Titanium Dioxide Presents a Different Profile in Dextran Sodium Sulphate-Induced Experimental Colitis in Mice Lacking the IBD Risk Gene Ptpn2 in Myeloid Cells
Abstract
1. Introduction
2. Results
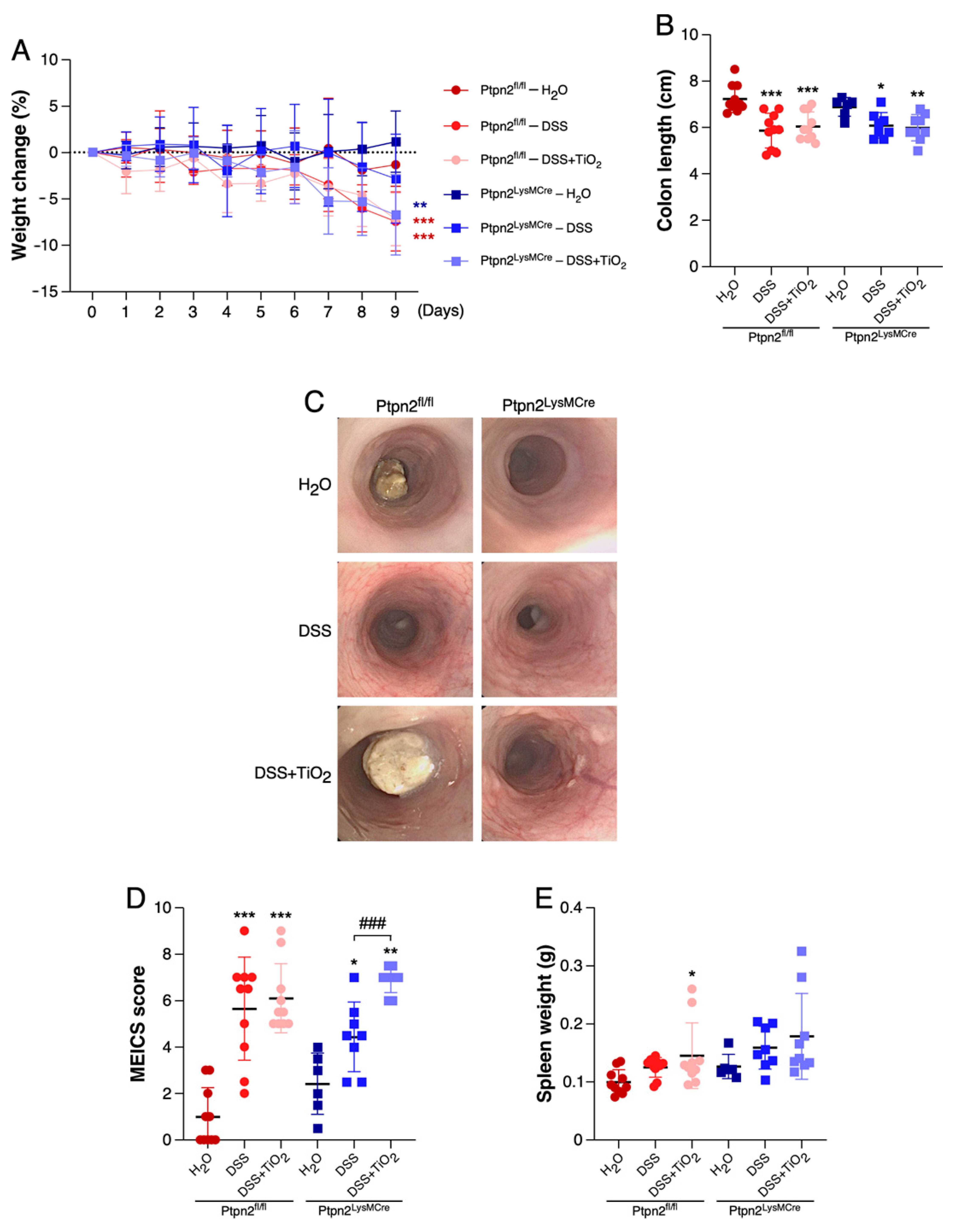
2.1. TiO2 Induces a Different Intestinal Inflammation Profile in Mice Featuring Loss of Ptpn2 in Myeloid Cells
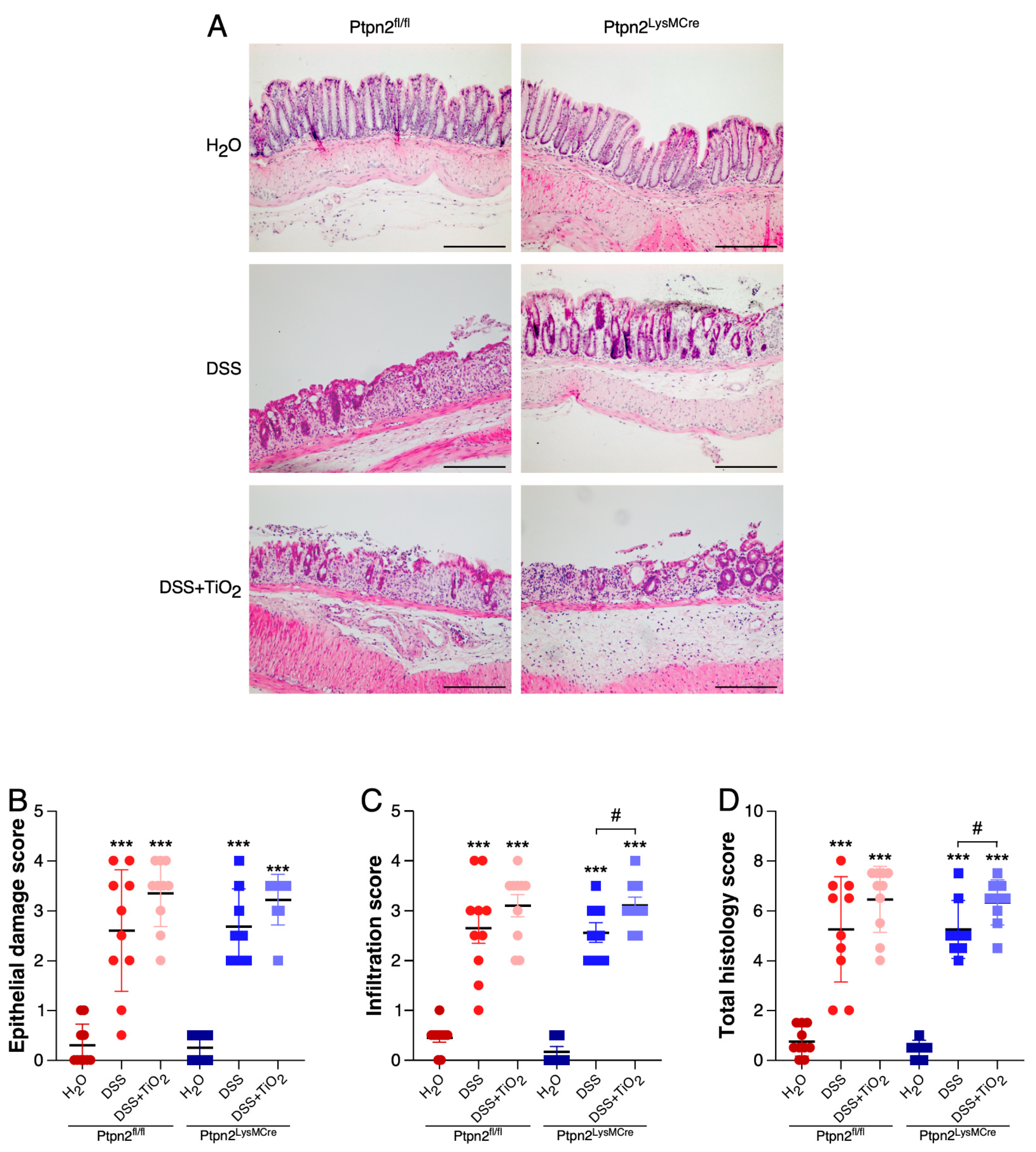
2.2. TiO2 Treatment Induces a Different Histologic Inflammation Profile in Mice Featuring Loss of Ptpn2 in Myeloid Cells
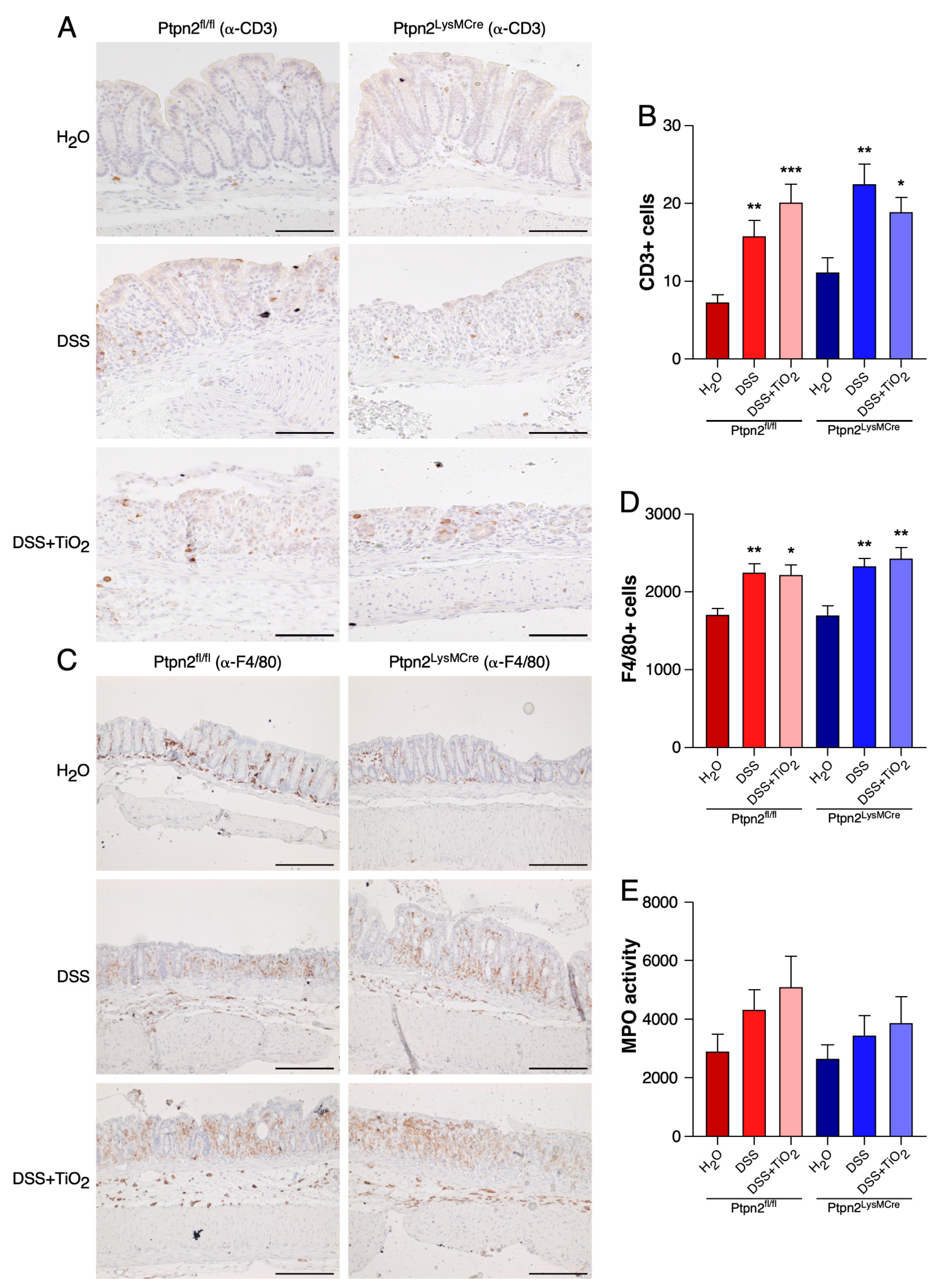
2.3. TiO2 Does Not Affect the Total Histological Numbers of T Cells and Macrophages or MPO Activity
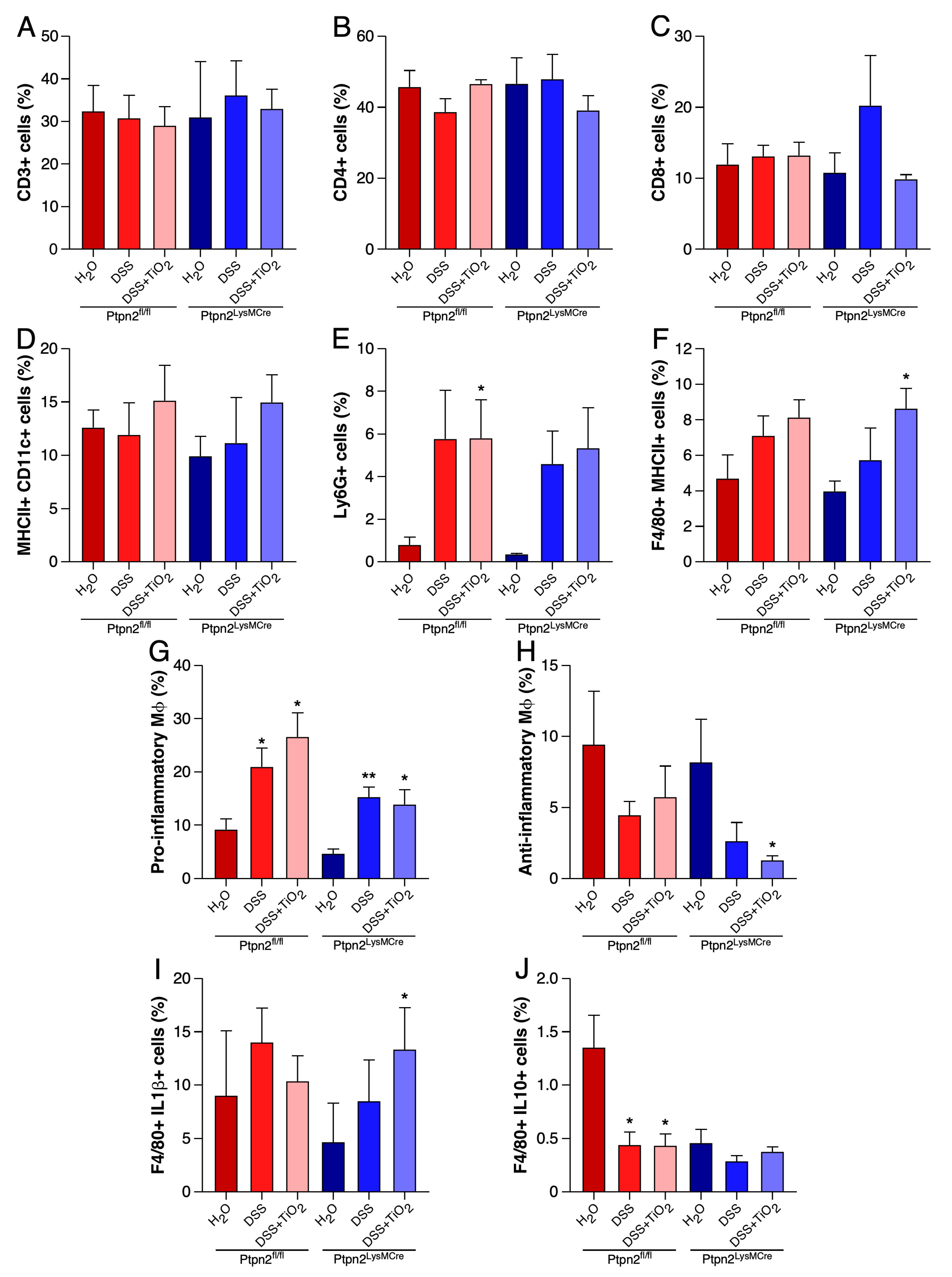
2.4. TiO2 Decreases the Number of Anti-Inflammatory Macrophages in LPLs
2.5. TiO2 Induces IL-1β and Represses IL-10 Expression in BMDMs
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Histology
4.3. Assessment of Histological Score
4.4. Cell Culture
4.5. Myeloperoxidase (MPO) Activity
4.6. Flow Cytometry
4.7. Western Blot
4.8. RNA Isolation and RT-qPCR
5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSS | Dextran Sodium Sulfate |
| IBD | Inflammatory Bowel Disease |
| TiO2 | Titanium dioxide |
| PTPN2 | Protein tyrosine phosptahatse non-receptor type 2 |
| LPL | Lamina propria lymphocytes |
| BMDMs | Bone marrow-derived macrophages |
| MAPK | Mitogen-activated protein kinase |
| IL | Interleukin |
| JNK–c-JUN | N-terminal kinase |
| ERK | Extracellular signal-regulated kinase |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| Tlr4 | Toll like receptor 4 |
References
