Crucial Role of FABP3 in αSyn-Induced Reduction of Septal GABAergic Neurons and Cognitive Decline in Mice
Abstract
1. Introduction
2. Results
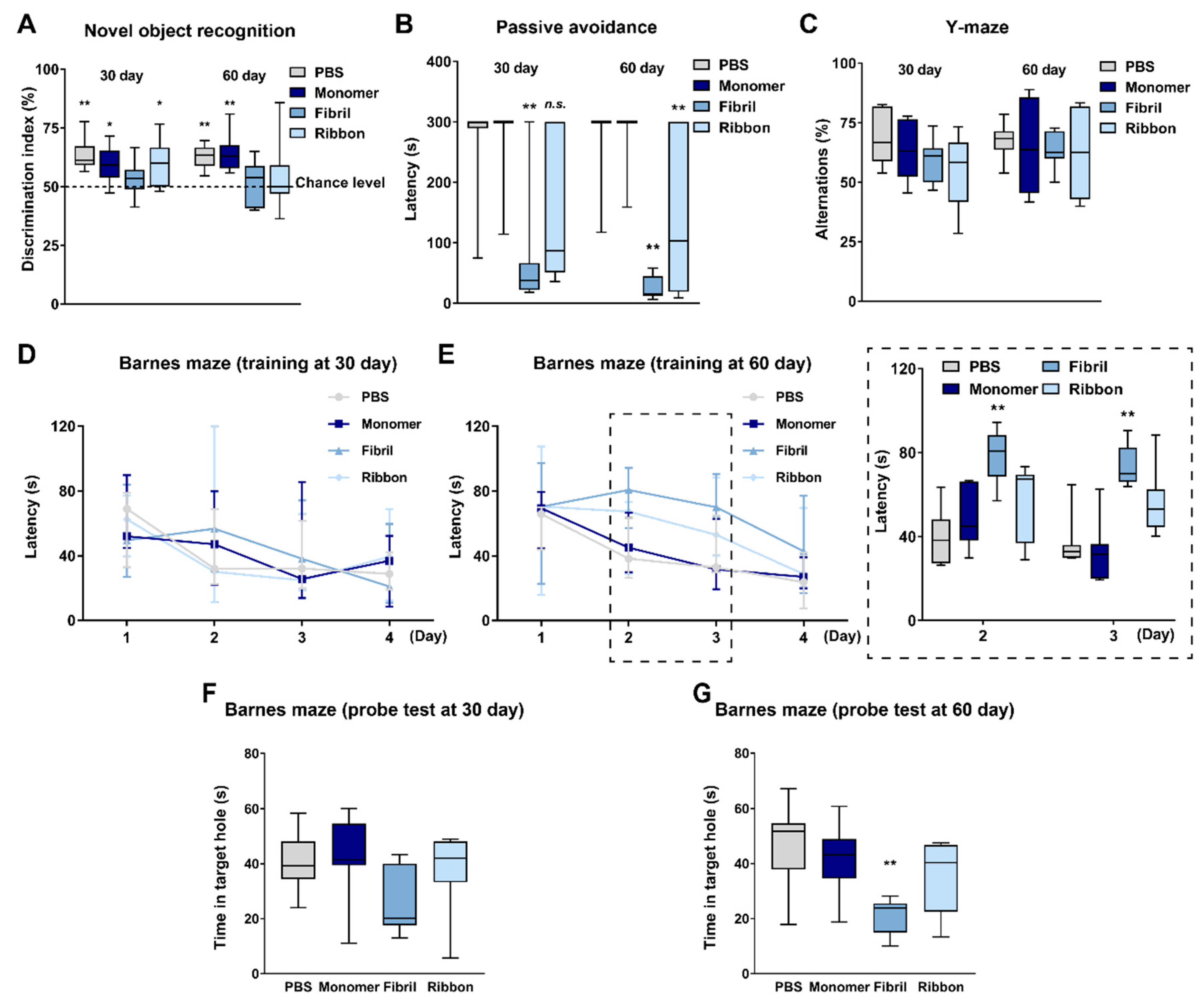
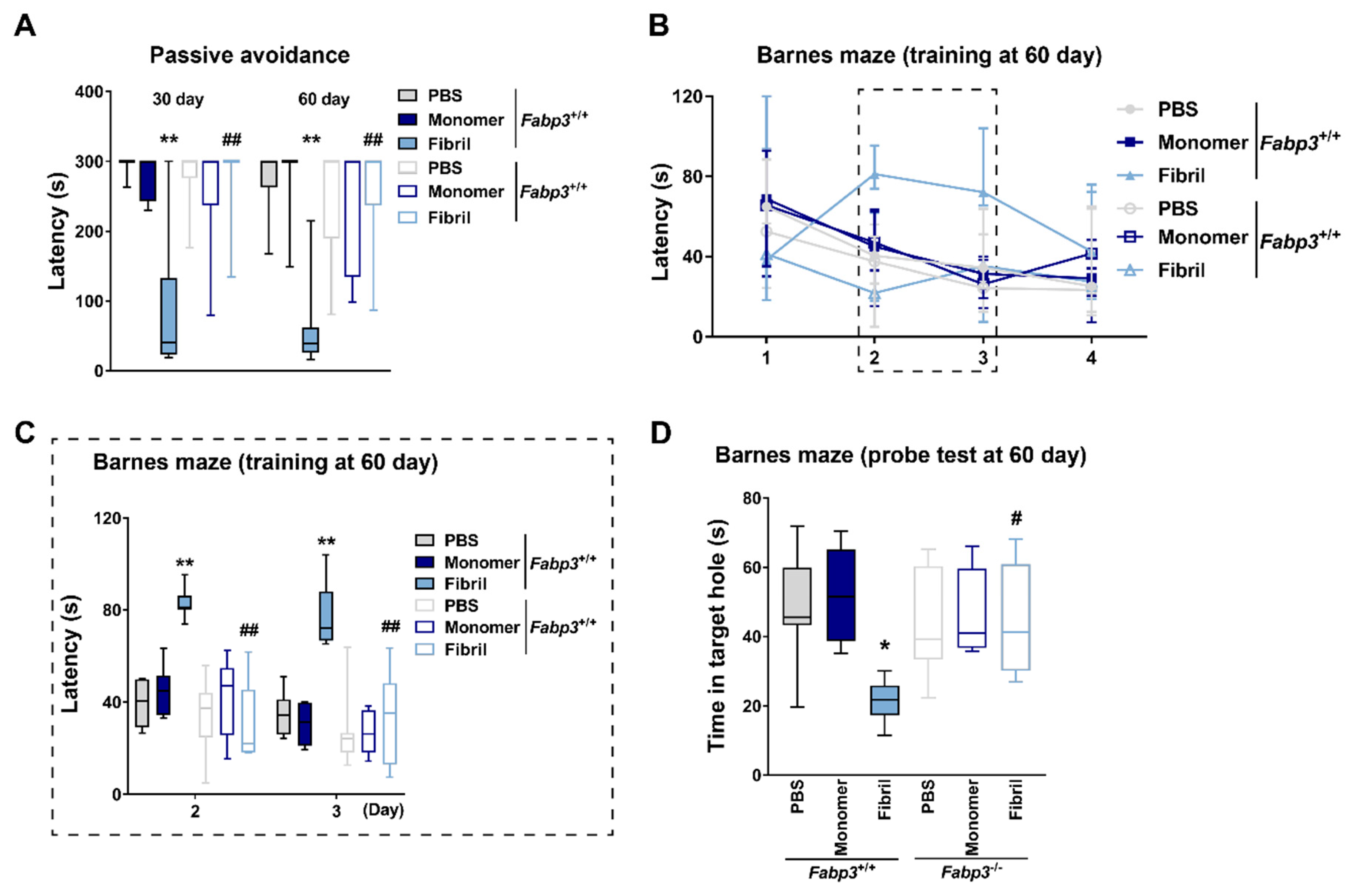
2.1. Distinct αSyn Strains Impair Cognition after Intrastriatal Injection in Mice
2.2. αSyn Fibrils Reduce Septal GABAergic Neurons after Intrastriatal Injection in Mice
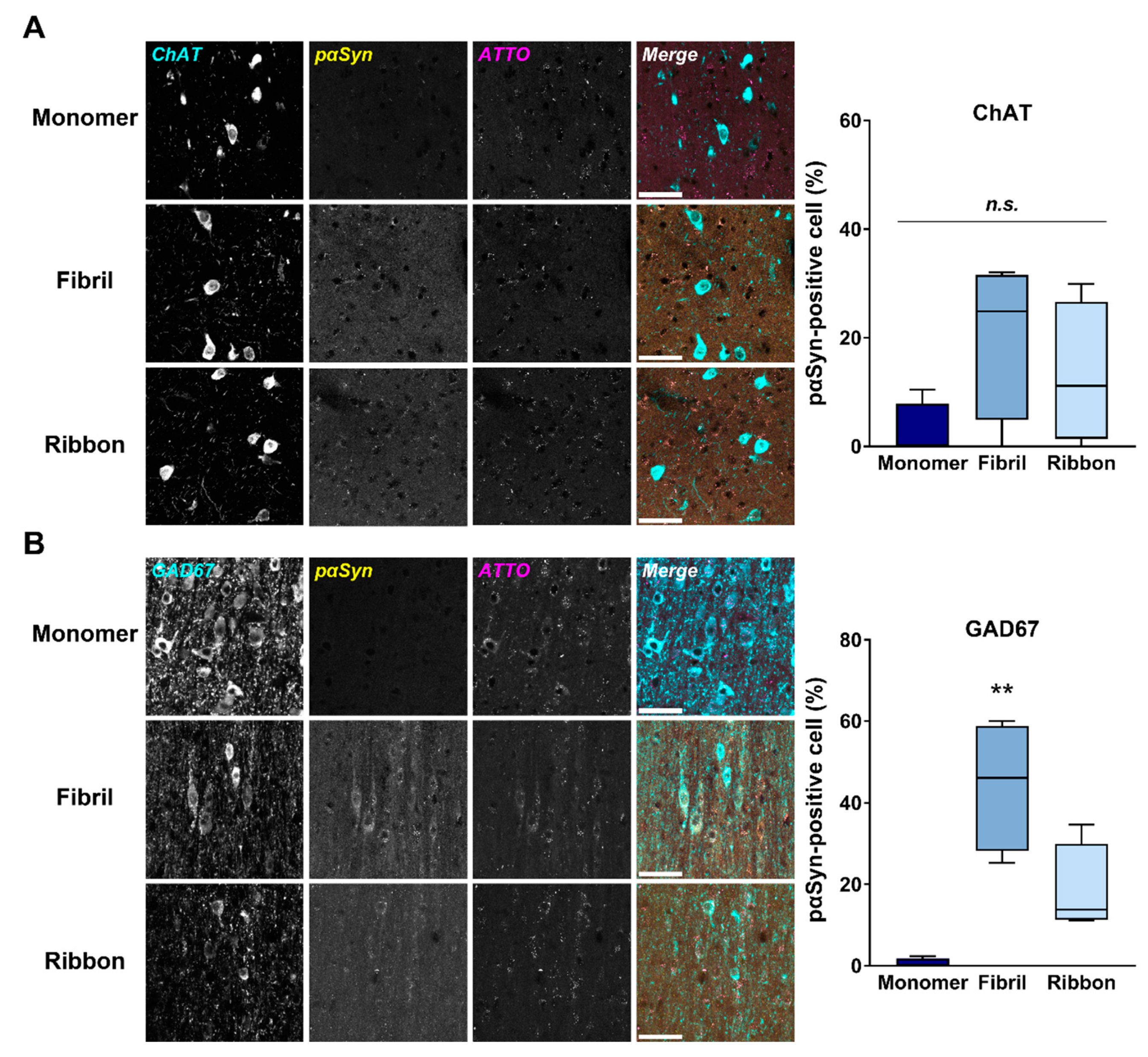
2.3. αSyn Fibrils Induce pαSyn Accumulation in Septal GABAergic Neurons after Intrastriatal Injection in Mice
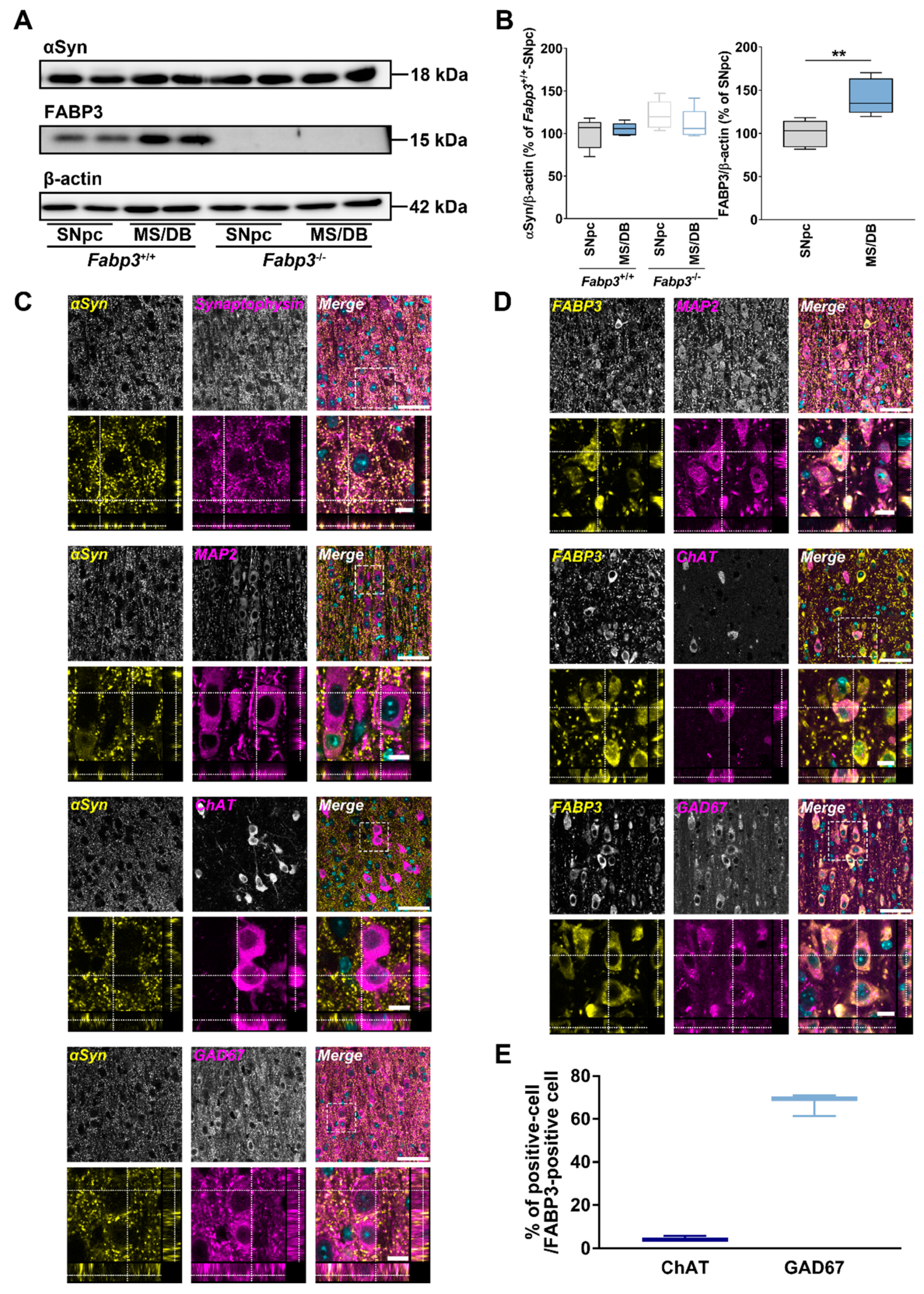
2.4. Properties of Expression and Localization of αSyn and FABP3 in MS/DB
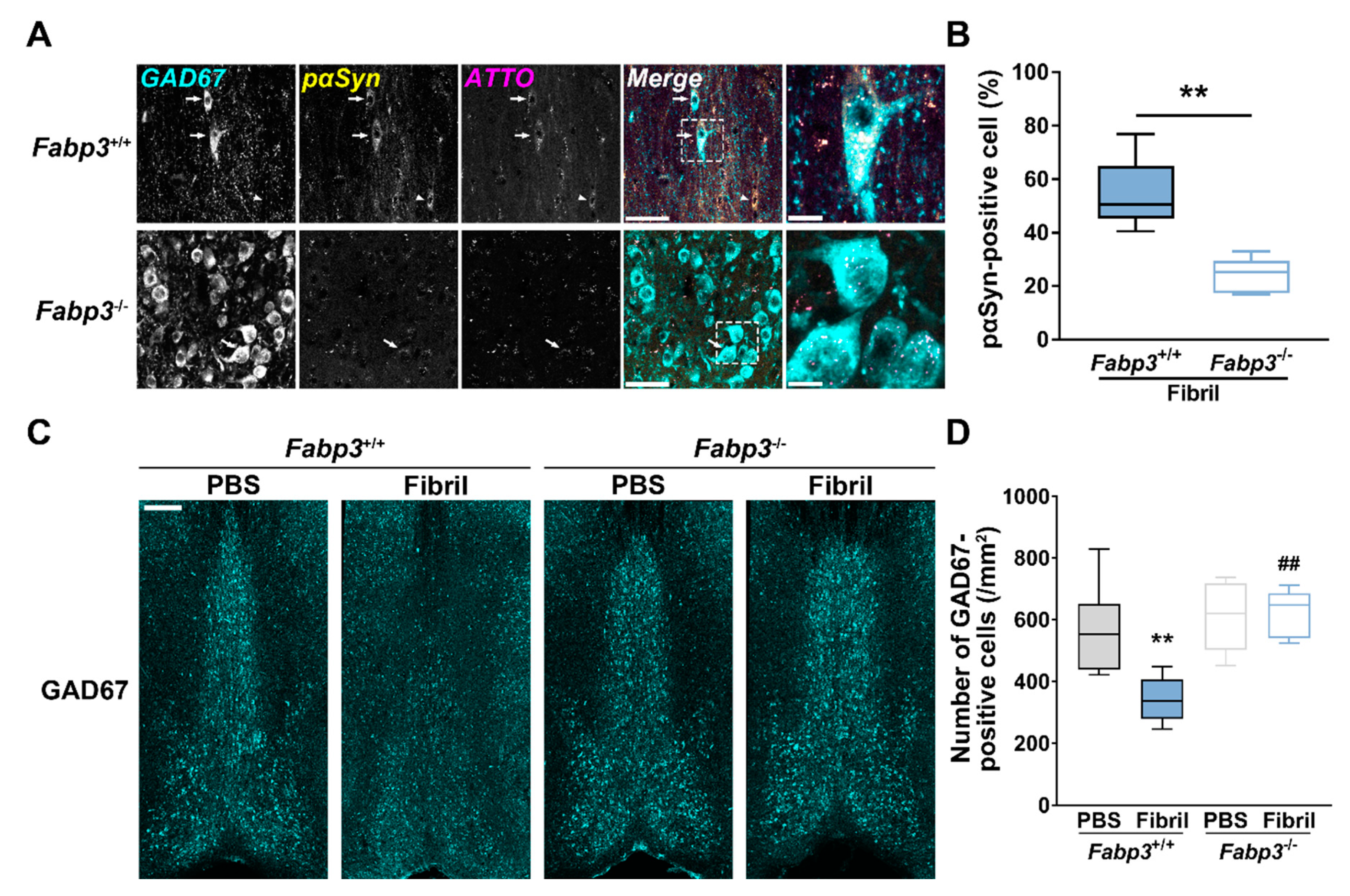
2.5. Fabp3 Deletion Inhibits pαSyn Accumulation and Reduction of GABAergic Neurons in MS/DB after Intrastriatal Injection of αSyn Fibrils in Mice
2.6. Fabp3 Deletion Restores αSyn Fibril-Induced Cognitive Impairments after Intrastriatal Injection in Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Stereotaxic Surgery
4.2. Behavioral Analyses
4.2.1. Novel Object Recognition Task
4.2.2. Step-Through Passive Avoidance Task
4.2.3. Y-Maze Task
4.2.4. Barnes Maze Task
4.3. Immunofluorescence and Quantification
4.4. Immunoblotting Analyses
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Buter, T.C.; van den Hout, A.; Matthews, F.E.; Larsen, J.P.; Brayne, C.; Aarsland, D. Dementia and survival in Parkinson disease: A 12-year population study. Neurology 2008, 70, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.M.; Rinne, J.O.; Helenius, H.; Dickson, D.W.; Röyttä, M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000, 100, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, D.H.; Roy, S.; Salmon, D.P.; Galasko, D.R.; Hansen, L.A.; Masliah, E.; Gage, F.H. Hippocampal α-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J. Neurosci. 2017, 37, 1675–1684. [Google Scholar] [CrossRef]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Bigl, V.; Woolf, N.J.; Butcher, L.L. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Res. Bull. 1982, 8, 727–749. [Google Scholar] [CrossRef]
- Freund, T.F.; Antal, M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 1988, 336, 170–173. [Google Scholar] [CrossRef]
