Single Administration of the T-Type Calcium Channel Enhancer SAK3 Reduces Oxidative Stress and Improves Cognition in Olfactory Bulbectomized Mice
Abstract
1. Introduction
2. Results
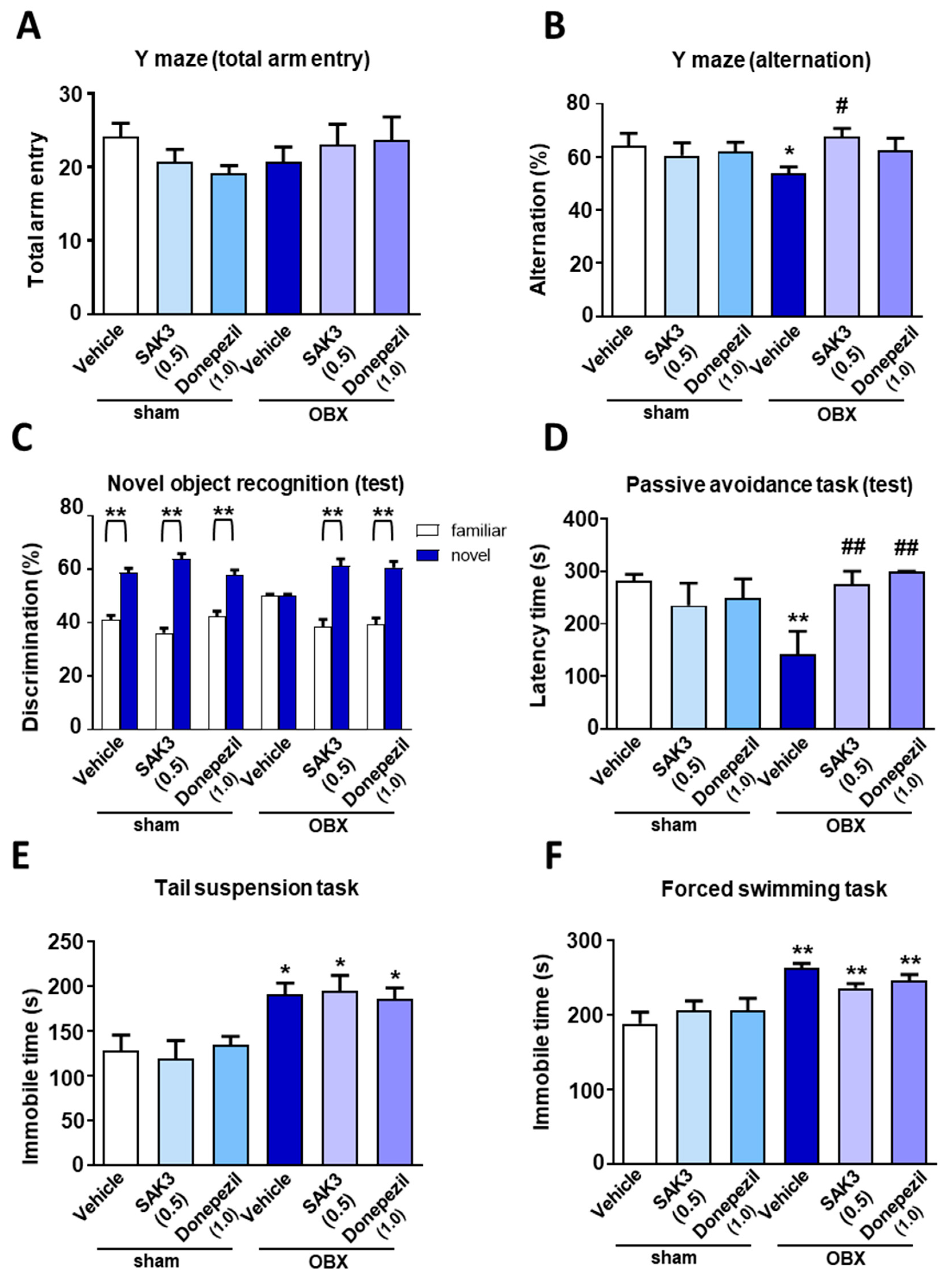
2.1. Effects of Acute SAK3 Administration on Spatial Memory, Cognitive Functions and Depressive-Like Behaviors in OBX Mice
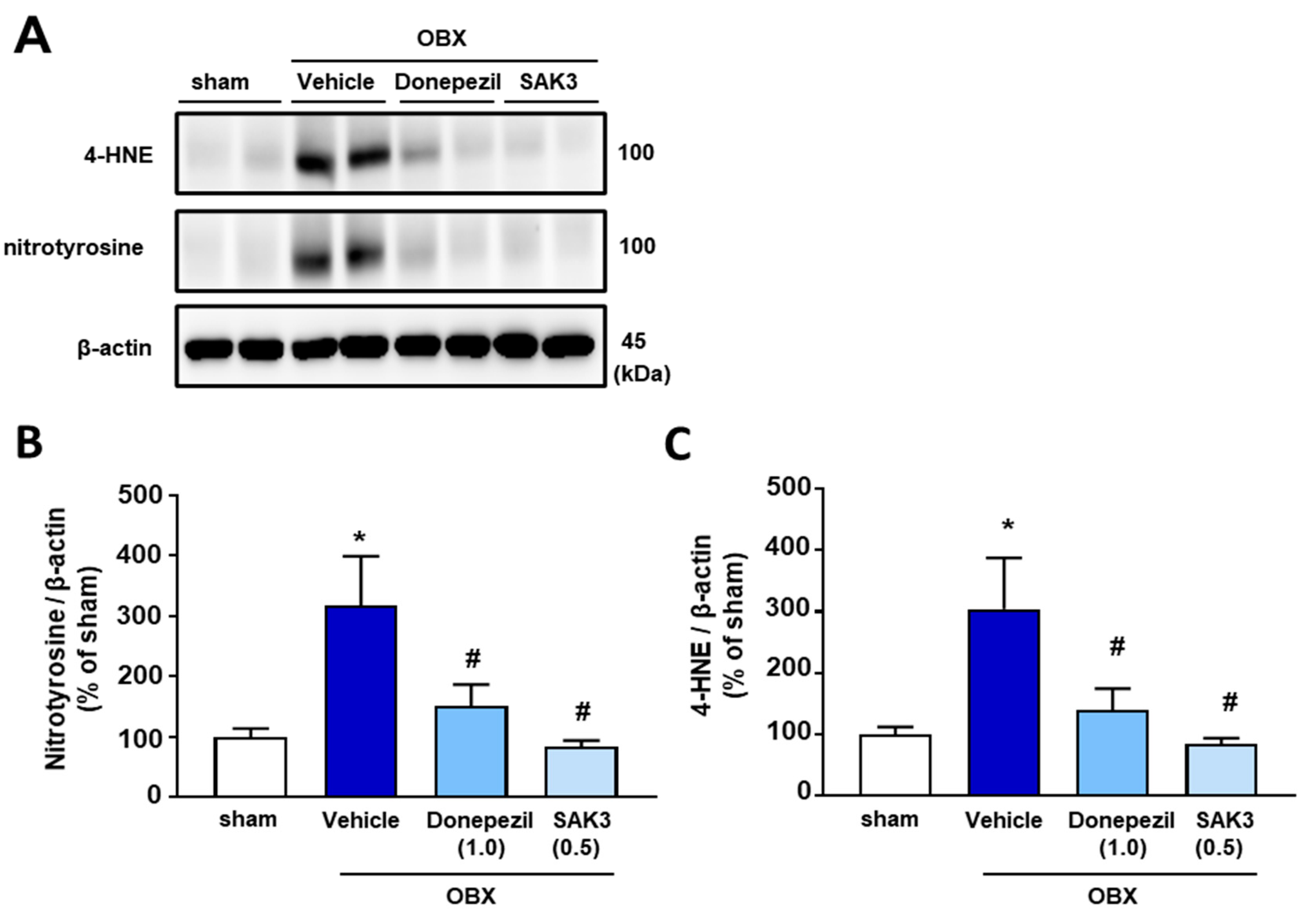
2.2. Elevated 4-HNE and Nitrotyrosine Protein Levels Were Suppressed by SAK3 Treatment in OBX Mice
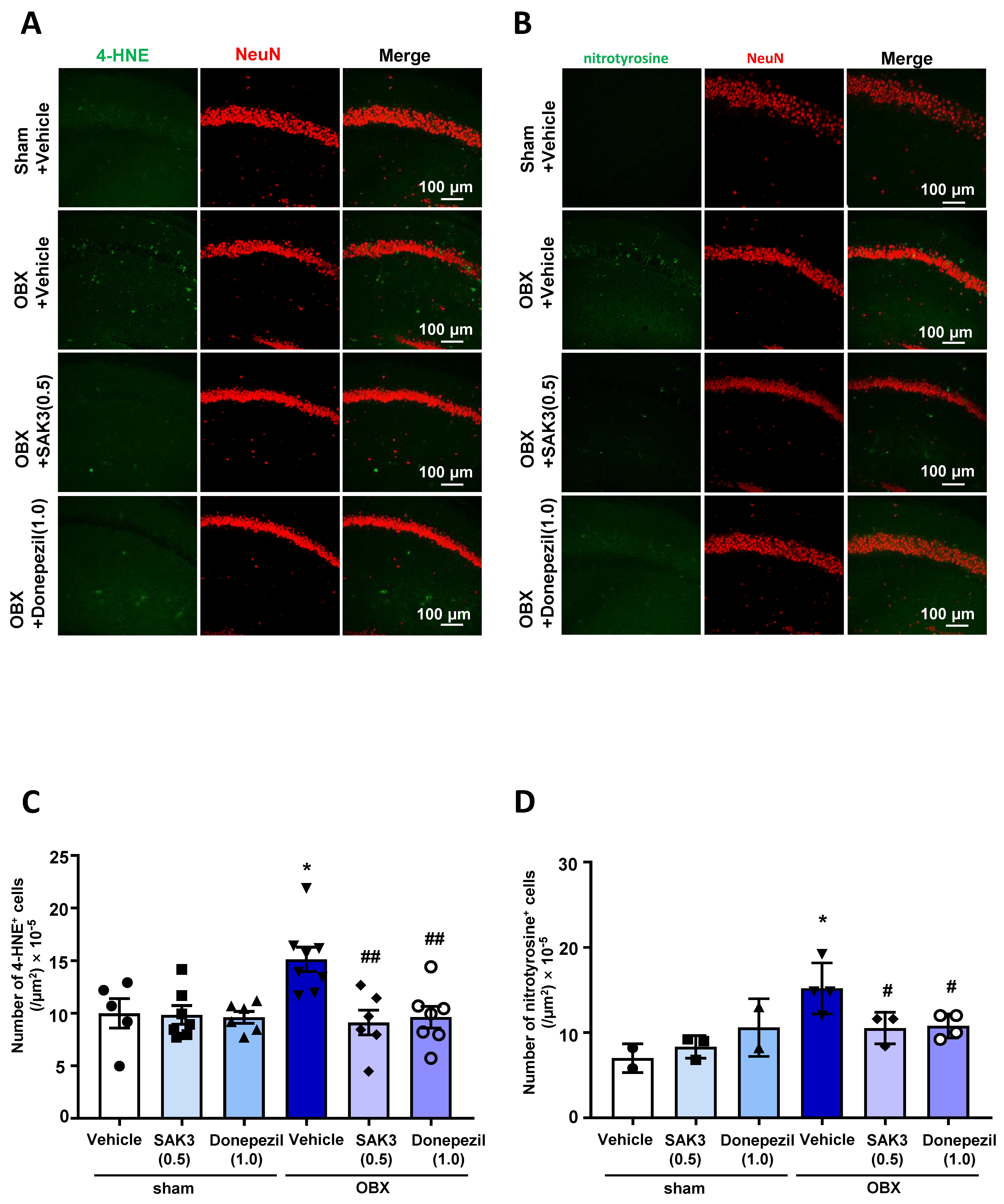
2.3. The Number of 4-HNE-and Nitrotyrosine-Positive Cells Decreased after SAK3 Treatment in OBX Mice
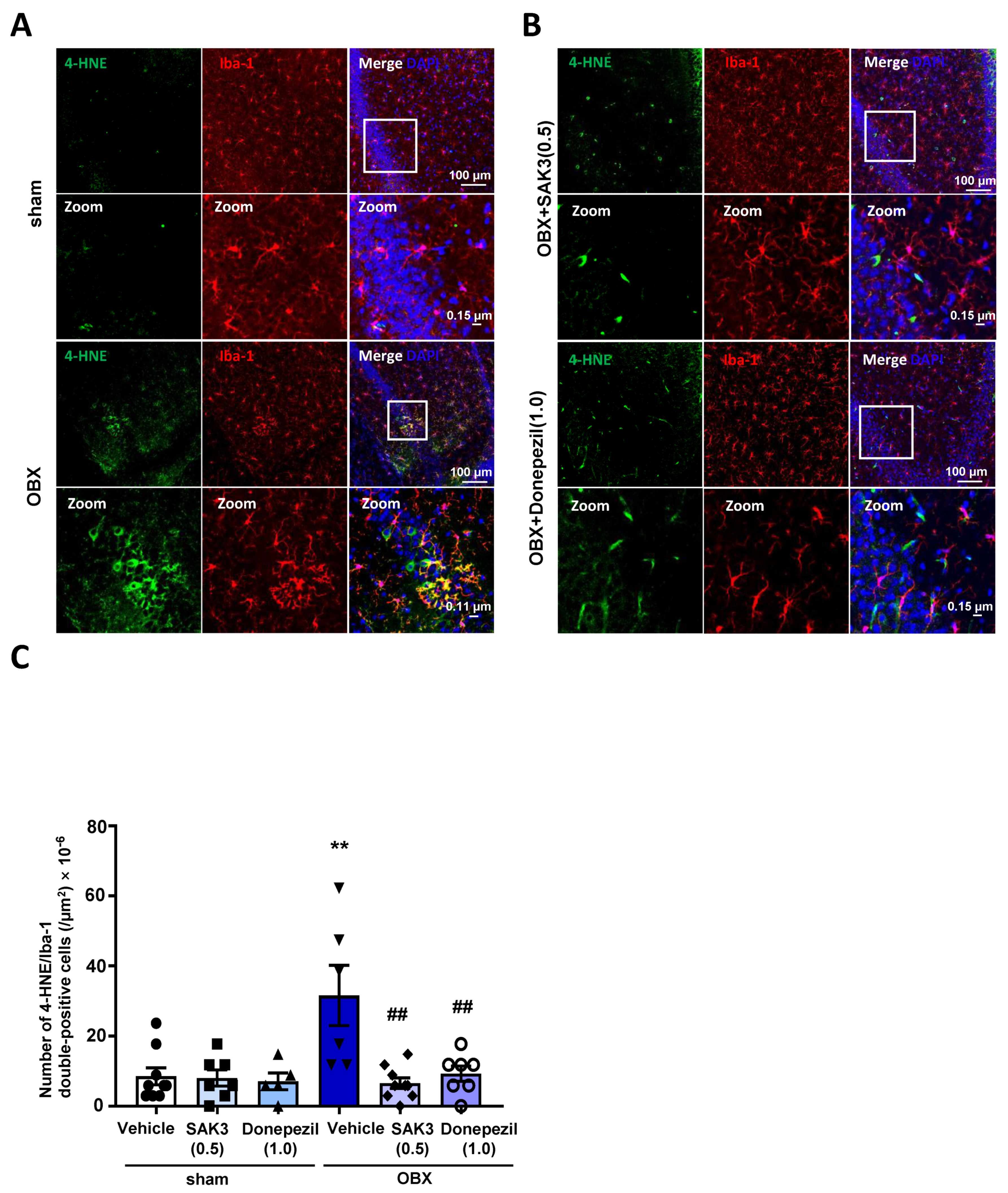
2.4. Acute SAK3 Oral Administration Reduced the Numbers of 4-HNE and Ionized Calcium Binding Adaptor Molecule 1 (IBA-1) Double-Positive Cells in the Hippocampus of OBX Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Surgery
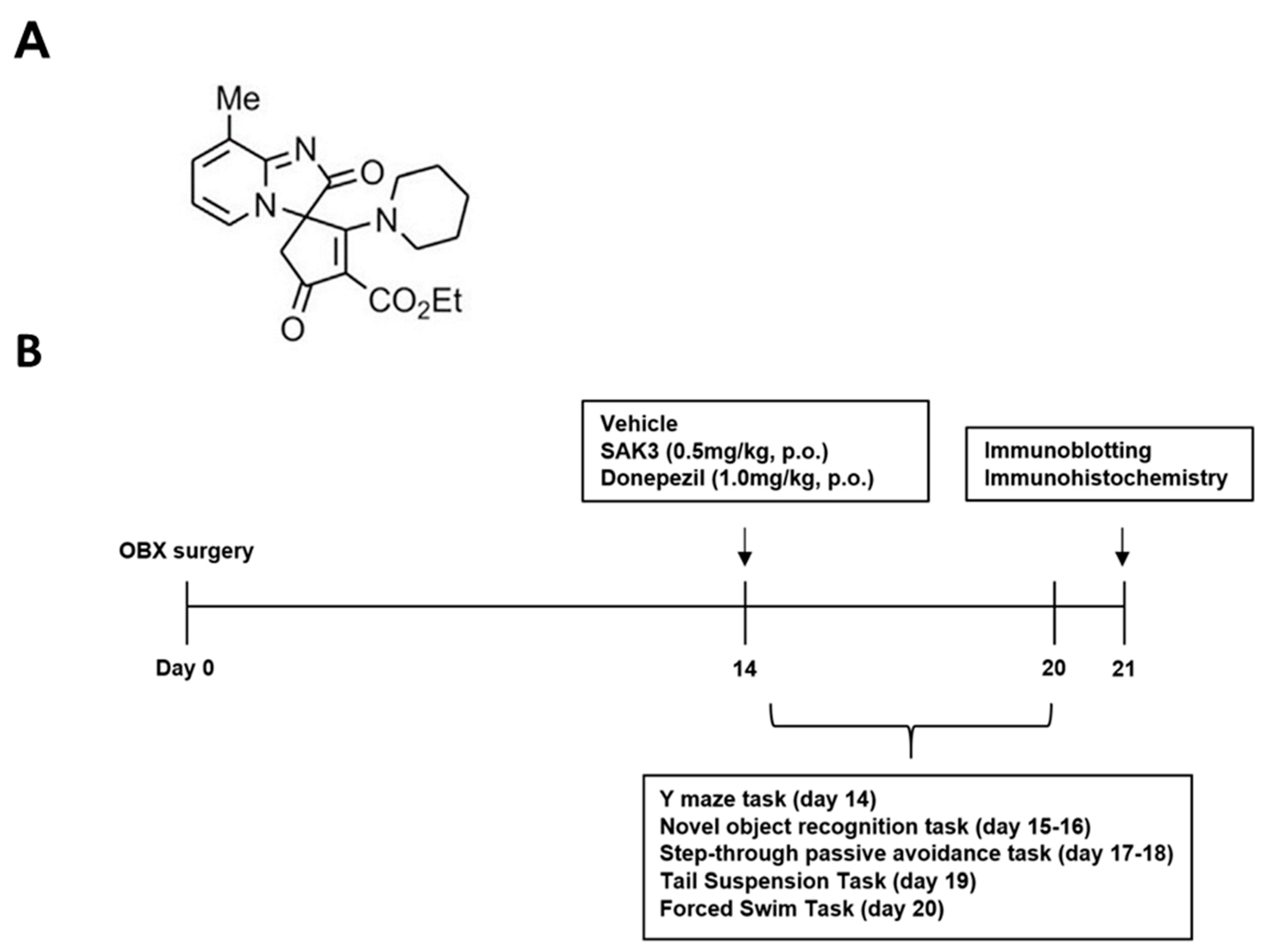
4.2. Drug Administration and Experimental Design
4.3. Behavioral Tasks
4.3.1. Y-Maze Task
4.3.2. Novel Object Recognition Task
4.3.3. Step-Through Passive Avoidance Task
4.3.4. TST
4.3.5. FST
4.4. Western Blot Analysis
4.5. Immunofluorescence Staining
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| SAK3 ethyl-8-methyl-2,4-dioxo-2-(piperidin-1-yl)-2H-spiro[cyclopentane-1,3-imidazo [1,2-a] pyridin]-2-ene-3- | carboxylate |
| OBX | olfactory bulbectomized |
| Aβ | β-amyloid |
| 4-HNE | 4-hydroxynonenal |
| ACh | acetylcholine |
| TST | tail suspension task |
| IBA-1 | ionized calcium binding adaptor molecule 1 |
| FST | forced swimming task |
| IL | interleukin |
| LPS | lipopolysaccharides |
| DAPI | 4,6-diamidino-2-phenylindole |
| SEM | standard error of mean |
| PBS | phosphate-buffered saline |
References
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V.; Bobkova, N.V.; Kolosova, N.G.; Samokhin, A.N.; Stepanichev, M.Y.; Stefanova, N.A. Molecular and cellular mechanisms of sporadic Alzheimer’s disease: Studies on rodent models in vivo. Biochem. Mosc. 2017, 10, 1088–1102. [Google Scholar] [CrossRef] [PubMed]
