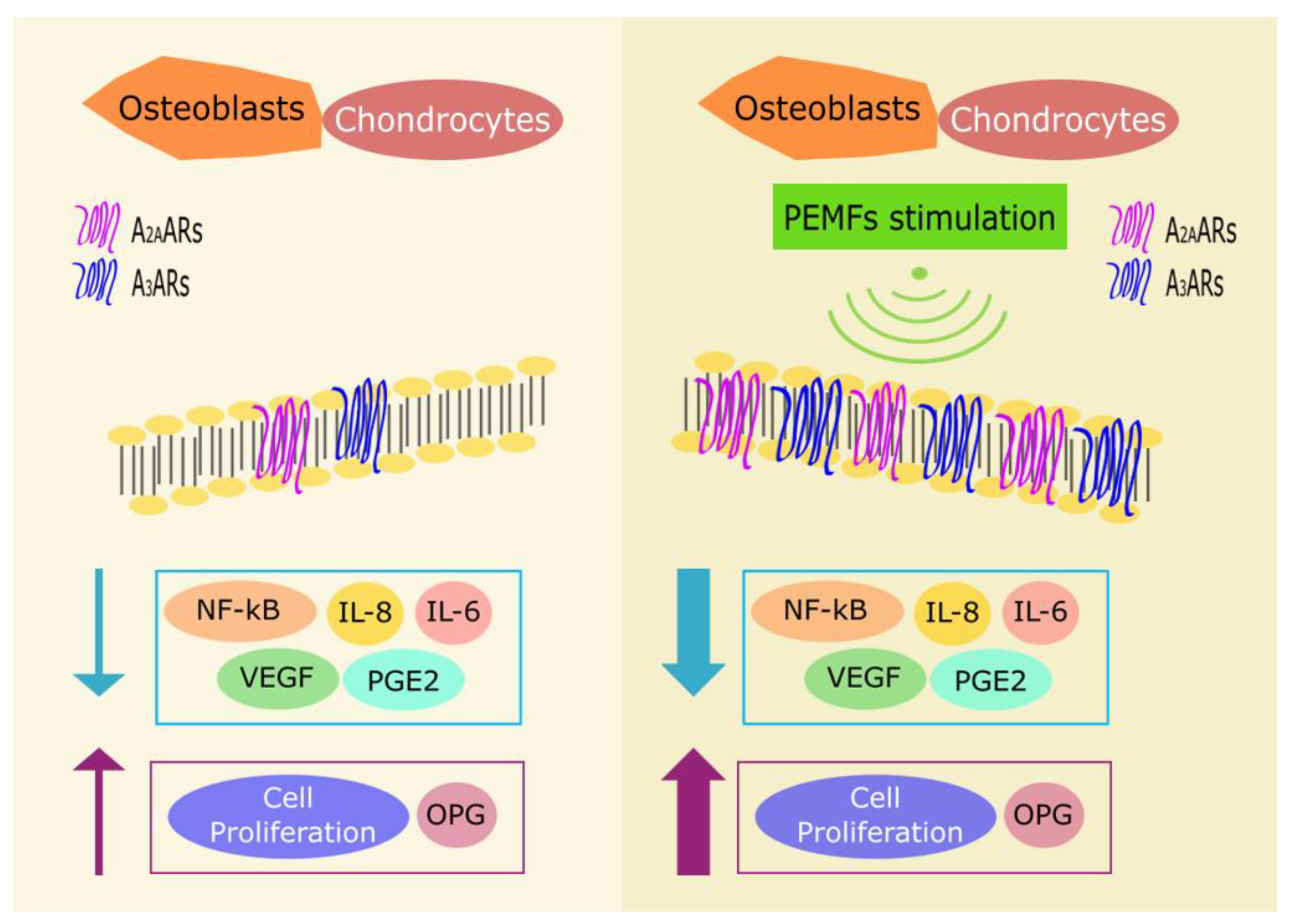
3. Adenosine Agonist Effect Induced by Pulsed Electromagnetic Fields
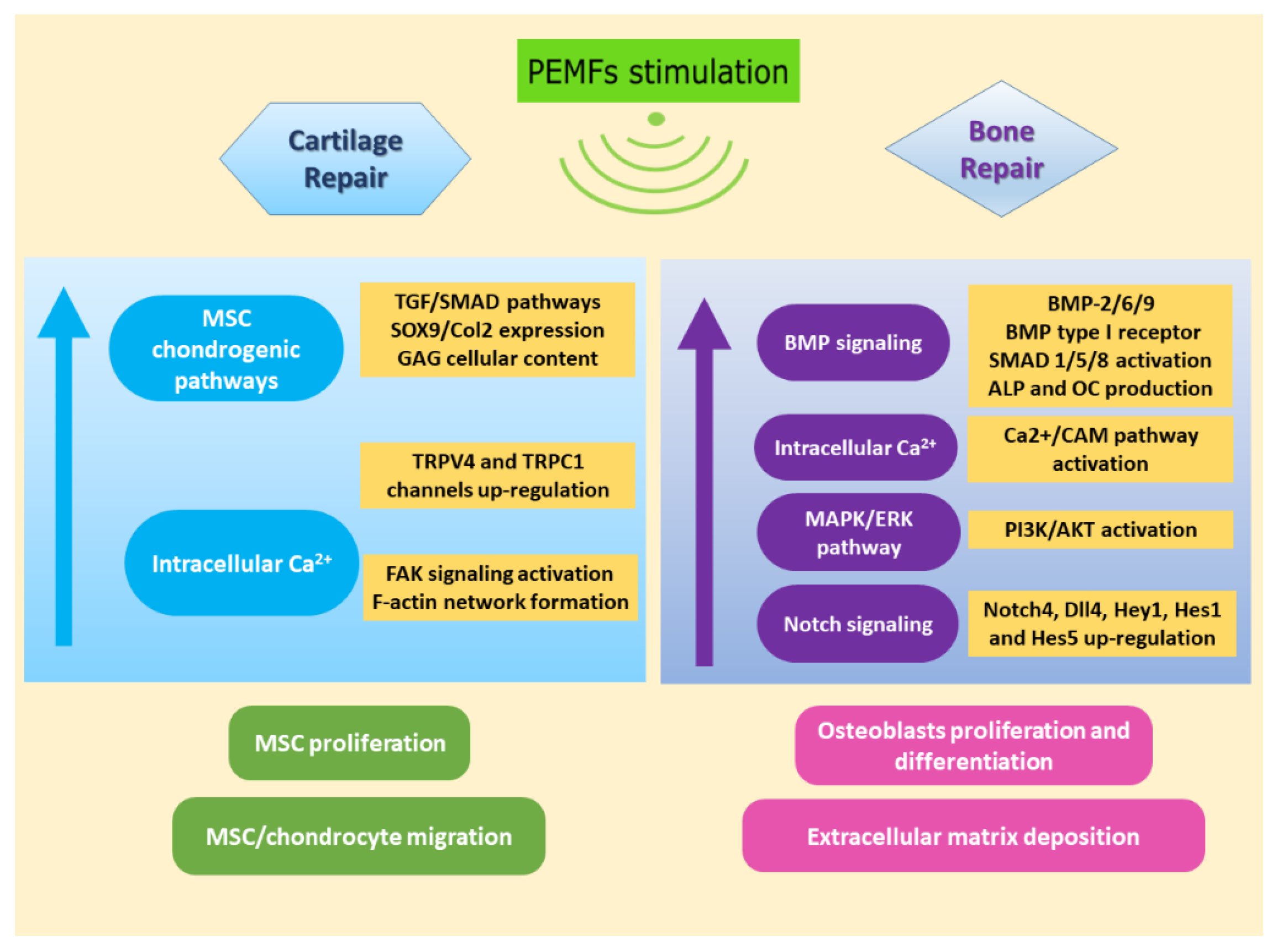
4. Chondrogenic Effects and Pathways Activated by Pulsed Electromagnetic Fields
5. Therapeutic Implications for Cartilage Repair Approaches
6. Osteogenic Effects and Pathways Activated by Pulsed Electromagnetic Fields
7. Therapeutic Implications for Bone Healing
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal Stem Cells |
| hBM-MSCs | Human Bone Marrow Mesenchymal Stem Cells |
| hADMSCs | Human Adipose Derived Mesenchymal Stem Cells |
| hPDLSC | Human periodontal ligament stem cells |
| PEMFs | Pulsed Electromagnetic Fields |
| TE | Tissue Engineering |
| BMP-2 | Bone Morphogenetic Protein-2 |
| EMF | Electromagnetic field |
| BMPs | Bone Morphogenetic Proteins |
| TGF-β | Transforming Growth Factor beta |
| MAPKs | Mitogen-Activated Protein Kinases |
| ALP | Alkaline Phosphatase |
| OC | Osteocalcin |
| SOST | Sclerostin |
| VEGF | Vascular-Endothelial Growth Factor |
| Ca2+/CaM | Ca2+/Calmodulin |
References
- Pittenger, M.F. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Braga Osorio Gomes Salgado, A.J.; Goncalves Reis, R.L.; Jorge Carvalho Sousa, N.; Gimble, J.M.; Salgado, A.J.; Reis, R.L.; Sousa, N. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem Cell Res. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Escacena, N.; Quesada-Hernández, E.; Capilla-Gonzalez, V.; Soria, B.; Hmadcha, A. Bottlenecks in the Efficient Use of Advanced Therapy Medicinal Products Based on Mesenchymal Stromal Cells. Stem Cells Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.N.F.B.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Richter, W. Mesenchymal Stem Cells and Cartilage In Situ Regeneration. J. Intern. Med. 2009, 266, 390–405. [Google Scholar] [CrossRef]
- Mamidi, M.K.; Das, A.K.; Zakaria, Z.; Bhonde, R. Mesenchymal Stromal Cells for Cartilage Repair in Osteoarthritis. Osteoarthr. Cart. 2016, 24, 1307–1316. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical Stimulation of Bone and Cartilage: State of the Art and Future Perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef]
- Bassett, C.A.; Pawluk, R.J.; Pilla, A.A. Augmentation of Bone Repair by Inductively Coupled Electromagnetic Fields. Science 1974, 184, 575–577. [Google Scholar] [PubMed]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Bassett, C.A.; Becker, R.O. Generation of Electric Potentials by Bone in Response to Mechanical Stress. Science 1962, 137, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A. Biological Effects of Electric and Magnetic Fields; Carpenter, D.O., Aĭrapetíàn, S.N., Eds.; Academic Press: San Diego, CA, USA, 1994; ISBN 978-0-12-160261-1. [Google Scholar]
- Massari, L.; Benazzo, F.; Moretti, B.; Dallari, D.; Perugia, D.; Meani, E.; Cadossi, R. Electrical Stimulation of Osteogenesis: Efficacy and Technologies Compared. GIOT 2011, 6, 1–8. [Google Scholar]
- Simonis, R.B.; Parnell, E.J.; Ray, P.S.; Peacock, J.L. Electrical Treatment of Tibial Non-Union: A Prospective, Randomised, Double-Blind Trial. Injury 2003, 34, 357–362. [Google Scholar] [CrossRef]