- Colom, L.V.; Castaneda, M.T.; Reyna, T.; Hernandez, S.; Garrido-Sanabria, E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse 2005, 58, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Petsche, H.; Stumpf, C.; Gogolak, G. The significance of the rabbit’s septum as a relay station between the midbrain and the hippocampus I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr. Clin. Neurophysiol. 1962, 14, 202–211. [Google Scholar] [CrossRef]
- Fuhrmann, F.; Justus, D.; Sosulina, L.; Kaneko, H.; Beutel, T.; Friedrichs, D.; Schoch, S.; Schwarz, M.K.; Fuhrmann, M.; Remy, S. Locomotion, theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron 2015, 86, 1253–1264. [Google Scholar] [CrossRef]
- Huerta, P.T.; Lisman, J.E. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 1995, 15, 1053–1063. [Google Scholar] [CrossRef]
- Hyman, J.M.; Wyble, B.P.; Goyal, V.; Rossi, C.A.; Hasselmo, M.E. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J. Neurosci. 2003, 23, 11725–11731. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.; Zhou, J.; Cui, G.; Yakel, J.L. Septal cholinergic neurons gate hippocampal output to entorhinal cortex via oriens lacunosum moleculare interneurons. Proc. Natl. Acad. Sci. USA 2018, 115, E1886–E1895. [Google Scholar] [CrossRef] [PubMed]
- Unal, G.; Crump, M.G.; Viney, T.J.; Éltes, T.; Katona, L.; Klausberger, T.; Somogyi, P. Spatio-temporal specialization of GABAergic septo-hippocampal neurons for rhythmic network activity. Brain Struct. Funct. 2018, 223, 2409–2432. [Google Scholar] [CrossRef] [PubMed]
- Salib, M.; Joshi, A.; Katona, L.; Howarth, M.; Micklem, B.R.; Somogyi, P.; Viney, T.J. GABAergic medial septal neurons with low-rhythmic firing innervating the dentate gyrus and hippocampal area CA3. J. Neurosci. 2019, 39, 4527–4549. [Google Scholar] [CrossRef]
- Sans-Dublanc, A.; Razzauti, A.; Desikan, S.; Pascual, M.; Monyer, H.; Sindreu, C. Septal GABAergic inputs to CA1 govern contextual memory retrieval. Sci. Adv. 2020, 6, eaba5003. [Google Scholar] [CrossRef]
- Lee, E.H.Y.; Lin, Y.P.; Yin, T.H. Effects of lateral and medial septal lesions on various activity and reactivity measures in rats. Physiol. Behav. 1988, 42, 97–102. [Google Scholar] [CrossRef]