- Minati, L.; Edginton, T.; Bruzzone, M.G.; Giaccone, G. Reviews: Current Concepts in Alzheimer’s Disease: A Multidisciplinary Review. Am. J. Alzheimers Dis. Other Dement. 2009, 24, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Sultana, R. Redox proteomics identification of oxidatively modified brain proteins in Alzheimer’s disease and mild cognitive impairment: Insights into the progression of this dementing disorder. J. Alzheimers Dis. 2007, 12, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Atukeren, P.; Cengiz, M.; Yavuzer, H.; Gelisgen, R.; Altunoglu, E.; Oner, S.; Erdenen, F.; Yuceakın, D.; Derici, H.; Cakatay, U.; et al. The efficacy of donepezil administration on acetylcholinesterase activity and altered redox homeostasis in Alzheimer’s disease. Biomed. Pharm. 2017, 90, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Sato, K.; Kojima, K.; Saito, T.; Saido, T.C.; Fukunaga, K. Oral glutathione administration inhibits the oxidative stress and the inflammatory responses in App knock-in mice. Neuropharmacology 2020, 168, 108026. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, I.Y.; Kuvichkin, V.V.; Kashparov, I.A.; Medvinskaya, N.I.; Nesterova, I.V.; Lunin, S.M.; Samokhin, A.N.; Bobkova, N.V. Increased level of beta-amyloid in the brain of bulbectomized mice. Biochemistry 2004, 69, 176–180. [Google Scholar] [PubMed]
- Bobkova, N.; Vorobyov, V.; Medvinskaya, N.; Nesterova, I.; Tatarnikova, O.; Nekrasov, P.; Samokhin, A.; Deev, A.; Sengpiel, F.; Koroev, D.; et al. Immunization against Specific Fragments of Neurotrophin p75 Receptor Protects Forebrain Cholinergic Neurons in the Olfactory Bulbectomized Mice. J. Alzheimers Dis. 2016, 53, 289–301. [Google Scholar] [CrossRef]