- Shi, H.; Xiong, J.; Chen, Y.; Wang, J.; Qiu, X.; Wang, Y.; Qiu, Y. Early Application of Pulsed Electromagnetic Field in the Treatment of Postoperative Delayed Union of Long-Bone Fractures: A Prospective Randomized Controlled Study. BMC Musculoskelet. Disord. 2013, 14. [Google Scholar] [CrossRef]
- Faldini, C.; Cadossi, M.; Luciani, D.; Betti, E.; Chiarello, E.; Giannini, S. Electromagnetic Bone Growth Stimulation in Patients with Femoral Neck Fractures Treated with Screws: Prospective Randomized Double-Blind Study. Curr. Orthop. Pract. 2010, 21, 282–287. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pathological Overproduction: The Bad Side of Adenosine: Bad Sides of Adenosine. Br. J. Pharm. 2017, 174, 1945–1960. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signaling Guardian Angel in Human Diseases: When, Where and How Does It Exert Its Protective Effects? Trends Pharm. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Noronha-Matos, J.B.; Correia-de-Sá, P. Mesenchymal Stem Cells Ageing: Targeting the “Purinome” to Promote Osteogenic Differentiation and Bone Repair: OSTEOGENIC DIFFERENTIATION OF AGED MSCs BY PURINES. J. Cell. Physiol. 2016, 231, 1852–1861. [Google Scholar] [CrossRef]
- Costa, M.A.; Barbosa, A.; Neto, E.; Sá-e-Sousa, A.; Freitas, R.; Neves, J.M.; Magalhães-Cardoso, T.; Ferreirinha, F.; Correia-de-Sá, P. On the Role of Subtype Selective Adenosine Receptor Agonists during Proliferation and Osteogenic Differentiation of Human Primary Bone Marrow Stromal Cells. J. Cell. Physiol. 2011, 226, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Cardoso, R.; Pereira-Costa, F.; Pedro Faria, J.; Bandarrinha, P.; Bessa-Andrês, C.; Correia-de-Sá, P.; Bernardo Noronha-Matos, J. Adenosinergic Signaling in Chondrogenesis and Cartilage Homeostasis: Friend or Foe? Biochem. Pharm. 2020, 174, 113784. [Google Scholar] [CrossRef] [PubMed]
- Tesch, A.M.; MacDonald, M.H.; Kollias-Baker, C.; Benton, H.P. Endogenously Produced Adenosine Regulates Articular Cartilage Matrix Homeostasis: Enzymatic Depletion of Adenosine Stimulates Matrix Degradation. Osteoarthr. Cartil. 2004, 12, 349–359. [Google Scholar] [CrossRef]
- Corciulo, C.; Lendhey, M.; Wilder, T.; Schoen, H.; Cornelissen, A.S.; Chang, G.; Kennedy, O.D.; Cronstein, B.N. Endogenous Adenosine Maintains Cartilage Homeostasis and Exogenous Adenosine Inhibits Osteoarthritis Progression. Nat. Commun. 2017, 8, 15019. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, P.; Charlebois, R.; Rejtar, P.; El Bikai, R.; Allard, B.; Stagg, J. CD73 Plays a Protective Role in Collagen-Induced Arthritis. J. Immunol. 2015, 194, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Webb, N.E.; Song, Y.; Tuan, R.S. Identification and Functional Analysis of Candidate Genes Regulating Mesenchymal Stem Cell Self-Renewal and Multipotency. Stem Cells 2006, 24, 1707–1718. [Google Scholar] [CrossRef]
- Mediero, A.; Cronstein, B.N. Adenosine and Bone Metabolism. Trends Endocrinol. Metab. 2013, 24, 290–300. [Google Scholar] [CrossRef]
- Bar-Yehuda, S.; Rath-Wolfson, L.; Del Valle, L.; Ochaion, A.; Cohen, S.; Patoka, R.; Zozulya, G.; Barer, F.; Atar, E.; Piña-Oviedo, S.; et al. Induction of an Antiinflammatory Effect and Prevention of Cartilage Damage in Rat Knee Osteoarthritis by CF101 Treatment. Arthritis Rheum. 2009, 60, 3061–3071. [Google Scholar] [CrossRef]
