Systemic Inflammation and the Breakdown of Intestinal Homeostasis Are Key Events in Chronic Spinal Cord Injury Patients
Abstract
1. Introduction
2. Results
2.1. Demographic Profile of Chronic SCI Patients
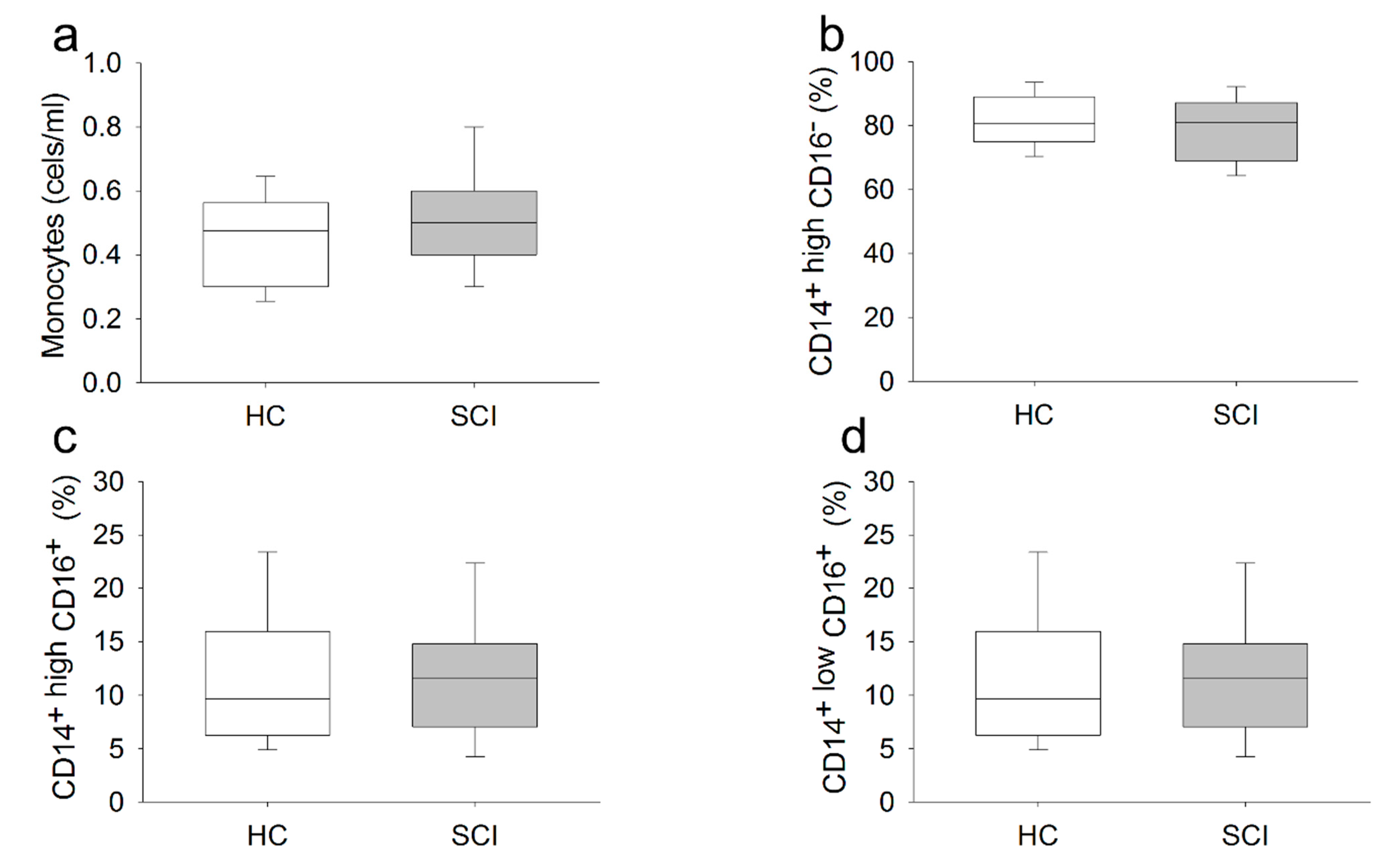
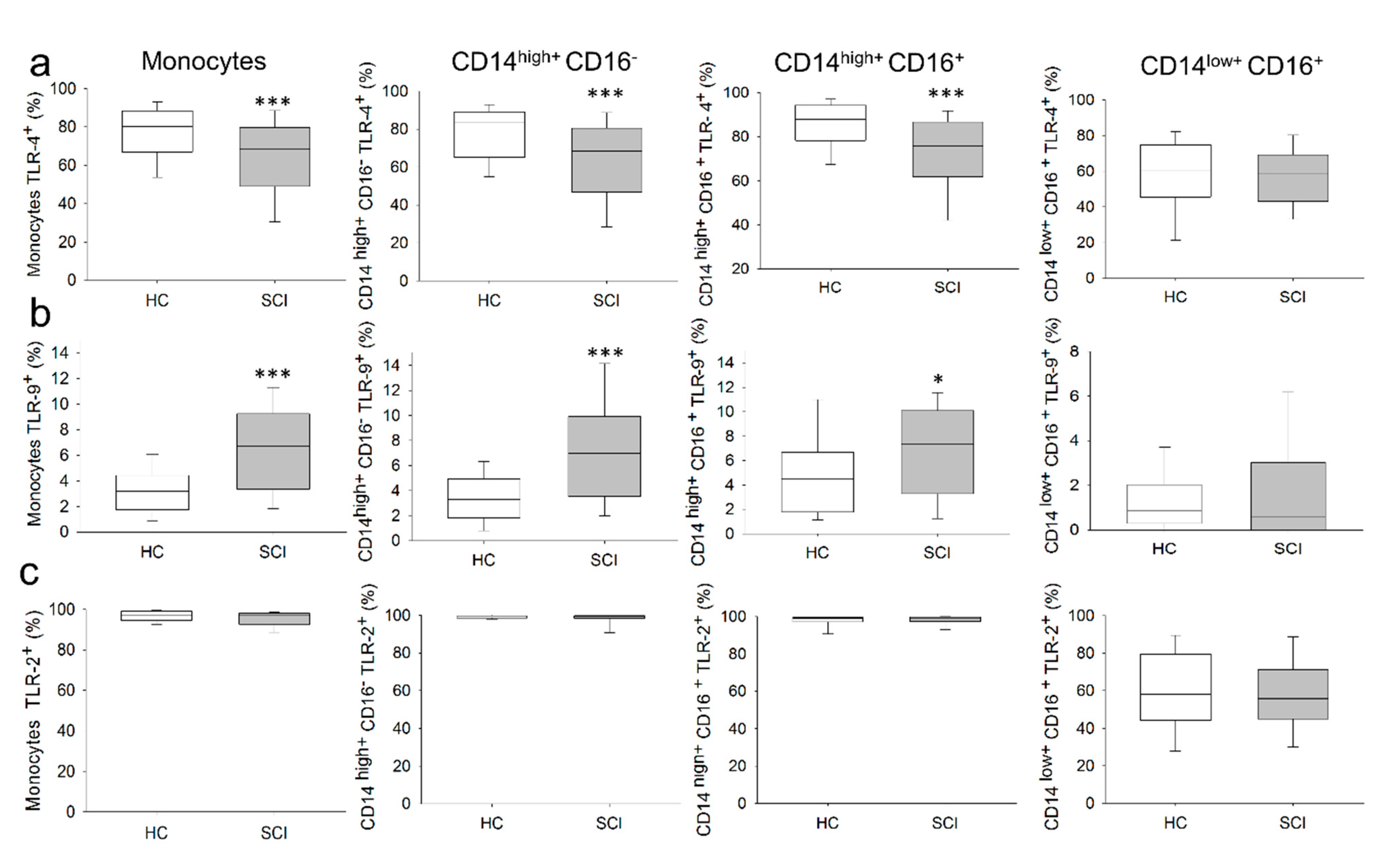
2.2. Chronic SCI Patients Show Normal Monocyte Subset Distributions and Cell Counts but Abnormal TLR Expression
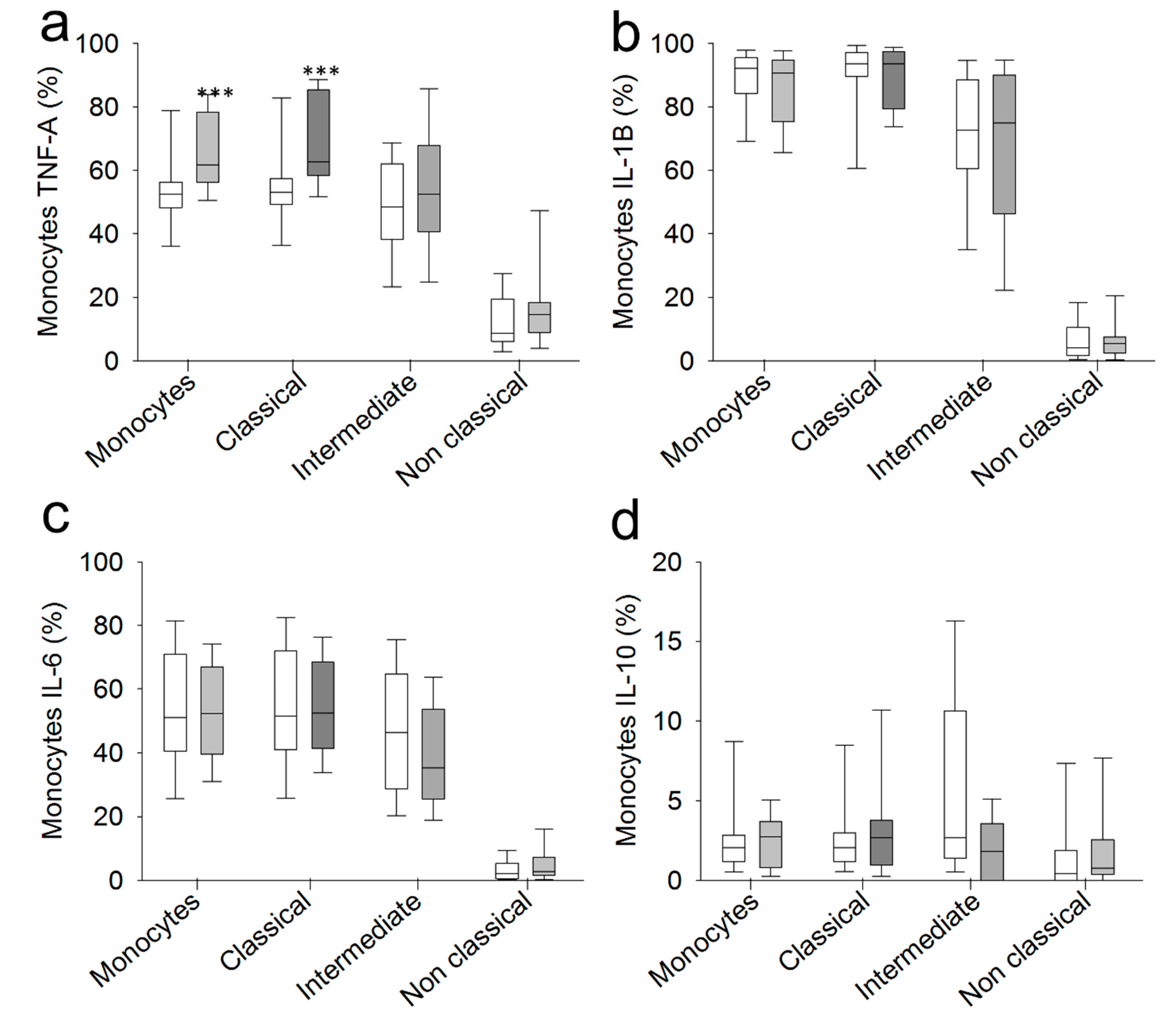
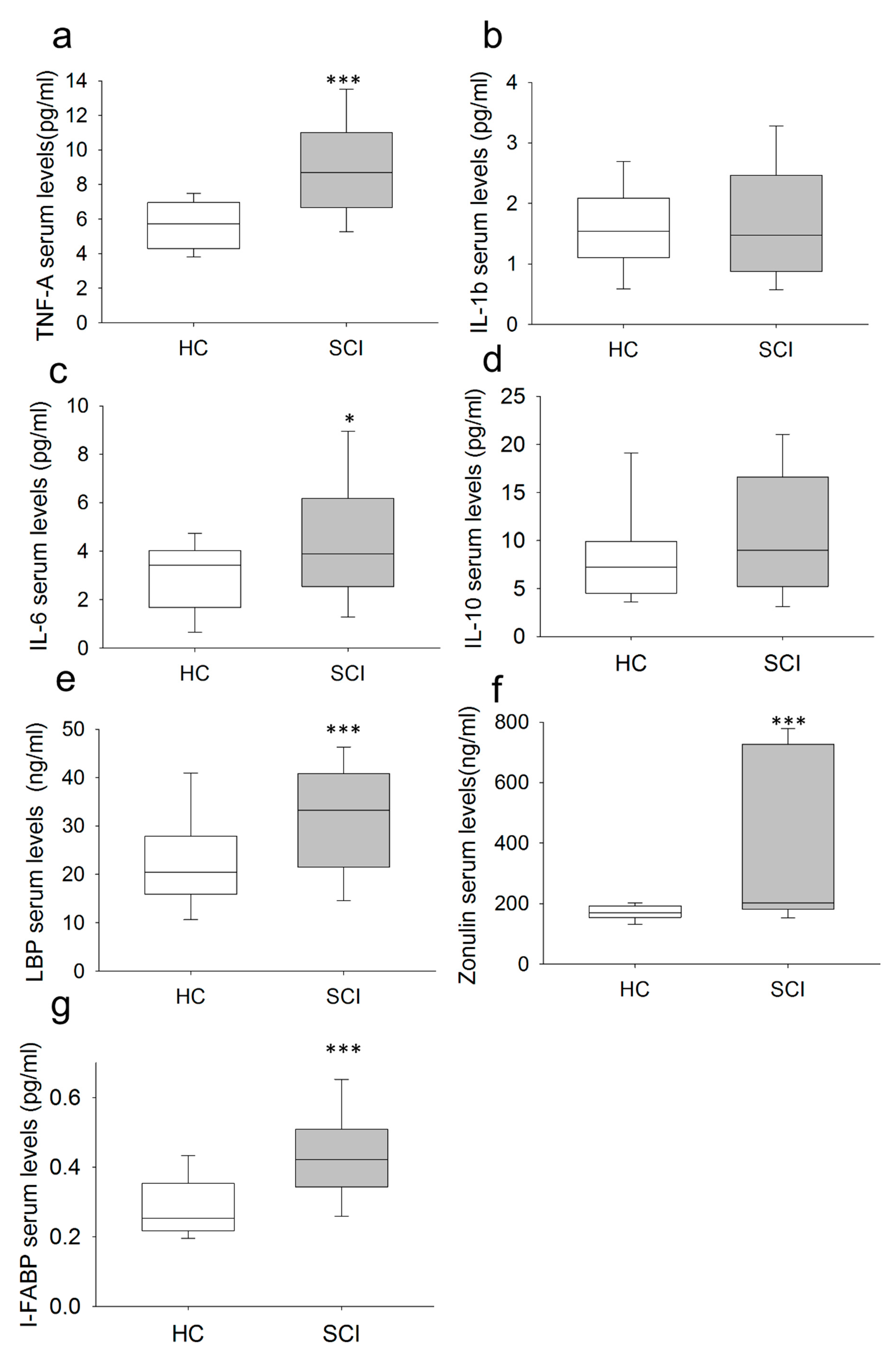
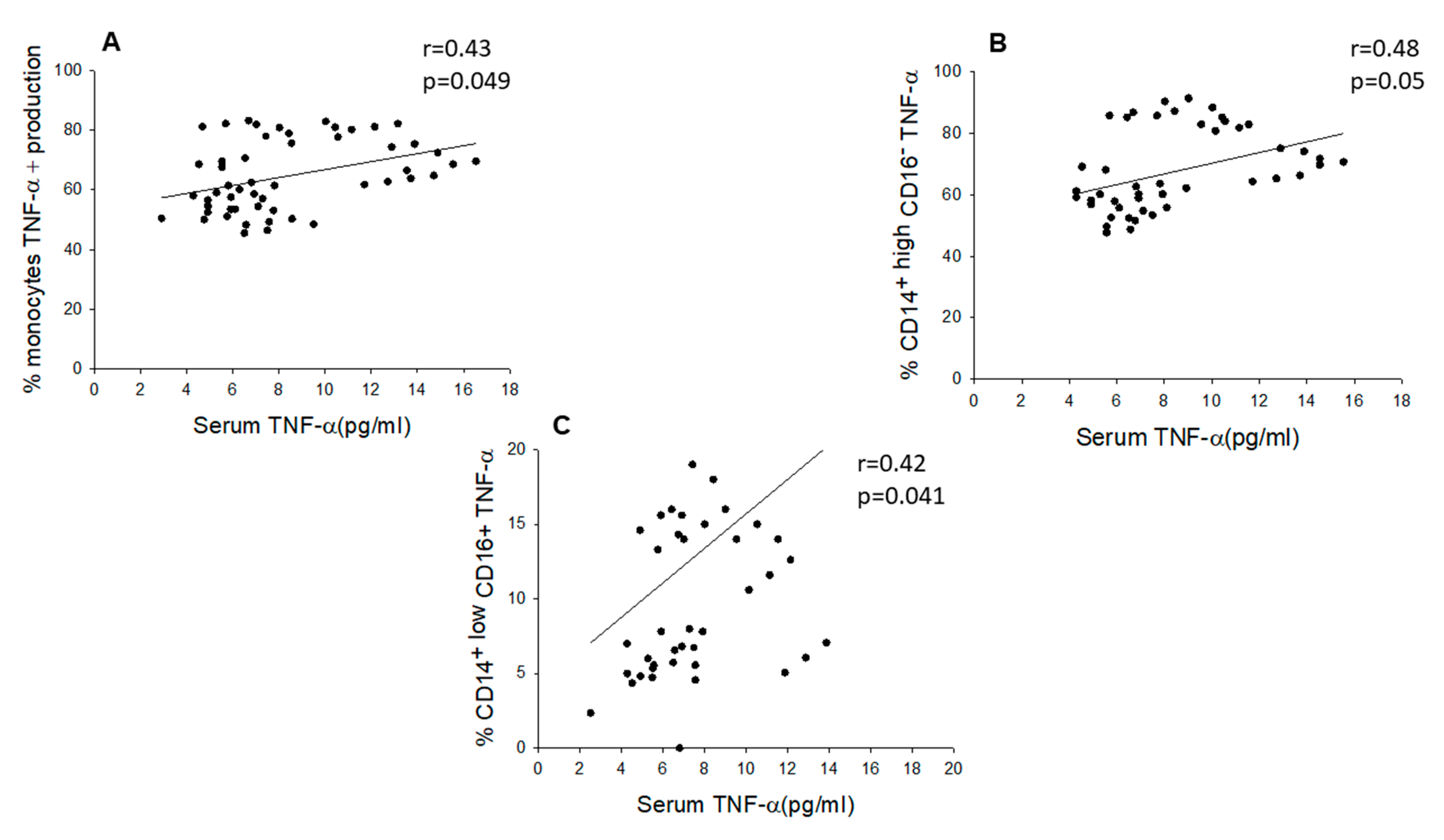
2.3. Chronic SCI Patients Exhibit TNF-α Overproduction by Monocytes and Increased Serum Levels of the Proinflammatory Cytokines TNF-α and IL-6
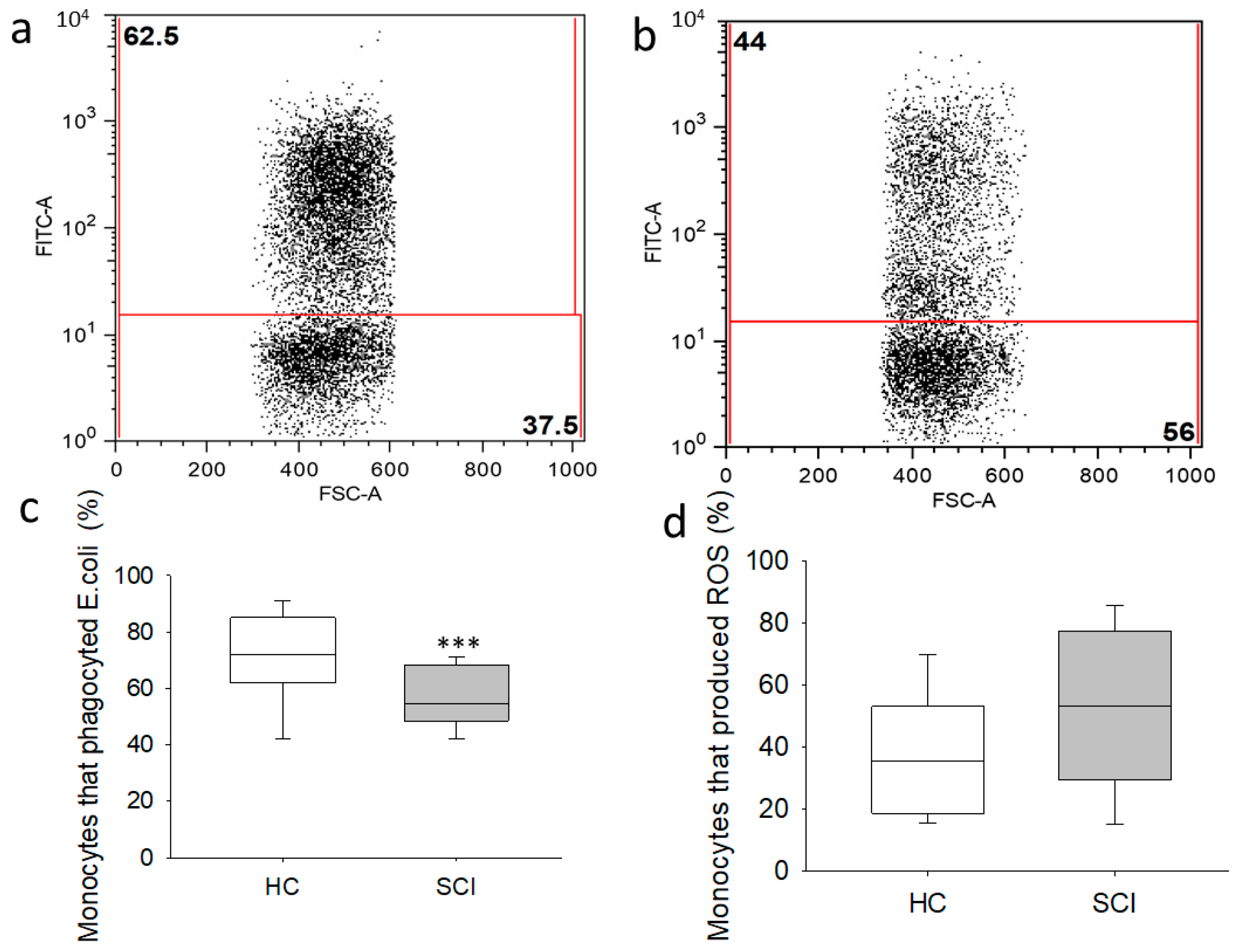
2.4. Monocytes from Chronic SCI Patients Show Defective Phagocytic Effector Functions
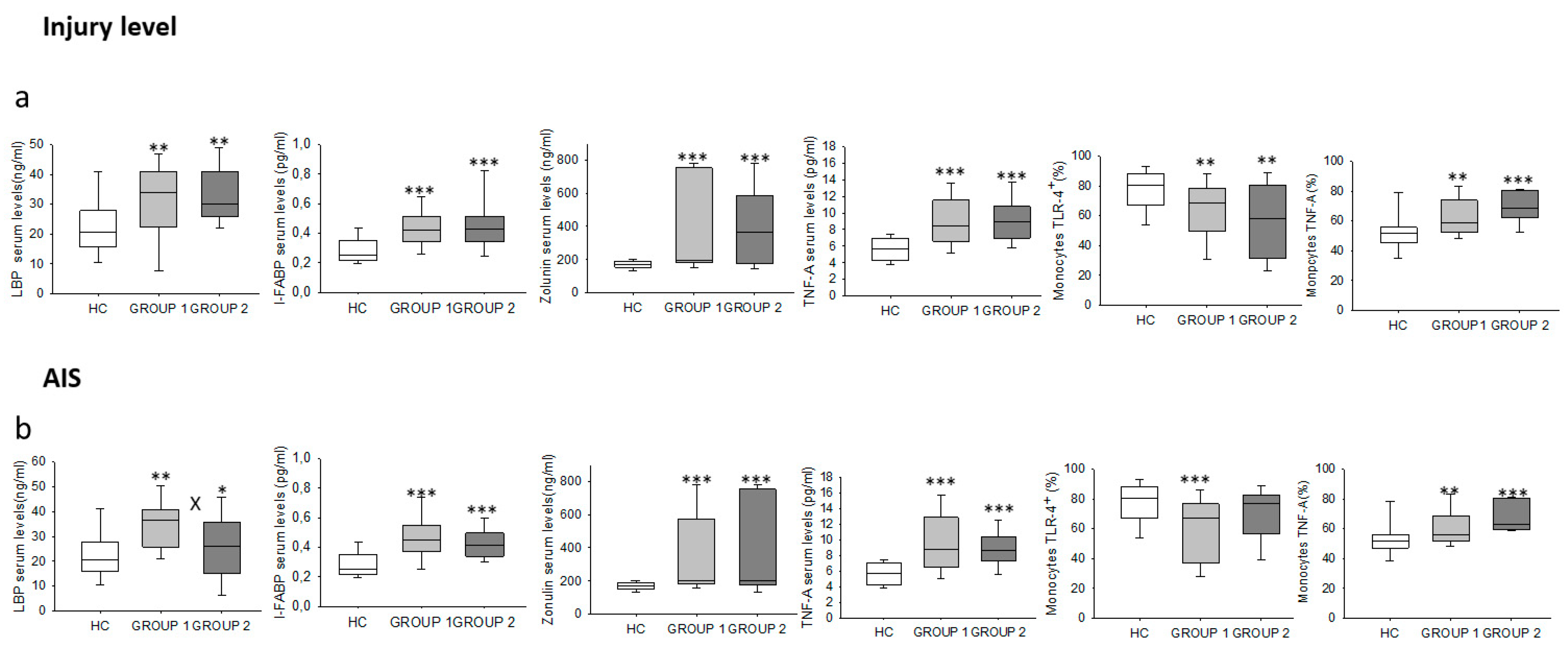
2.5. Chronic SCI Patients Show Increased Levels of Circulating LBP, I-FABP and Zonulin
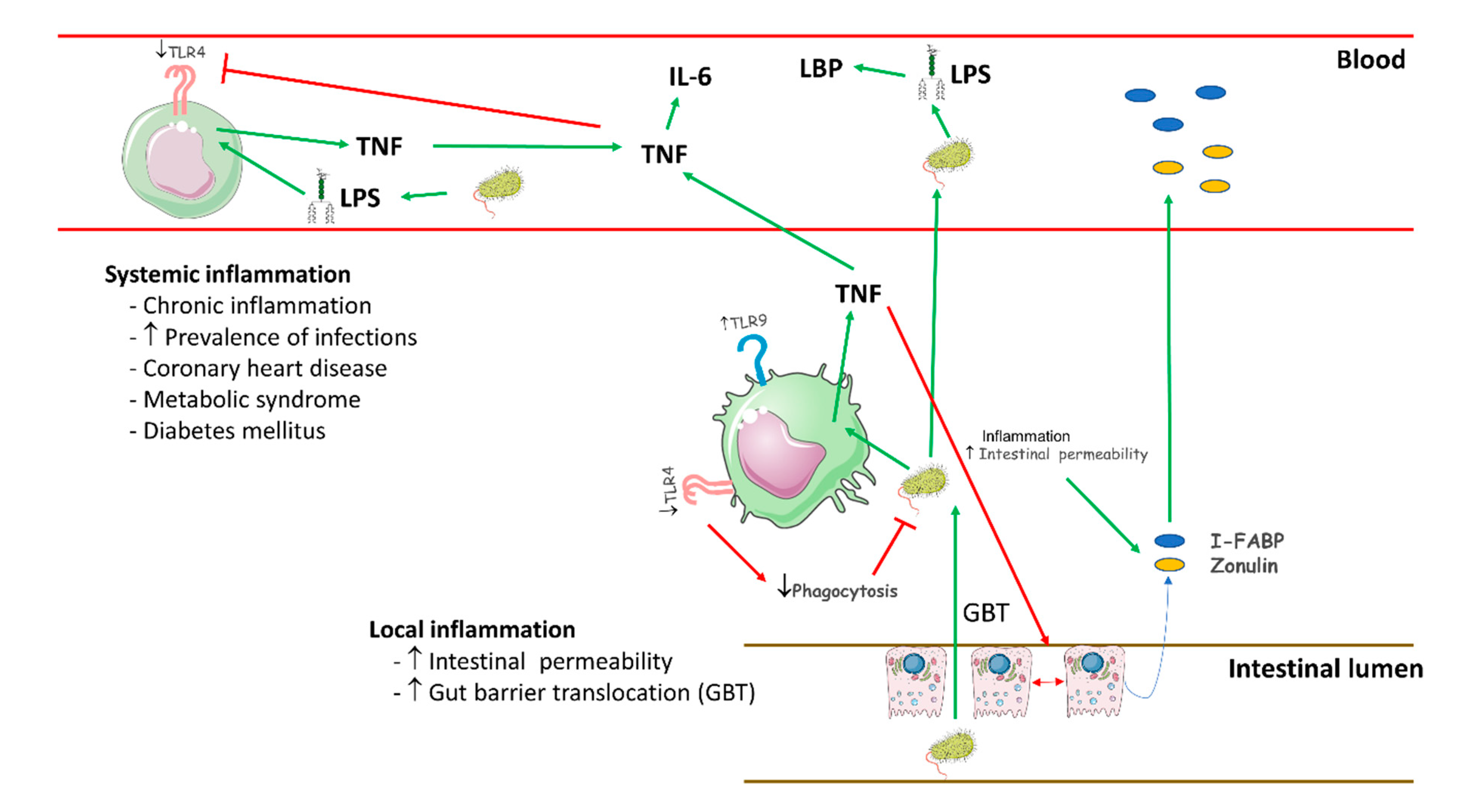
3. Discussion
4. Materials and Methods
4.1. Study Protocol
4.2. Isolation of Peripheral Blood Mononuclear Cells
4.3. Immunophenotype Studies
4.4. Intracellular Cytokines
4.5. Flow Cytometry Studies of Oxidation and Phagocytic Activity
4.6. Quantification of Serum Cytokines Using Luminex
4.7. Study of Damage to the Intestinal Barrier
4.8. Study of the Acute Phase Response
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Ethical Approval
References
- DeVivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Bowes, A.L.; Yip, P.K. Modulating inflammatory cell responses to spinal cord injury: All in good time. J. Neurotrauma 2014, 31, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Walsh, J.T.; Smirnov, I.; Zheng, J.; Kipnis, J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 2015, 85, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Lymphocytes and autoimmunity after spinal cord injury. Exp. Neurol. 2014, 258, 78–90. [Google Scholar] [CrossRef]