- Srividya, R.; Mallick, H.N.; Kumar, V.M. Sleep changes produced by destruction of medial septal neurons in rats. Neuroreport 2004, 15, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, T.A.; Servatius, R.J.; Pang, K.C.H. Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J. Neurosci. 2007, 27, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Knox, D.; Keller, S.M. Cholinergic neuronal lesions in the medial septum and vertical limb of the diagonal bands of Broca induce contextual fear memory generalization and impair acquisition of fear extinction. Hippocampus 2016, 26, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Del Tredici, K.; Braak, H. Idiopathic Parkinson’s disease: Staging an α-synucleinopathy with a predictable pathoanatomy. In Molecular Mechanisms in Parkinson’s Disease; Kahle, P., Haass, C., Eds.; Landes Bioscience: Austin, TX, USA, 2004; pp. 1–32. [Google Scholar]
- Jellinger, K.A. A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders. Biochim. Biophys. Acta 2009, 1792, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Masuda-Suzukake, M.; Nonaka, T.; Hosokawa, M.; Oikawa, T.; Arai, T.; Akiyama, H.; Mann, D.M.A.; Hasegawa, M. Prion-like spreading of pathological α-synuclein in brain. Brain 2013, 136, 1128–1138. [Google Scholar] [CrossRef]
- Lohmann, S.; Bernis, M.E.; Tachu, B.J.; Ziemski, A.; Grigoletto, J.; Tamgüney, G. Oral and intravenous transmission of α-synuclein fibrils to mice. Acta Neuropathol. 2019, 138, 515–533. [Google Scholar] [CrossRef]
- Uemura, N.; Uemura, M.T.; Lo, A.; Bassil, F.; Zhang, B.; Luk, K.C.; Lee, V.M.Y.; Takahashi, R.; Trojanowski, J.Q. Slow progressive accumulation of oligodendroglial alpha-synuclein (α-syn) pathology in synthetic α-syn fibril-induced mouse models of synucleinopathy. J. Neuropathol. Exp. Neurol. 2019, 78, 877–890. [Google Scholar] [CrossRef]
- Harauma, A.; Hatanaka, E.; Yasuda, H.; Nakamura, M.T.; Salem, N.; Moriguchi, T. Effects of arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid on brain development using artificial rearing of delta-6-desaturase knockout mice. Prostaglandins Leukot. Essent. Fat. Acids 2017, 127, 32–39. [Google Scholar] [CrossRef]
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 2001, 276, 41958–41962. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Frosch, M.P.; Walsh, D.M.; Hamilton, J.A.; Selkoe, D.J. The formation of highly soluble oligomers of α-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 2003, 37, 583–595. [Google Scholar] [CrossRef]
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta 1998, 1391, 287–306. [Google Scholar] [CrossRef]