- Shkhyan, R.; Lee, S.; Gullo, F.; Li, L.; Peleli, M.; Carlstrom, M.; Chagin, A.S.; Banks, N.W.; Limfat, S.; Liu, N.Q.; et al. Genetic Ablation of Adenosine Receptor A3 Results in Articular Cartilage Degeneration. J. Mol. Med. 2018, 96, 1049–1060. [Google Scholar] [CrossRef]
- Varani, K.; Gessi, S.; Merighi, S.; Iannotta, V.; Cattabriga, E.; Spisani, S.; Cadossi, R.; Borea, P.A. Effect of Low Frequency Electromagnetic Fields on A2A Adenosine Receptors in Human Neutrophils. Br. J. Pharm. 2002, 136, 57–66. [Google Scholar] [CrossRef]
- Varani, K.; De Mattei, M.; Vincenzi, F.; Gessi, S.; Merighi, S.; Pellati, A.; Ongaro, A.; Caruso, A.; Cadossi, R.; Borea, P.A. Characterization of Adenosine Receptors in Bovine Chondrocytes and Fibroblast-like Synoviocytes Exposed to Low Frequency Low Energy Pulsed Electromagnetic Fields. Osteoarthr. Cartil. 2008, 16, 292–304. [Google Scholar] [CrossRef] [PubMed]
- De Mattei, M.; Varani, K.; Masieri, F.F.; Pellati, A.; Ongaro, A.; Fini, M.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Caruso, A. Adenosine Analogs and Electromagnetic Fields Inhibit Prostaglandin E2 Release in Bovine Synovial Fibroblasts. Osteoarthr. Cartil. 2009, 17, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Vincenzi, F.; Tosi, A.; Targa, M.; Masieri, F.; Ongaro, A.; De Mattei, M.; Massari, L.; Borea, P. Expression and Functional Role of Adenosine Receptors in Regulating Inflammatory Responses in Human Synoviocytes: Adenosine Receptors in Human Synoviocytes. Br. J. Pharm. 2010, 160, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.; Varani, K.; Masieri, F.F.; Pellati, A.; Massari, L.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Fini, M.; Caruso, A.; et al. Electromagnetic Fields (EMFs) and Adenosine Receptors Modulate Prostaglandin E2 and Cytokine Release in Human Osteoarthritic Synovial Fibroblasts. J. Cell. Physiol. 2012, 227, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Targa, M.; Corciulo, C.; Gessi, S.; Merighi, S.; Setti, S.; Cadossi, R.; Goldring, M.B.; Borea, P.A.; Varani, K. Pulsed Electromagnetic Fields Increased the Anti-Inflammatory Effect of A2A and A3 Adenosine Receptors in Human T/C-28a2 Chondrocytes and HFOB 1.19 Osteoblasts. PLoS ONE 2013, 8, e65561. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Wagner, S.; Passberger, A.; Sievers, B.; Aigner, J.; Summer, B.; Schiergens, T.S.; Jansson, V.; Müller, P.E. Effects of Low Frequency Electromagnetic Fields on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Bioelectromagnetics 2011, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.-S.; Fu, Y.-C.; Wang, C.-K.; Wu, S.-C.; Wang, G.-J.; Eswaramoorthy, R.; Wang, Y.-H.; Wang, C.-Z.; Wang, Y.-H.; et al. Electromagnetic Fields Enhance Chondrogenesis of Human Adipose-Derived Stem Cells in a Chondrogenic Microenvironment In Vitro. J. Appl. Physiol. 2013, 114, 647–655. [Google Scholar] [CrossRef]
- Esposito, M.; Lucariello, A.; Costanzo, C.; Fiumarella, A.; Giannini, A.; Riccardi, G.; Riccio, I. Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells, WJ-MSCs, into Chondrogenic Cells in the Presence of Pulsed Electromagnetic Fields. Vivo Athens Greece 2013, 27, 495–500. [Google Scholar]