- Laginha, I.; Kopp, M.A.; Druschel, C.; Schaser, K.D.; Brommer, B.; Hellmann, R.C.; Watzlawick, R.; Ossami-Saidi, R.R.; Prüss, H.; Failli, V.; et al. Natural killer (NK) cell functionality after human spinal cord injury (SCI): Protocol of a prospective, longitudinal study. BMC Neurol. 2016, 16, 170. [Google Scholar] [CrossRef]
- Pajoohesh-Ganji, A.; Knoblach, S.M.; Faden, A.I.; Byrnes, K.R. Characterization of inflammatory gene expression and galectin-3 function after spinal cord injury in mice. Brain Res. 2012, 1475, 96–105. [Google Scholar] [CrossRef]
- DiMarco, A.F.; Dawson, N.V. Risk factors for mortality in spinal cord injury. J. Spinal Cord Med. 2014, 37, 670–671. [Google Scholar] [CrossRef][Green Version]
- Strauss, D.J.; DeVivo, M.J.; Paculdo, D.R.; Shavelle, R.M. Trends in life expectancy after spinal cord injury. Arch. Phys. Med. Rehabil. 2006, 87, 1079–1085. [Google Scholar] [CrossRef]
- Khan, B.; Bauman, W.A.; Sinha, A.K.; Kahn, N.N. Non-conventional hemostatic risk factors for coronary heart disease in individuals with spinal cord injury. Spinal Cord 2011, 49, 858–866. [Google Scholar] [CrossRef][Green Version]
- Lavela, S.L.; Weaver, F.M.; Goldstein, B.; Chen, K.; Miskevics, S.; Rajan, S.; Gater, D.R., Jr. Diabetes mellitus in individuals with spinal cord injury or disorder. J. Spinal Cord Med. 2006, 29, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.A.; Druschel, C.; Meisel, C.; Liebscher, T.; Prilipp, E.; Watzlawick, R.; Cinelli, P.; Niedeggen, A.; Schaser, K.D.; Wanner, G.A.; et al. The SCIentinel study–prospective multicenter study to define the spinal cord injury-induced immune depression syndrome (SCI-IDS)–study protocol and interim feasibility data. BMC Neurol. 2013, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Walsh, J.T.; Lukens, J.R.; Kipnis, J. Dealing with danger in the CNS: The response of the immune system to injury. Neuron 2015, 87, 47–62. [Google Scholar] [CrossRef]
- Chaudhry, R.; Madden-Fuentes, R.J.; Ortiz, T.K.; Balsara, Z.; Tang, Y.; Nseyo, U.; Wiener, J.S.; Ross, S.S.; Seed, P.C. Inflammatory response to Escherichia coli urinary tract infection in the neurogenic bladder of the spinal cord injured host. J. Urol. 2014, 191, 1454–1461. [Google Scholar] [CrossRef]