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Wasinger, V.C.; Yau, Y.Y.; Chuang, E.; Yajnik, V.; Leong, R.W. Molecular Pathophysiology of Epithelial Barrier Dysfunction in Inflammatory Bowel Diseases. Proteomes 2018, 6, 17. [Google Scholar] [CrossRef]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef]
- Franke, A.; Balschun, T.; Karlsen, T.H.; Hedderich, J.; May, S.; Lu, T.; Schuldt, D.; Nikolaus, S.; Rosenstiel, P.; Krawczak, M.; et al. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nat. Genet. 2008, 40, 713–715. [Google Scholar] [CrossRef]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases-Sustaining Healthcare Delivery into the 21st Century. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Amamou, A.; Savoye, G.; Ghosh, S. Inflammatory Bowel Diseases and Food Additives: To Add Fuel on the Flames! Nutrients 2019, 11, 1111. [Google Scholar] [CrossRef]
- Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Coumarin. EFSA J. 2004, 2, 104. [CrossRef]
- Talamini, L.; Gimondi, S.; Violatto, M.B.; Fiordaliso, F.; Pedica, F.; Tran, N.L.; Sitia, G.; Aureli, F.; Raggi, A.; Nelissen, I.; et al. Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology 2019, 13, 1087–1101. [Google Scholar] [CrossRef]
- Heringa, M.B.; Peters, R.J.B.; Bleys, R.L.A.W.; van der Lee, M.K.; Tromp, P.C.; van Kesteren, P.C.E.; van Eijkeren, J.C.H.; Undas, A.K.; Oomen, A.G.; Bouwmeester, H. Detection of titanium particles in human liver and spleen and possible health implications. Part. Fibre Toxicol. 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Ainley, C.C.; Harvey, R.S.; Mason, I.M.; Kendall, M.D.; Sankey, E.A.; Dhillon, A.P.; Thompson, R.P. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut 1996, 38, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Ortega, I.M.; Garduño-Balderas, L.G.; Delgado-Buenrostro, N.L.; Freyre-Fonseca, V.; Flores-Flores, J.O.; González-Robles, A.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Rodríguez-Sosa, M.; León-Cabrera, S.; et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; Anderson, C.A.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef]
- Scharl, M.; Mwinyi, J.; Fischbeck, A.; Leucht, K.; Eloranta, J.J.; Arikkat, J.; Pesch, T.; Kellermeier, S.; Mair, A.; Kullak-Ublick, G.A.; et al. Crohn’s disease-associated polymorphism within the PTPN2 gene affects muramyl-dipeptide-induced cytokine secretion and autophagy. Inflamm. Bowel Dis. 2012, 18, 900–912. [Google Scholar] [CrossRef]
- Glas, J.; Wagner, J.; Seiderer, J.; Olszak, T.; Wetzke, M.; Beigel, F.; Tillack, C.; Stallhofer, J.; Friedrich, M.; Steib, C.; et al. PTPN2 gene variants are associated with susceptibility to both Crohn’s disease and ulcerative colitis supporting a common genetic disease background. PLoS ONE 2012, 7, e33682. [Google Scholar] [CrossRef]
- Todd, J.A.; Walker, N.M.; Cooper, J.D.; Smyth, D.J.; Downes, K.; Plagnol, V.; Bailey, R.; Nejentsev, S.; Field, S.F.; Payne, F.; et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 2007, 39, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Aradi, B.; Kato, M.; Filkova, M.; Karouzakis, E.; Klein, K.; Scharl, M.; Kolling, C.; Michel, B.A.; Gay, R.E.; Buzas, E.I.; et al. Protein tyrosine phosphatase nonreceptor type 2: An important regulator of lnterleukin-6 production in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol. (Hoboken, N.J.) 2015, 67, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Scharl, M.; McCole, D.F.; Weber, A.; Vavricka, S.R.; Frei, P.; Kellermeier, S.; Pesch, T.; Fried, M.; Rogler, G. Protein tyrosine phosphatase N2 regulates TNFα-induced signalling and cytokine secretion in human intestinal epithelial cells. Gut 2011, 60, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, K.M.; Nestel, F.P.; Newell, E.W.; Charette, G.; Seemayer, T.A.; Tremblay, M.L.; Lapp, W.S. T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood 2004, 103, 3457–3464. [Google Scholar] [CrossRef]
- Scharl, M.; Paul, G.; Weber, A.; Jung, B.C.; Docherty, M.J.; Hausmann, M.; Rogler, G.; Barrett, K.E.; McCole, D.F. Protection of Epithelial Barrier Function by the Crohn’s Disease Associated Gene Protein Tyrosine Phosphatase N2. Gastroenterology 2009, 137, 2030–2040.e5. [Google Scholar] [CrossRef]
- Scharl, M.; Hruz, P.; McCole, D.F. Protein tyrosine phosphatase non-receptor Type 2 regulates IFN-γ-induced cytokine signaling in THP-1 monocytes. Inflamm. Bowel Dis. 2010, 16, 2055–2064. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Kasper, S.; Chassard, C.; Raselli, T.; Frey-Wagner, I.; Gottier, C.; Lang, S.; Atrott, K.; Vavricka, S.R.; Mair, F.; et al. PTPN2 controls differentiation of CD4+ T cells and limits intestinal inflammation and intestinal dysbiosis. Mucosal Immunol. 2015, 8, 918–929. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Manzini, R.; Hering, L.; Riggs, J.B.; Gottier, C.; Lang, S.; Atrott, K.; Fettelschoss, A.; Olomski, F.; Kündig, T.M.; et al. PTPN2 Regulates Inflammasome Activation and Controls Onset of Intestinal Inflammation and Colon Cancer. Cell Rep. 2018, 22, 1835–1848. [Google Scholar] [CrossRef]