- Kavand, H.; Haghighipour, N.; Zeynali, B.; Seyedjafari, E.; Abdemami, B. Extremely Low Frequency Electromagnetic Field in Mesenchymal Stem Cells Gene Regulation: Chondrogenic Markers Evaluation: Chondrogenic Markers as EMF Targets. Artif. Organs 2016, 40, 929–937. [Google Scholar] [CrossRef]
- Chen, X.; Qin, Z.; Zhao, J.; Yan, X.; Ye, J.; Ren, E.; Wang, J.; Yang, X.; Heng, S.; Zheng, L.; et al. Pulsed Magnetic Field Stimuli Can Promote Chondrogenic Differentiation of Superparamagnetic Iron Oxide Nanoparticles-Labeled Mesenchymal Stem Cells in Rats. J. Biomed. Nanotechnol. 2018, 14, 2135–2145. [Google Scholar] [CrossRef]
- Wang, J.; Tang, N.; Xiao, Q.; Zhang, L.; Li, Y.; Li, J.; Wang, J.; Zhao, Z.; Tan, L. Pulsed Electromagnetic Field May Accelerate in Vitro Endochondral Ossification: PEMF Stimulation of Endochondral Ossification. Bioelectromagnetics 2015, 36, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of Mesenchymal Stem Cell Chondrogenesis with Short-Term Low Intensity Pulsed Electromagnetic Fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, C.; Matta, C.; Foldvari, Z.; Juhász, T.; Katona, É.; Takács, Á.; Hajdú, T.; Dobrosi, N.; Gergely, P.; Zákány, R. Polymodal Transient Receptor Potential Vanilloid (TRPV) Ion Channels in Chondrogenic Cells. Int. J. Mol. Sci. 2015, 16, 18412–18438. [Google Scholar] [CrossRef] [PubMed]
- Gavenis, K.; Schumacher, C.; Schneider, U.; Eisfeld, J.; Mollenhauer, J.; Schmidt-Rohlfing, B. Expression of Ion Channels of the TRP Family in Articular Chondrocytes from Osteoarthritic Patients: Changes between Native and in Vitro Propagated Chondrocytes. Mol. Cell. Biochem. 2009, 321, 135–143. [Google Scholar] [CrossRef]
- Ongaro, A.; Pellati, A.; Setti, S.; Masieri, F.F.; Aquila, G.; Fini, M.; Caruso, A.; De Mattei, M. Electromagnetic Fields Counteract IL-1 β Activity during Chondrogenesis of Bovine Mesenchymal Stem Cells: EMFs Counteract IL-1 β during Chondrogenesis. J. Tissue Eng. Regen. Med. 2015, 9, E229–E238. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Xu, H.; Yang, Y.; Li, W.; Wu, H.; Liu, C. Extremely Low Frequency Electromagnetic Fields Promote Mesenchymal Stem Cell Migration by Increasing Intracellular Ca2+ and Activating the FAK/Rho GTPases Signaling Pathways In Vitro. Stem Cell Res. 2018, 9, 143. [Google Scholar] [CrossRef]
- Celik, C.; Franco-Obregón, A.; Lee, E.H.; Hui, J.H.; Yang, Z. Directionalities of Magnetic Fields and Topographic Scaffolds Synergise to Enhance MSC Chondrogenesis. Acta Biomater. 2021, 119, 169. [Google Scholar] [CrossRef]
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.H.P.; Franco-Obregón, A.; Yang, Z. Pulsed Electromagnetic Fields Potentiate the Paracrine Function of Mesenchymal Stem Cells for Cartilage Regeneration. Stem Cell Res. 2020, 11, 46. [Google Scholar] [CrossRef]
- Veronesi, F.; Fini, M.; Giavaresi, G.; Ongaro, A.; De Mattei, M.; Pellati, A.; Setti, S.; Tschon, M. Experimentally Induced Cartilage Degeneration Treated by Pulsed Electromagnetic Field Stimulation; an In Vitro Study on Bovine Cartilage. BMC Musculoskelet. Disord. 2015, 16. [Google Scholar] [CrossRef]