- Morales-Medina, J.C.; Iannitti, T.; Freeman, A.; Caldwell, H.K. The olfactory bulbectomized rat as a model of depression: The hippocampal pathway. Behav. Brain Res. 2017, 317, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Túnez, I.; Drucker-Colín, R.; Montilla, P.; Peña, J.; Jimena, I.; Medina, F.J.; Tasset, I. Protective effect of nicotine on oxidative and cell damage in rats with depression induced by olfactory bulbectomy. Eur. J. Pharmacol. 2010, 627, 115–118. [Google Scholar] [CrossRef]
- Tunez, I. Effect of 17β-estradiol on olfactory bulbectomy-induced oxidative stress and behavioral changes in rats. Neuropsychiatr. Dis. Treat. 2008, 4, 441. [Google Scholar] [CrossRef][Green Version]
- Martinez, B.; Peplow, P.V. Amelioration of Alzheimer’s disease pathology and cognitive deficits by immunomodulatory agents in animal models of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1158–1176. [Google Scholar] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Uchiyama, K.; Naito, Y. Oxidative Stress Involvement in Diabetic Nephropathy and Its Prevention by Astaxanthin. Oxidative Stress Dis. 2005, 21, 235–242. [Google Scholar]
- Lovell, M.A.; Ehmann, W.D.; Butler, S.M.; Markesbery, W.R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 1995, 45, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Su, J.H.; Cotman, C.W. DNA damage and apoptosis in Alzheimer’s disease: Colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J. Neurosci. 1996, 16, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Cervellati, C.; Cortelazzo, A.; Cervellati, F.; Sticozzi, C.; Mirasole, C.; Guerranti, R.; Trentini, A.; Zolla, L.; Savelli, V.; et al. Proteomic analysis of 4-hydroxynonenal and nitrotyrosine modified proteins in RTT fibroblasts. Int. J. Biochem. Cell Biol. 2016, 81, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Arai, T.; Kondo, H.; Tanno, E.; Haga, C.; Ikeda, K. Cell Mediators of Inflammation in the Alzheimer Disease Brain. Alzheimer Dis. Assoc. Disord. 2000, 14, S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.-F.; Brachova, L.; Civin, H.W.; Rogers, J. Inflammation, Aβ Deposition, and Neurofibrillary Tangle Formation as Correlates of Alzheimer’s Disease Neurodegeneration. J. Neuropathol. Exp. Neurol. 