- Schedlowski, M.; Engler, H.; Grigoleit, J.S. Endotoxin-induced experimental systemic inflammation in humans: A model to disentangle immune-to-brain communication. Brain Behav. Immun. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Kwon, B.K.; Stammers, A.M.T.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M.; et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef]
- Hausmann, O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003, 41, 369–378. [Google Scholar] [CrossRef]
- Iversen, P.O.; Hjeltnes, N.; Holm, B.; Flatebø, T.; Strøm-Gundersen, I.; Rønning, W.; Stanghelle, J.; Benestad, H.B. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood 2000, 96, 2081–2083. [Google Scholar] [CrossRef]
- Stein, A.; Panjwani, A.; Sison, C.; Rosen, L.; Chugh, R.; Metz, C.; Bank, M.; Bloom, O. Pilot study: Elevated circulating levels of the proinflammatory cytokine macrophage migration inhibitory factor in patients with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, 1498–1507. [Google Scholar] [CrossRef]
- Monahan, R.; Stein, A.; Gibbs, K.; Bank, M.; Bloom, O. Circulating T cell subsets are altered in individuals with chronic spinal cord injury. Immunol. Res. 2015, 63, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Murray, P.J. Immune regulation by monocytes. Semin. Immunol. 2018, 35, 12–18. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Mostacada, K.; Popovich, P.G. Gut microbiota are disease-modifying factors after traumatic spinal cord injury. Neurotherapeutics 2018, 15, 60–67. [Google Scholar] [CrossRef]
- Schumann, R.R.; Latz, E. Lipopolysaccharide-binding protein. Chem. Immunol. 2000, 74, 42–60. [Google Scholar]
- Albillos, A.; de-la-Hera, A.; Alvarez-Mon, M. Serum lipopolysaccharide-binding protein prediction of severe bacterial infection in cirrhotic patients with ascites. Lancet 2004, 363, 1608–1610. [Google Scholar] [CrossRef]