- Stefani, R.M.; Barbosa, S.; Tan, A.R.; Setti, S.; Stoker, A.M.; Ateshian, G.A.; Cadossi, R.; Vunjak-Novakovic, G.; Aaron, R.K.; Cook, J.L.; et al. Pulsed Electromagnetic Fields Promote Repair of Focal Articular Cartilage Defects with Engineered Osteochondral Constructs. Biotechnol. Bioeng. 2020, 117, 1584–1596. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, Y.; Ni, Z.; Chen, L. Effects and Mechanisms of Exogenous Electromagnetic Field on Bone Cells: A Review. Bioelectromagnetics 2020, 41, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Simon, B.J.; Duran, M.A.; Barabino, G.; Chaudhri, R.; Boyan, B.D. Pulsed Electromagnetic Fields Enhance BMP-2 Dependent Osteoblastic Differentiation of Human Mesenchymal Stem Cells. J. Orthop. Res. 2008, 26, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.; Pellati, A.; Bagheri, L.; Fortini, C.; Setti, S.; De Mattei, M. Pulsed Electromagnetic Fields Stimulate Osteogenic Differentiation in Human Bone Marrow and Adipose Tissue Derived Mesenchymal Stem Cells: PEMFs on Osteogenic Differentiation of MSCs. Bioelectromagnetics 2014, 35, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Seeliger, C.; Unger, M.; Falldorf, K.; Balmayor, E.R.; van Griensven, M. Osteogenic Effect and Cell Signaling Activation of Extremely Low-Frequency Pulsed Electromagnetic Fields in Adipose-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Heydari Asl, S.; Hosseinpoor, H.; Parivar, K.; Hayati Roodbari, N.; Hanaee-Ahvaz, H. Physical Stimulation and Scaffold Composition Efficiently Support Osteogenic Differentiation of Mesenchymal Stem Cells. Tissue Cell 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Bloise, N.; Petecchia, L.; Ceccarelli, G.; Fassina, L.; Usai, C.; Bertoglio, F.; Balli, M.; Vassalli, M.; Cusella De Angelis, M.G.; Gavazzo, P.; et al. The Effect of Pulsed Electromagnetic Field Exposure on Osteoinduction of Human Mesenchymal Stem Cells Cultured on Nano-TiO2 Surfaces. PLoS ONE 2018, 13, e0199046. [Google Scholar] [CrossRef]
- Saino, E.; Fassina, L.; Van Vlierberghe, S.; Avanzini, M.A.; Dubruel, P.; Magenes, G.; Visai, L.; Benazzo, F. Effects of Electromagnetic Stimulation on Osteogenic Differentiation of Human Mesenchymal Stromal Cells Seeded onto Gelatin Cryogel. Int. J. Immunopathol. Pharm. 2011, 24, 1–6. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q.; Lai, A.; Tian, J. Pulsed Electromagnetic Field Induces Ca2+-Dependent Osteoblastogenesis in C3H10T1/2 Mesenchymal Cells through the Wnt-Ca2+/Wnt-β-Catenin Signaling Pathway. Biochem. Biophys. Res. Commun. 2018, 503, 715–721. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Dolkart, O.; Salai, M.; Barak, S.; Piattelli, A.; Amir-Barak, H.; Zavan, B. Pulsed Electromagnetic Fields Increase Osteogenetic Commitment of MSCs via the MTOR Pathway in TNF-α Mediated Inflammatory Conditions: An In-Vitro Study. Sci. Rep. 2018, 8, 5108. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, P.; Cao, Z.; Wang, X.; Wang, D.; Shen, Y.; Jing, D.; Luo, E.; Tang, W. Effects of BMP9 and Pulsed Electromagnetic Fields on the Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells: BMP9 and PEMF Regulate Osteogenesis on PDLSCs. Bioelectromagnetics 2017, 38, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Electromagnetic Fields Act via Activation of Voltage-gated Calcium Channels to Produce Beneficial or Adverse Effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Petecchia, L.; Sbrana, F.; Utzeri, R.; Vercellino, M.; Usai, C.; Visai, L.; Vassalli, M.; Gavazzo, P. Electro-Magnetic Field Promotes Osteogenic Differentiation of BM-HMSCs through a Selective Action on Ca2+-Related Mechanisms. Sci. Rep. 2015, 5, 13856. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.; Pellati, A.; Mazzoni, E.; Salati, S.; Caruso, G.; Contartese, D.; De Mattei, M. Bone Morphogenetic Protein-2 Signaling in the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Induced by Pulsed Electromagnetic Fields. Int. J. Mol. Sci. 2020, 21, 2104. [Google Scholar] [CrossRef]