- Shioda, N.; Yabuki, Y.; Kobayashi, Y.; Onozato, M.; Owada, Y.; Fukunaga, K. FABP3 protein promotes α-synuclein oligomerization associated with 1-Methyl-1,2,3,6-tetrahydropiridine-induced neurotoxicity. J. Biol. Chem. 2014, 289, 18957–18965. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Giraudo, S.; Corpillo, D.; Bergamasco, B.; Lopiano, L.; Fasano, M. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteomics 2004, 4, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Matsuo, K.; Kawahata, I.; Fukui, N.; Mizobata, T.; Kawata, Y.; Owada, Y.; Shioda, N.; Fukunaga, K. Fatty acid binding protein 3 enhances the spreading and toxicity of α-synuclein in mouse brain. Int. J. Mol. Sci. 2020, 21, 2230. [Google Scholar] [CrossRef]
- Matsuo, K.; Cheng, A.; Yabuki, Y.; Takahata, I.; Miyachi, H.; Fukunaga, K. Inhibition of MPTP-induced α-synuclein oligomerization by fatty acid-binding protein 3 ligand in MPTP-treated mice. Neuropharmacology 2019, 150, 164–174. [Google Scholar] [CrossRef]
- Matsuo, K.; Kawahata, I.; Melki, R.; Bousset, L.; Owada, Y.; Fukunaga, K. Suppression of α-synuclein propagation after intra-striatal injection in FABP3 null mice. Brain Res. Under review.
- Peelaerts, W.; Bousset, L.; Van Der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van Den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J.F. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; Delon, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Fujishiro, H.; Umegaki, H.; Isojima, D.; Akatsu, H.; Iguchi, A.; Kosaka, K. Depletion of cholinergic neurons in the nucleus of the medial septum and the vertical limb of the diagonal band in dementia with Lewy bodies. Acta Neuropathol. 2006, 111, 109–114. [Google Scholar] [CrossRef]
- Mehla, J.; Lacoursiere, S.G.; Lapointe, V.; McNaughton, B.L.; Sutherland, R.J.; McDonald, R.J.; Mohajerani, M.H. Age-dependent behavioral and biochemical characterization of single APP knock-in mouse (APPNL-G-F/NL-G-F) model of Alzheimer’s disease. Neurobiol. Aging 2019, 75, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Erskine, D.; Patterson, L.; Alexandris, A.; Hanson, P.S.; McKeith, I.G.; Attems, J.; Morris, C.M. Regional levels of physiological α-synuclein are directly associated with Lewy body pathology. Acta Neuropathol. 