- Tudesq, J.J.; Dunyach-Remy, C.; Combescure, C.; Doncesco, R.; Laureillard, D.; Lavigne, J.P.; Sotto, A. Microbial translocation is correlated with HIV evolution in HIV-HCV co-infected patients. PLoS ONE 2017, 12, e0183372. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, D.I.; Bartlett, J.A.; Keller, S.E. Influence of neurological level on immune function following spinal cord injury: A review. J. Spinal Cord Med. 2000, 23, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, D.I.; Dixon, D.; Schwartz, J.; Bartlett, J.A.; Keller, S.E. Altered innate immunity following spinal cord injury. Spinal Cord 2008, 46, 477–481. [Google Scholar] [CrossRef]
- Campagnolo, D.I.; Keller, S.E.; DeLisa, J.A.; Glick, T.J.; Sipski, M.L.; Schleifer, S.J. Alteration of immune system function in tetraplegics. Am. J. Phys. Med. Rehabil. 1994, 73, 387–393. [Google Scholar] [CrossRef]
- Gucluler, G.; Adiguzel, E.; Gungor, B.; Kahraman, T.; Gursel, M.; Yilmaz, B.; Gursel, I. Impaired toll like receptor-7 and 9 induced immune activation in chronic spinal cord injured patients contributes to immune dysfunction. PLoS ONE 2017, 12, e0171003. [Google Scholar] [CrossRef]
- Riegger, T.; Conrad, S.; Liu, K.; Schluesener, H.J.; Adibzahdeh, M.; Schwab, J.M. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur. J. Neurosci. 2007, 25, 1743–1747. [Google Scholar] [CrossRef]
- Gurses, K.M.; Kocyigit, D.; Yalcin, M.U.; Canpinar, H.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Oto, M.A.; Ozer, N.; Guc, D.; et al. Monocyte toll-like receptor expression in patients with atrial fibrillation. Am. J. Cardiol. 2016, 117, 1463–1467. [Google Scholar] [CrossRef]
- Salomão, R.; Martins, P.S.; Brunialti, M.K.C.; da Luz Fernandes, M.; Martos, L.S.W.; Mendes, M.E.; Gomes, N.E.; Rigato, O. TLR signaling pathway in patients with sepsis. Shock 2008, 30, 73–77. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; van’t Veer, C.; van den Pangaart, P.S.; Dondorp, A.M.; Day, N.P.; Peacock, S.J.; van der Poll, T. Immunosuppression associated with interleukin-1R-associated-kinase-M upregulation predicts mortality in Gram-negative sepsis (melioidosis). Crit. Care Med. 2009, 37, 569–576. [Google Scholar] [CrossRef]