- Bagheri, L.; Pellati, A.; Rizzo, P.; Aquila, G.; Massari, L.; De Mattei, M.; Ongaro, A. Notch Pathway Is Active during Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Induced by Pulsed Electromagnetic Fields. J. Tissue Eng. Regen. Med. 2018, 12, 304–315. [Google Scholar] [CrossRef]
- De Mattei, M.; Grassilli, S.; Pellati, A.; Brugnoli, F.; De Marchi, E.; Contartese, D.; Bertagnolo, V. Pulsed Electromagnetic Fields Modulate MiRNAs During Osteogenic Differentiation of Bone Mesenchymal Stem Cells: A Possible Role in the Osteogenic-Angiogenic Coupling. Stem Cell Rev. Rep. 2020, 16, 1005–1012. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, R.-W.; Chang, C.-W.; Wang, G.-J.; Lai, K.-A. Single-Pulsed Electromagnetic Field Therapy Increases Osteogenic Differentiation through Wnt Signaling Pathway and Sclerostin Downregulation: SPEMF Enhance Bone Growth by Wnt/Sost Control. Bioelectromagnetics 2015, 36, 494–505. [Google Scholar] [CrossRef]
- Tan, Y.; Fei, D.; He, X.; Dai, J.; Xu, R.; Xu, X.; Wu, J.; Li, B. L-type Voltage-gated Calcium Channels in Stem Cells and Tissue Engineering. Cell Prolif. 2019, 52. [Google Scholar] [CrossRef]
- Schupbach, D.; Comeau-Gauthier, M.; Harvey, E.; Merle, G. Wnt Modulation in Bone Healing. Bone 2020, 138, 115491. [Google Scholar] [CrossRef]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. P38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef]
- Novak, S.; Roeder, E.; Sinder, B.P.; Adams, D.J.; Siebel, C.W.; Grcevic, D.; Hankenson, K.D.; Matthews, B.G.; Kalajzic, I. Modulation of Notch1 Signaling Regulates Bone Fracture Healing. J. Orthop. Res. 2020, 38, 2350–2361. [Google Scholar] [CrossRef]
- Qian, G.; Wang, M.; Dong, Y.; Hong, Y.; Yu, Y.; Mei, J. Effect of Combined Treatment with Pulsed Electromagnetic Field Stimulation and Sclerostin Monoclonal Antibody on Changes in Bone Metabolism and Pedicle Screw Augmentation in Rabbits with Ovariectomy-Induced Osteoporosis. Ann. Palliat. Med. 2020, 9, 20. [Google Scholar] [CrossRef]
- Dishowitz, M.I.; Zhu, F.; Sundararaghavan, H.G.; Ifkovits, J.L.; Burdick, J.A.; Hankenson, K.D. Jagged1 Immobilization to an Osteoconductive Polymer Activates the Notch Signaling Pathway and Induces Osteogenesis: Jagged1 Induces Osteogenesis. J. Biomed. Mater. Res. A 2014, 102, 1558–1567. [Google Scholar] [CrossRef]
- Youngstrom, D.W.; Senos, R.; Zondervan, R.L.; Brodeur, J.D.; Lints, A.R.; Young, D.R.; Mitchell, T.L.; Moore, M.E.; Myers, M.H.; Tseng, W.-J.; et al. Intraoperative Delivery of the Notch Ligand Jagged-1 Regenerates Appendicular and Craniofacial Bone Defects. NPJ Regen. Med. 2017, 2, 32. [Google Scholar] [CrossRef]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing Application of Mesenchymal Stem Cell-Based Bone Tissue Regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef]