2018, 135, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Okuzumi, A.; Kurosawa, M.; Hatano, T.; Takanashi, M.; Nojiri, S.; Fukuhara, T.; Yamanaka, T.; Miyazaki, H.; Yoshinaga, S.; Furukawa, Y.; et al. Rapid dissemination of alpha-synuclein seeds through neural circuits in an in-vivo prion-like seeding experiment. Acta Neuropathol. Commun. 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.X.; Cornblath, E.J.; Darwich, A.; Zhang, B.; Brown, H.; Gathagan, R.J.; Sandler, R.M.; Bassett, D.S.; Trojanowski, J.Q.; Lee, V.M.Y. Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat. Neurosci. 2019, 22, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Do, J.P.; Xu, M.; Lee, S.H.; Chang, W.C.; Zhang, S.; Chung, S.; Yung, T.J.; Fan, J.L.; Miyamichi, K.; Luo, L.; et al. Cell type-specific long-range connections of basal forebrain circuit. eLife 2016, 5, e13214. [Google Scholar] [CrossRef]
- Agostinelli, L.J.; Geerling, J.C.; Scammell, T.E. Basal forebrain subcortical projections. Brain Struct. Funct. 2019, 224, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Owada, Y.; Yoshimoto, T.; Kondo, H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J. Chem. Neuroanat. 1996, 12, 113–122. [Google Scholar] [CrossRef]
- Yabuki, Y.; Takahata, I.; Matsuo, K.; Owada, Y.; Fukunaga, K. Ramelteon improves post-traumatic stress disorder-like behaviors exhibited by fatty acid-binding protein 3 null mice. Mol. Neurobiol. 2018, 55, 3577–3591. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kida, H.; Kagawa, Y.; Yasumoto, Y.; Miyazaki, H.; Islam, A.; Ogata, M.; Yanagawa, Y.; Mitsushima, D.; Fukunaga, K.; et al. FABP3 in the anterior cingulate cortex modulates the methylation status of the glutamic acid decarboxylase 67 promoter region. J. Neurosci. 2018, 38, 10411–10423. [Google Scholar] [CrossRef]
- Richieri, G.V.; Ogata, R.T.; Kleinfeld, A.M. Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J. Biol. Chem. 1994, 269, 23918–23930. [Google Scholar]
- Richieri, G.V.; Ogata, R.T.; Zimmerman, A.W.; Veerkamp, J.H.; Kleinfeld, A.M. Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions. Biochemistry 2000, 39, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Golovko, M.Y.; Rosenberger, T.A.; Færgeman, N.J.; Feddersen, S.; Cole, N.B.; Pribill, I.; Berger, J.; Nussbaum, R.L.; Murphy, E.J. Acyl-CoA synthetase activity links wild-type but not mutant α-synuclein to brain arachidonate metabolism. Biochemistry 2006, 45, 6956–6966. [Google Scholar] [CrossRef] [PubMed]