- Eaton-Bassiri, A.; Dillon, S.B.; Cunningham, M.; Rycyzyn, M.A.; Mills, J.; Sarisky, R.T.; Mbow, M.L. Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect. Immun. 2004, 72, 7202–7211. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Ewald, S.E.; Lee, B.L.; Lau, L.; Wickliffe, K.E.; Shi, G.P.; Chapman, H.A.; Barton, G.M. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 2008, 456, 658–662. [Google Scholar] [CrossRef]
- Baiyee, E.E.; Flohe, S.; Lendemans, S.; Bauer, S.; Mueller, N.; Kreuzfelder, E.; Grosse-Wilde, H. Expression and function of Toll-like receptor 9 in severely injured patients prone to sepsis. Clin. Exp. Immunol. 2006, 145, 456–462. [Google Scholar] [CrossRef]
- Xu, N.; Yao, H.P.; Sun, Z.; Chen, Z. Toll-like receptor 7 and 9 expression in peripheral blood mononuclear cells from patients with chronic hepatitis B and related hepatocellular carcinoma. Acta Pharmacol. Sin. 2008, 29, 239–244. [Google Scholar] [CrossRef]
- Vieira, É.L.M.; Keesen, T.S.L.; Machado, P.R.; Guimarães, L.H.; Carvalho, E.M.; Dutra, W.O.; Gollob, K.J. Immunoregulatory profile of monocytes from cutaneous leishmaniasis patients and association with lesion size. Parasite Immunol. 2013, 35, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Wong, P.T.Y.; Tam, L.S.; Li, E.K.; Chen, D.P.; Lam, C.W.K. Activation profile of Toll-like receptors of peripheral blood lymphocytes in patients with systemic lupus erythematosus. Clin. Exp. Immunol. 2010, 159, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Thiesen, S.; Janciauskiene, S.; Uronen-Hansson, H.; Agace, W.; Högerkorp, C.M.; Spee, P.; Håkansson, K.; Grip, O. CD14hi HLA-DRdim macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn’s disease. J. Leukoc. Biol. 2014, 95, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Seiler, S.; Zawada, A.M.; Reichart, B.; Herath, E.; Roth, D.; Ulrich, C.; Fliser, D.; Heine, G.H. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur. Heart J. 2010, 32, 84–92. [Google Scholar] [CrossRef]