1996, 55, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Millington, C.; Sonego, S.; Karunaweera, N.; Rangel, A.; Aldrich-Wright, J.R.; Campbell, I.L.; Gyengesi, E.; Münch, G. Chronic Neuroinflammation in Alzheimer’s Disease: New Perspectives on Animal Models and Promising Candidate Drugs. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.L.; Müller, N. Neuroinflammation, Microglia and Implications for Anti-Inflammatory Treatment in Alzheimer’s Disease. Int. J. Alzheimers Dis. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.-E.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The Role of Microglia in the Healthy Brain. J. Neurosci. 2011, 31, 16064–16069. [Google Scholar] [CrossRef]
- Arends, Y.M.; Duyckaerts, C.; Rozemuller, J.M.; Eikelenboom, P.; Hauw, J.-J. Microglia, amyloid and dementia in Alzheimer disease. Neurobiol. Aging 2000, 21, 39–47. [Google Scholar] [CrossRef]
- Koenigsknecht-Talboo, J. Microglial Phagocytosis Induced by Fibrillar-Amyloid and IgGs Are Differentially Regulated by Proinflammatory Cytokines. J. Neurosci. 2005, 25, 8240–8249. [Google Scholar] [CrossRef] [PubMed]
- Seabrook, T.J.; Jiang, L.; Maier, M.; Lemere, C.A. Minocycline affects microglia activation, Aβ deposition, and behavior in APP-tg mice. Glia 2006, 53, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Brantefjord, M.; Hansson, E.; Rönnbäck, L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-α. Glia 2005, 51, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Dziegielewska, B.; Gray, L.S.; Dziegielewski, J. T-type calcium channels blockers as new tools in cancer therapies. Pflug. Arch. 2014, 466, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, S.M.; Jevtovic-Todorovic, V. Targeting of CaV3.2 T-type calcium channels in peripheral sensory neurons for the treatment of painful diabetic neuropathy. Pflug. Arch. 2014, 466, 701–706. [Google Scholar] [CrossRef]