- Van der Perren, A.; Gelders, G.; Fenyi, A.; Bousset, L.; Brito, F.; Peelaerts, W.; Van den Haute, C.; Gentleman, S.; Melki, R.; Baekelandt, V. The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020, 139, 977–1000. [Google Scholar] [CrossRef] [PubMed]
- Strohäker, T.; Jung, B.C.; Liou, S.H.; Fernandez, C.O.; Riedel, D.; Becker, S.; Halliday, G.M.; Bennati, M.; Kim, W.S.; Lee, S.J.; et al. Structural heterogeneity of α-synuclein fibrils amplified from patient brain extracts. Nat. Commun. 2019, 10, 5535. [Google Scholar] [CrossRef]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Böckmann, A.; Meier, B.H.; et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef]
- Rey, N.L.; Bousset, L.; George, S.; Madaj, Z.; Meyerdirk, L.; Schulz, E.; Steiner, J.A.; Melki, R.; Brundin, P. α-Synuclein conformational strains spread, seed and target neuronal cells differentially after injection into the olfactory bulb. Acta Neuropathol. Commun. 2019, 7, 221. [Google Scholar] [CrossRef]
- Lippa, C.F.; Smith, T.W.; Perry, E. Dementia with Lewy bodies: Choline acetyltransferase parallels nucleus basalis pathology. J. Neural Transm. 1999, 106, 525–535. [Google Scholar] [CrossRef]
- Mori, S.; Mori, E.; Iseki, E.; Kosaka, K. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: Preliminary findings from an open-label study. Psychiatry Clin. Neurosci. 2006, 60, 190–195. [Google Scholar] [CrossRef]
- Ikeda, M.; Mori, E.; Kosaka, K.; Iseki, E.; Hashimoto, M.; Matsukawa, N.; Matsuo, K.; Nakagawa, M.; Katayama, S.; Higashi, Y.; et al. Long-term safety and efficacy of donepezil in patients with dementia with Lewy bodies: Results from a 52-week, open-label, multicenter extension study. Dement. Geriatr. Cogn. Disord. 2013, 36, 229–241. [Google Scholar] [CrossRef]
- Ikeda, M.; Mori, E.; Matsuo, K.; Nakagawa, M.; Kosaka, K. Donepezil for dementia with Lewy bodies: A randomized, placebo-controlled, confirmatory phase III trial. Alzheimer’s Res. Ther. 2015, 7, 4. [Google Scholar] [CrossRef]
- Mori, E.; Ikeda, M.; Nagai, R.; Matsuo, K.; Nakagawa, M.; Kosaka, K. Long-term donepezil use for dementia with Lewy bodies: Results from an open-label extension of Phase III trial. Alzheimer’s Res. Ther. 2015, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.C.H.; Jiao, X.; Sinha, S.; Beck, K.D.; Servatius, R.J. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: Effects on proactive interference. Hippocampus 2011, 21, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Dashniani, M.G.; Burjanadze, M.A.; Chkhikvishvili, N.C.; Solomonia, R.O.; Kandashvili, M.; Naneishvili, T.L.; Beselia, G.V.; Kruashvili, L.B.; Chighladze, M.R. Modulation of spatial memory and expression of hippocampal neurotransmitter receptors by selective lesion of medial septal cholinergic and GABAergic neurons. Exp. Brain Res. 2020, 238, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, G.; Shin, J.; Kim, S.W.; Kim, A.; Paydar, A.; Kim, D.S.; Miyazaki, T.; Watanabe, M.; Yanagawa, Y.; Kim, J.; et al. Medial septal GABAergic projection neurons promote object exploration behavior and type 2 theta rhythm. Proc. Natl. Acad. Sci. USA 2016, 113, 6550–6555. [Google Scholar] [CrossRef]
- Gulyás, A.I.; Seress, L.; Tóth, K.; Acsády, L.; Antal, M.; Freund, T.F. Septal GABAergic neurons innervate inhibitory interneurons in the hippocampus of the macaque monkey. Neuroscience 1991, 41, 381–390. [Google Scholar] [CrossRef]
- Hijazi, S.; Heistek, T.S.; van der Loo, R.; Mansvelder, H.D.; Smit, A.B.; van Kesteren, R.E. Hyperexcitable parvalbumin interneurons render hippocampal circuitry vulnerable to amyloid beta. iScience 2020, 23, 101271. [Google Scholar] [CrossRef]
- Bernstein, H.G.; Johnson, M.; Perry, R.H.; LeBeau, F.E.N.; Dobrowolny, H.; Bogerts, B.; Perry, E.K. Partial loss of parvalbumin-containing hippocampal interneurons in dementia with Lewy bodies. Neuropathology 2011, 31, 1–10. [Google Scholar] [CrossRef]
- Freichel, C.; Neumann, M.; Ballard, T.; Müller, V.; Woolley, M.; Ozmen, L.; Borroni, E.; Kretzschmar, H.A.; Haass, C.; Spooren, W.; et al. Age-dependent cognitive decline and amygdala pathology in α-synuclein transgenic mice. Neurobiol. Aging 2007, 28, 1421–1435. [Google Scholar] [CrossRef]
- Robson, E.; Tweedy, C.; Manzanza, N.; Taylor, J.P.; Atkinson, P.; Randall, F.; Reeve, A.; Clowry, G.J.; LeBeau, F.E.N. Impaired fast network oscillations and mitochondrial dysfunction in a mouse model of alpha-synucleinopathy (A30P). Neuroscience 2018, 377, 161–173. [Google Scholar] [CrossRef]
- Stylianou, M.; Zaaimi, B.; Thomas, A.; Taylor, J.P.; LeBeau, F.E.N. Early disruption of cortical sleep-related oscillations in a mouse model of dementia with Lewy bodies (DLB) expressing human mutant (A30P) alpha-synuclein. Front. Neurosci. 2020, 14, 579867. [Google Scholar] [CrossRef]