- Rossol, M.; Kraus, S.; Pierer, M.; Baerwald, C.; Wagner, U. The CD14brightCD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012, 64, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Carmo, A.M.; Brunialti, M.K.; Machado, F.R.; Azevedo, L.C.; Assunção, M.; Trevelin, S.C.; Cunha, F.Q.; Salomao, R. Modulation of monocytes in septic patients: Preserved phagocytic activity, increased ROS and NO generation, and decreased production of inflammatory cytokines. Intensive Care Med. Exp. 2016, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liu, Y.; Chen, T. Correlations of chemokine CXCL16 and TNF-α with coronary atherosclerotic heart disease. Exp. Ther. Med. 2018, 15, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Hayes, K.C.; Dekaban, G.A. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, 1384–1393. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Albillos, A.; de la Hera, A.; González, M.; Moya, J.L.; Calleja, J.L.; Monserrat, J.; Ruiz-del-Arbol, L.; Alvarez-Mon, M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 2003, 37, 208–217. [Google Scholar] [CrossRef]
- Albillos, A.n.; de la Hera, A.; Reyes, E.; Monserrat, J.; Muñoz, L.; Nieto, M.; Prieto, A.; Sanz, E.; Alvarez-Mon, M. Tumour necrosis factor-alpha expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: Amelioration with norfloxacin. J. Hepatol. 2004, 40, 624–631. [Google Scholar] [CrossRef]
- Blairon, L.; Wittebole, X.; Laterre, P.F. Lipopolysaccharide-binding protein serum levels in patients with severe sepsis due to gram-positive and fungal infections. J. Infect. Dis. 2003, 187, 287–291. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Hall, J.C.E.; Wang, L.; Mo, X.; Yu, Z.; Popovich, P.G. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 2016, 213, 2603–2620. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernández-Real, J.M. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 2012, 7, e37160. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Sugai, E.; Niveloni, S.; Vázquez, H.; Pedreira, S.; Mazure, R.; Moreno, M.L.; Label, M.; Mauriño, E.; Fasano, A.; et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin. Gastroenterol. Hepatol. 