- Azadian, E.; Arjmand, B.; Khodaii, Z.; Ardeshirylajimi, A. A Comprehensive Overview on Utilizing Electromagnetic Fields in Bone Regenerative Medicine. Electromagn. Biol. Med. 2019, 38, 1–20. [Google Scholar] [CrossRef]
- Tschon, M.; Veronesi, F.; Contartese, D.; Sartori, M.; Martini, L.; Vincenzi, F.; Ravani, A.; Varani, K.; Fini, M. Effects of Pulsed Electromagnetic Fields and Platelet Rich Plasma in Preventing Osteoclastogenesis in an In Vitro Model of Osteolysis. J. Cell. Physiol. 2018, 233, 2645–2656. [Google Scholar] [CrossRef]
- Veronesi, F.; Cadossi, M.; Giavaresi, G.; Martini, L.; Setti, S.; Buda, R.; Giannini, S.; Fini, M. Pulsed Electromagnetic Fields Combined with a Collagenous Scaffold and Bone Marrow Concentrate Enhance Osteochondral Regeneration: An In Vivo Study. BMC Musculoskelet. Disord. 2015, 16, 233. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R. Adenosine Receptors as a Biological Pathway for the Anti-Inflammatory and Beneficial Effects of Low Frequency Low Energy Pulsed Electromagnetic Fields. Mediat. Inflamm. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Cadossi, M.; Setti, S.; Borea, P.A.; Cadossi, R. Role of Adenosine Receptors in Clinical Biophysics Based on Pulsed Electromagnetic Fields. In The Adenosine Receptors; The Receptors; Humana Press: Cham, Switzerland, 2018; Volume 34. [Google Scholar] [CrossRef]
| Cell Source | PEMF Parameters | PEMF Effects |
|---|---|---|
| Rat BM-MSCs | 75Hz, 1–5 mT 3 h/day for 4 weeks. Chengdu Miracle Chemical device. | Inhibit the maintenance of the cartilaginous phenotype [42] |
| Rabbit ADMSCs | 75 Hz, 1.8 mT, 8 h/day for 21 days | Increase collagen type II expression and ECM deposition [40] |
| Bovine MSCs from synovial fluid | Trapezoidal wave, 75 Hz, 1.5 mT, 3–5 weeks. IGEA device. | Counteract the IL-1β-induced inhibition of chondrogenesis [46] |
| Human ADMSCs | Sinusoidal wave, 1 T, 3 min/day, 3-5-7-10 days. | Increase collagen type II expression and glycosaminoglycan (GAG) content [38] |
| Enhance chondrogenic differentiation in 2D and 3D cultures [38] | ||
| Human umbilical cord-derived MSCs | Trapezoidal wave, 75 Hz, 1.5 mT, 8 h/day, 21 days. IGEA device. | Enhance cellular proliferation [39] |
| Increase chondrogenic differentiation [39] | ||
| Human BM-MSCs | Sinusoidal wave, 15 Hz, 5 mT, 45 min every 8 h, for 21 days. TNeue Magnetodyn device. | Increase chondrogenic differentiation [37] |
| 15 Hz, 1–4 mT, 5–60 min, single and multiple exposures. | Upregulate Sox9, Col2 and aggrecan mRNA expression [43] | |
| Increase chondrogenic ECM deposition [43] | ||
| Upregulate TRPV4 and TRPC1 [43] | ||
| Sinusoidal wave, 7.5–75 Hz, 1 mT, 24 h. Naval University of Engineering. | Promote MSC migration [47] | |
| Intracellular Ca2+ increase [47] | ||
| FAK activation [47] | ||
| enhanced Rho GTPase activity [47] | ||
| increased F-actin network formation [47] | ||
| 15 Hz, 0–3 mT, 5–30 min, single and multiple exposures. | Perpendicular PEMFs enhance chondrogenic differentiation in MSCs cultured on randomly oriented scaffolds [48] | |
| 15 Hz, 0.5–4 mT, 10 min. | Enhance paracrine function of MSCs for cartilage regeneration [49] |
| Cell Source | PEMF Parameters | PEMF Effects |
|---|---|---|