- Bermejo, P.E.; Anciones, B. Review: A review of the use of zonisamide in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2009, 2, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Bayazitov, I.T.; Westmoreland, J.J.; Zakharenko, S.S. Forward Suppression in the Auditory Cortex Is Caused by the Cav3.1 Calcium Channel-Mediated Switch from Bursting to Tonic Firing at Thalamocortical Projections. J. Neurosci. 2013, 33, 18940–18950. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-J.; Li, Z.-J.; Chen, Z.-X.; Fang, Z.-Y.; Yang, C.-X.; Li, H.; Zeng, Y.-M. Intrathecal administration of Cav3.2 and Cav3.3 antisense oligonucleotide reverses tactile allodynia and thermal hyperalgesia in rats following chronic compression of dorsal root of ganglion. Acta Pharmacol. Sin. 2006, 27, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Matsuo, K.; Izumi, H.; Haga, H.; Yoshida, T.; Wakamori, M.; Kakei, A.; Sakimura, K.; Fukuda, T.; Fukunaga, K. Pharmacological properties of SAK3, a novel T-type voltage-gated Ca channel enhancer. Neuropharmacology 2017, 117, 1–13. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shioda, N.; Feng, H.A.N.; Moriguchi, S.; Fukunaga, K. Donepezil-induced Neuroprotection of Acetylcholinergic Neurons in Olfactory Bulbectomized Mice. Yakugaku Zasshi 2010, 130, 717–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, C.; Leonard, B.E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 2005, 29, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Thakare, V.N.; Aswar, M.K.; Kulkarni, Y.P.; Patil, R.R.; Patel, B.M. Silymarin ameliorates experimentally induced depressive like behavior in rats: Involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response. Physiol. Behav. 2017, 179, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Gonsette, R.E. Neurodegeneration in multiple sclerosis: The role of oxidative stress and excitotoxicity. J. Neurol. Sci. 2008, 274, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, S.M.; Lashuel, H.A. Amyloidogenic protein-membrane interactions: Mechanistic insight from model systems. Angew. Chem. Int. Ed. Engl. 2010, 49, 5628–5654. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Shinoda, Y.; Saito, T.; Saido, T.C.; Sato, K.; Yabuki, Y.; Matsumoto, Y.; Kanemitsu, Y.; Tomioka, Y.; Abolhassani, N.; et al. The Disease-modifying Drug Candidate, SAK3 Improves Cognitive Impairment and Inhibits Amyloid beta Deposition in App Knock-in Mice. Neuroscience 2018, 377, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Roediger, F.; Jordan, B.; Mattson, M.P.; Keller, J.N.; Waeg, G.; Butterfield, D.A. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J. Neurochem. 1997, 69, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Terni, B.; Boada, J.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer’s disease pathology. Brain Pathol. 2010, 20, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Bentlage, H.; de Coo, R.; Laak, H.; Sengers, R.; Trijbels, F.; Ruitenbeek, W.; Schlote, W.; Pfeiffer, K.; Gencic, S.; Jagow, G.; et al. Human Diseases with Defects in Oxidative Phosphorylation. 1. Decreased Amounts of Assembled Oxidative Phosphorylation Complexes in Mitochondrial Encephalomyopathies. Eur. J. Biochem. 1995, 227, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-Z.; Yin, J.-B.; Yang, J.-J.; Cao, L. Regulatory factor X1 depresses ApoE-dependent Aβ uptake by miRNA-124 in microglial response to oxidative stress. Neuroscience 2017, 344, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Smith, M.A.; Avilá, J.; DeBernardis, J.; Kansal, M.; Takeda, A.; Zhu, X.; Nunomura, A.; Honda, K.; Moreira, P.I.; et al. Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Radic. Biol. Med. 2005, 38, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.K.; Tsirka, S.E. The Diverse Roles of Microglia in the Neurodegenerative Aspects of Central Nervous System (CNS) Autoimmunity. Int. J. Mol. Sci. 2017, 18, 504. [Google Scholar] [CrossRef] [PubMed]
- Watters, J.J.; Pocock, J.M. Microglial Physiology. In Microglia Health Disease; Springer: New York, NY, USA, 2014; pp. 47–79. [Google Scholar]