2005, 3, 335–341. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Oropallo, M.A.; Held, K.S.; Goenka, R.; Ahmad, S.A.; O’Neill, P.J.; Steward, O.; Lane, T.E.; Cancro, M.P. Chronic spinal cord injury impairs primary antibody responses but spares existing humoral immunity in mice. J. Immunol. 2012, 188, 5257–5266. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, D.D.; Hoffman, J.M.; Kirshblum, S.; McKinley, W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: A multicenter analysis11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabil. 2004, 85, 1757–1763. [Google Scholar] [CrossRef]
- De Vivo, M.J.; Stuart Krause, J.; Lammertse, D.P. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch. Phys. Med. Rehabil. 1999, 80, 1411–1419. [Google Scholar] [CrossRef]
- Anand, R.J.; Kohler, J.W.; Cavallo, J.A.; Li, J.; Dubowski, T.; Hackam, D.J. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J. Pediatr. Surg. 2007, 42, 927–933. [Google Scholar] [CrossRef]
- Lam, T.D.; Lammers, S.; Munoz, C.; Tamayo, A.; Spence, J.D. Diabetes, intracranial stenosis and microemboli in asymptomatic carotid stenosis. Can. J. Neurol. Sci. 2013, 40, 177–181. [Google Scholar] [CrossRef][Green Version]
- Ghattas, A.; Griffiths, H.R.; Devitt, A.; Lip, G.Y.H.; Shantsila, E. Monocytes in coronary artery disease and atherosclerosis. J. Am. Coll. Cardiol. 2013, 62, 1541–1551. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta 2014, 1842, 446–462. [Google Scholar] [CrossRef]
- Biering-Sørensen, F.; Charlifue, S.; DeVivo, M.; Noonan, V.; Post, M.; Stripling, T.; Wing, P. International spinal cord injury data sets. Spinal Cord 2006, 44, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
| Variables | Healthy Controls | Chronic SCI | AIS Group 1 | AIS Group 2 | Injury Level Group 1 | Injury Level Group 2 |
|---|---|---|---|---|---|---|
| (n = 28) | Patients (n = 55) | (A–B) (n = 30) | (C–D) (n = 25) | (C1–T6) (n = 35) | (T7–L6) (n = 20) | |
| Age (years) | 25.03 ± 2.86 | 26.92 ± 12.87 | 24.96 ± 12.90 | 30.65 ± 13.30 | 23.28 ± 13.64 | 27.21 ± 12.69 |