| C3H10T1/2 murine mesenchymal stem cell line | 30 Hz, 1 mT, 2 h/day for 20 days. EBI device. | Increase ALP activity, mineralization, Runx2, Osx [61] |
| Increase intracellular Ca2+ concentration [61] | ||
| Upregulate Wnt1, phospho-Lrp6, and β-catenin [61] | ||
| Human ADMSCs | 26 Hz ELF-PEMF 7 min/day for 14 days. Somagen® device. | Increase ALP, mineralization, COL-I and OC gene expression [57] Increase Akt, p70 S6 kinase, S6 ribosomal protein, and ERK1/2 phosphorylation [57] |
| 12 MHz microwave and 30 mT PEMF, frequency range 50–400 Hz, 8 h/day, 14 days. STRC device. | Increase ALP activity, mineralization, ALP and Runx2 gene expression [58] | |
| 30 days, MED device. | Increase ALP activity, mineralization, ALP and OSP gene expression [62] | |
| Activate Akt and mTOR pathway [62] | ||
| Human periodontal ligament stem cells (hPDLSC) | Rectangular wave, 15 Hz, 1.8 or 2.4 mT, 1 h/day. GHY-III device. | Increase ALP, OPN, mineralization, Runx2 [63] |
| Synergistic effect with BMP-9 [63] | ||
| Human BM-MSCs | Trapezoidal wave, 75 Hz, 2 mT, 10 min/day for 28 days. | Increase Runx-2, COL-I, FN, OSP, Osx, OC, BMP-2, ALP gene expression; ALP activity; BMP-2, DCN, COL-I protein in cells cultured on nano-TiO2 surfaces [59] |
| Increase L-type voltage gated Ca channels (VGCCs) expression [59] | ||
| Increase ALP, COL-I, OPN, DCN proteins [64] | ||
| Increase Ca2+ fluxes by L-type voltage-gated Ca channels (VGCCs) [65] | ||
| Activation of the Ca2+/CaM pathway [65] | ||
| Increase Ca2+ fluxes by L-type voltage-gated Ca channels (VGCCs) [65] | ||
| Activation of the Ca2+/CaM pathway [65] | ||
| Trapezoidal wave, 75 Hz, 1.5 mT, 28 days. IGEA device. | Increase ALP activity, OC production, mineralization, Runx2, Dlx5 [66] | |
| Activate SMAD1/5/8 and p38 MAPK [66] | ||
| Upregulate BMP-2, BMP-6, BMP type I receptor [66] | ||
| Increase ALP activity, OC level, mineralization, Runx2, Dlx5, Osterix gene expression [67] | ||
| Increase Notch4, Dll4, Hey1, Hes1 and Hes5 expression [67] | ||
| Increase ALP activity, OC production, Runx2 and Dlx5 gene expression [68] | ||
| Increase miR-26a, miR-29b, miR-210 expression or extracellular release [68] | ||
| Increase VEGF expression and release [68] | ||
| Asymmetrical hemi-sine wave, 5-ms pulse every 5 s, 1 T, 3 min/day, days 1–5. Oriental Advance Technology device. | Upregulate Wnt1, Wnt3a, Wnt10b, Fzd9, BMP2 [69] | |
| Downregulate SOST [69] | ||
| Increase ALP activity, mineralization [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. https://doi.org/10.3390/ijms22020809
Varani K, Vincenzi F, Pasquini S, Blo I, Salati S, Cadossi M, De Mattei M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. International Journal of Molecular Sciences. 2021; 22(2):809. https://doi.org/10.3390/ijms22020809
Chicago/Turabian StyleVarani, Katia, Fabrizio Vincenzi, Silvia Pasquini, Irene Blo, Simona Salati, Matteo Cadossi, and Monica De Mattei. 2021. "Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications" International Journal of Molecular Sciences 22, no. 2: 809. https://doi.org/10.3390/ijms22020809
APA StyleVarani, K., Vincenzi, F., Pasquini, S., Blo, I., Salati, S., Cadossi, M., & De Mattei, M. (2021). Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. International Journal of Molecular Sciences, 22(2), 809. https://doi.org/10.3390/ijms22020809