- Colton, C.A.; Gilbert, D.L. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987, 223, 284–288. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Bian, H.; Guo, L.; Zhu, H. Activation of the α7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am. J. Transl. Res. 2017, 9, 971–985. [Google Scholar]
- Rossi, B.; Constantin, G. Live Imaging of Immune Responses in Experimental Models of Multiple Sclerosis. Front. Immunol. 2016, 7, 506. [Google Scholar] [CrossRef]
- Levin, E.D. Nicotinic systems and cognitive function. Psychopharmacology 1992, 108, 417–431. [Google Scholar] [CrossRef]
- Suzuki, T.; Hide, I.; Matsubara, A.; Hama, C.; Harada, K.; Miyano, K.; Andrä, M.; Matsubayashi, H.; Sakai, N.; Kohsaka, S.; et al. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 2006, 83, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Erausquin, M.; Marubio, L.M.; Klink, R.; Changeux, J.P. Nicotinic receptor function: New perspectives from knockout mice. Trends Pharmacol. Sci. 2000, 21, 211–217. [Google Scholar] [CrossRef]
- Yabuki, Y.; Jing, X.; Fukunaga, K. The T-type calcium channel enhancer SAK3 inhibits neuronal death following transient brain ischemia via nicotinic acetylcholine receptor stimulation. Neurochem. Int. 2017, 108, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kihara, T.; Shimohama, S.; Sawada, H.; Honda, K.; Nakamizo, T.; Shibasaki, H.; Kume, T.; Akaike, A. α7 Nicotinic Receptor Transduces Signals to Phosphatidylinositol 3-Kinase to Block A β-Amyloid-induced Neurotoxicity. J. Biol. Chem. 2001, 276, 13541–13546. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, S.Y.; Lee, H.G.; Kim, S.U.; Lee, Y.B. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar β amyloid peptide (1-42)-stimulated microglia. EMM 2008, 40, 11. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Shioda, N.; Han, F.; Moriguchi, S.; Fukunaga, K. The Novel Cognitive Enhancer ST101 Enhances Acetylcholine Release in Mouse Dorsal Hippocampus Through T-type Voltage-Gated Calcium Channel Stimulation. J. Pharmacol. Sci. 2013, 121, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yabuki, Y.; Yu, M.; Fukunaga, K. T-type calcium channel enhancer SAK3 produces anti-depressant-like effects by promoting adult hippocampal neurogenesis in olfactory bulbectomized mice. J. Pharmacol. Sci. 2018, 137, 333–341. [Google Scholar] [CrossRef]