| Sex (men%/women%) | 43.24/56.76 | 68.00/32.00 | 76.67/23.33 | 60.86/39.14 | 74.28/25.71 | 57.89/42.10 |
| Time of injury (years) | 12.00 ± 9.22 | 10.7 ± 9.15 | 12.91 ± 9.61 | 10.74 ± 9.69 | 14.23 ± 8.81 | |
| AIS (%) | ||||||
| A | 34.00 | 63.33 | 48.57 | 10.52 | ||
| B | 21.43 | 36.67 | 14.28 | 26.31 | ||
| C | 19.64 | 45.83 | 8.57 | 36.84 | ||
| D | 25.00 | 54.17 | 28.57 | 21.05 | ||
| Injury level (%) | ||||||
| C1–C4 | 23.21 | 20.00 | 25.00 | 34.28 | ||
| C5–C8 | 19.64 | 20.00 | 16.67 | 28.57 | ||
| T1–T6 | 23.22 | 33.33 | 12.50 | 37.14 | ||
| T7–T12 | 19.64 | 13.33 | 29.17 | 57.89 | ||
| L1–L6 | 14.29 | 13.33 | 16.67 | 42.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz, D.; Lopez-Dolado, E.; Haro, S.; Monserrat, J.; Martinez-Alonso, C.; Balomeros, D.; Albillos, A.; Alvarez-Mon, M. Systemic Inflammation and the Breakdown of Intestinal Homeostasis Are Key Events in Chronic Spinal Cord Injury Patients. Int. J. Mol. Sci. 2021, 22, 744. https://doi.org/10.3390/ijms22020744
Diaz D, Lopez-Dolado E, Haro S, Monserrat J, Martinez-Alonso C, Balomeros D, Albillos A, Alvarez-Mon M. Systemic Inflammation and the Breakdown of Intestinal Homeostasis Are Key Events in Chronic Spinal Cord Injury Patients. International Journal of Molecular Sciences. 2021; 22(2):744. https://doi.org/10.3390/ijms22020744
Chicago/Turabian StyleDiaz, David, Elisa Lopez-Dolado, Sergio Haro, Jorge Monserrat, Carlos Martinez-Alonso, Dimitrios Balomeros, Agustín Albillos, and Melchor Alvarez-Mon. 2021. "Systemic Inflammation and the Breakdown of Intestinal Homeostasis Are Key Events in Chronic Spinal Cord Injury Patients" International Journal of Molecular Sciences 22, no. 2: 744. https://doi.org/10.3390/ijms22020744
APA StyleDiaz, D., Lopez-Dolado, E., Haro, S., Monserrat, J., Martinez-Alonso, C., Balomeros, D., Albillos, A., & Alvarez-Mon, M. (2021). Systemic Inflammation and the Breakdown of Intestinal Homeostasis Are Key Events in Chronic Spinal Cord Injury Patients. International Journal of Molecular Sciences, 22(2), 744. https://doi.org/10.3390/ijms22020744