- Han, M.K.; Anderson, R.; Viennois, E.; Merlin, D. Examination of food consumption in United States adults and the prevalence of inflammatory bowel disease using National Health Interview Survey 2015. PLoS ONE 2020, 15, e0232157. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Romano, K.A.; Gu, M.; Sanidad, K.Z.; Kim, D.; Yang, J.; Schmidt, B.; Panigrahy, D.; Pei, R.; et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci. Transl. Med. 2018, 10, eaan4116. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, F.; Fan, M.; He, C.; Yang, X.; Huang, Z.; Fu, Z. The influence of titanium dioxide nanoparticles on their cellular response to macrophage cells. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2019, 223, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Dosh, R.H.; Jordan-Mahy, N.; Sammon, C.; Le Maitre, C. Interleukin 1 is a key driver of inflammatory bowel disease-demonstration in a murine IL-1Ra knockout model. Oncotarget 2019, 10, 3559–3575. [Google Scholar] [CrossRef] [PubMed]
- Shouval, D.S.; Biswas, A.; Kang, Y.H.; Griffith, A.E.; Konnikova, L.; Mascanfroni, I.D.; Redhu, N.S.; Frei, S.M.; Field, M.; Doty, A.L.; et al. Interleukin 1β Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 2016, 151, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Apte, R.N. IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron. 2015, 8, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-X.; Wang, B.; Li, B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Becker, C.; Fantini, M.C.; Neurath, M.F. High resolution colonoscopy in live mice. Nat. Protoc. 2006, 1, 2900–2904. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde, J.; Schwarzfischer, M.; Katkeviciute, E.; Häfliger, J.; Niechcial, A.; Brillant, N.; Manzini, R.; Bäbler, K.; Atrott, K.; Lang, S.; et al. Titanium Dioxide Presents a Different Profile in Dextran Sodium Sulphate-Induced Experimental Colitis in Mice Lacking the IBD Risk Gene Ptpn2 in Myeloid Cells. Int. J. Mol. Sci. 2021, 22, 772. https://doi.org/10.3390/ijms22020772
Conde J, Schwarzfischer M, Katkeviciute E, Häfliger J, Niechcial A, Brillant N, Manzini R, Bäbler K, Atrott K, Lang S, et al. Titanium Dioxide Presents a Different Profile in Dextran Sodium Sulphate-Induced Experimental Colitis in Mice Lacking the IBD Risk Gene Ptpn2 in Myeloid Cells. International Journal of Molecular Sciences. 2021; 22(2):772. https://doi.org/10.3390/ijms22020772
Chicago/Turabian StyleConde, Javier, Marlene Schwarzfischer, Egle Katkeviciute, Janine Häfliger, Anna Niechcial, Nathalie Brillant, Roberto Manzini, Katharina Bäbler, Kirstin Atrott, Silvia Lang, and et al. 2021. "Titanium Dioxide Presents a Different Profile in Dextran Sodium Sulphate-Induced Experimental Colitis in Mice Lacking the IBD Risk Gene Ptpn2 in Myeloid Cells" International Journal of Molecular Sciences 22, no. 2: 772. https://doi.org/10.3390/ijms22020772
APA StyleConde, J., Schwarzfischer, M., Katkeviciute, E., Häfliger, J., Niechcial, A., Brillant, N., Manzini, R., Bäbler, K., Atrott, K., Lang, S., & Scharl, M. (2021). Titanium Dioxide Presents a Different Profile in Dextran Sodium Sulphate-Induced Experimental Colitis in Mice Lacking the IBD Risk Gene Ptpn2 in Myeloid Cells. International Journal of Molecular Sciences, 22(2), 772. https://doi.org/10.3390/ijms22020772