- Yabuki, Y.; Fukunaga, K. Oral administration of glutathione improves memory deficits following transient brain ischemia by reducing brain oxidative stress. Neuroscience 2013, 250, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Izumi, H.; Shinoda, Y.; Fukunaga, K. Neuroprotective effects of protein tyrosine phosphatase 1B inhibitor on cerebral ischemia/reperfusion in mice. Brain Res. 2018, 1694, 1–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, D.; Cheng, A.; Kawahata, I.; Izumi, H.; Xu, J.; Fukunaga, K. Single Administration of the T-Type Calcium Channel Enhancer SAK3 Reduces Oxidative Stress and Improves Cognition in Olfactory Bulbectomized Mice. Int. J. Mol. Sci. 2021, 22, 741. https://doi.org/10.3390/ijms22020741
Yuan D, Cheng A, Kawahata I, Izumi H, Xu J, Fukunaga K. Single Administration of the T-Type Calcium Channel Enhancer SAK3 Reduces Oxidative Stress and Improves Cognition in Olfactory Bulbectomized Mice. International Journal of Molecular Sciences. 2021; 22(2):741. https://doi.org/10.3390/ijms22020741
Chicago/Turabian StyleYuan, Dian, An Cheng, Ichiro Kawahata, Hisanao Izumi, Jing Xu, and Kohji Fukunaga. 2021. "Single Administration of the T-Type Calcium Channel Enhancer SAK3 Reduces Oxidative Stress and Improves Cognition in Olfactory Bulbectomized Mice" International Journal of Molecular Sciences 22, no. 2: 741. https://doi.org/10.3390/ijms22020741
APA StyleYuan, D., Cheng, A., Kawahata, I., Izumi, H., Xu, J., & Fukunaga, K. (2021). Single Administration of the T-Type Calcium Channel Enhancer SAK3 Reduces Oxidative Stress and Improves Cognition in Olfactory Bulbectomized Mice. International Journal of Molecular Sciences, 22(2), 741. https://doi.org/10.3390/ijms22020741