- Masuda-Suzukake, M.; Nonaka, T.; Hosokawa, M.; Kubo, M.; Shimozawa, A.; Akiyama, H.; Hasegawa, M. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol. Commun. 2014, 2, 88. [Google Scholar] [CrossRef]
- Mezias, C.; Rey, N.; Brundin, P.; Raj, A. Neural connectivity predicts spreading of alpha-synuclein pathology in fibril-injected mouse models: Involvement of retrograde and anterograde axonal propagation. Neurobiol. Dis. 2020, 134, 104623. [Google Scholar] [CrossRef] [PubMed]
- Bergado, J.A.; Frey, S.; López, J.; Almaguer-Melian, W.; Frey, J.U. Cholinergic afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. Neurobiol. Learn. Mem. 2007, 88, 331–341. [Google Scholar] [CrossRef]
- Henrich, M.T.; Geibl, F.F.; Lee, B.; Chiu, W.H.; Koprich, J.B.; Brotchie, J.M.; Timmermann, L.; Decher, N.; Matschke, L.A.; Oertel, W.H. A53T-α-synuclein overexpression in murine locus coeruleus induces Parkinson’s disease-like pathology in neurons and glia. Acta Neuropathol. Commun. 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sulser, A.; Parthier, D.; Candela, A.; McClure, C.; Pastoll, H.; Garden, D.; Sürmeli, G.; Nolan, M.F. GABAergic projections from the medial septum selectively inhibit interneurons in the medial entorhinal cortex. J. Neurosci. 2014, 34, 16739–16743. [Google Scholar] [CrossRef] [PubMed]
- Desikan, S.; Koser, D.E.; Neitz, A.; Monyer, H. Target selectivity of septal cholinergic neurons in the medial and lateral entorhinal cortex. Proc. Natl. Acad. Sci. USA 2018, 115, E2644–E2652. [Google Scholar] [CrossRef]
- Binas, B.; Danneberg, H.; Mcwhir, J.; Mullins, L.; Clark, A.J. Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J. 1999, 13, 805–812. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Matsuo, K.; Yabuki, Y.; Fukunaga, K. 5-aminolevulinic acid inhibits oxidative stress and ameliorates autistic-like behaviors in prenatal valproic acid-exposed rats. Neuropharmacology 2020, 168, 107975. [Google Scholar] [CrossRef]
- Patil, S.S.; Sunyer, B.; Höger, H.; Lubec, G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav. Brain Res. 2009, 198, 58–68. [Google Scholar] [CrossRef]
- Sasaki, A.; Arawaka, S.; Sato, H.; Kato, T. Sensitive western blotting for detection of endogenous Ser129-phosphorylated α-synuclein in intracellular and extracellular spaces. Sci. Rep. 2015, 5, 14211. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, K.; Yabuki, Y.; Melki, R.; Bousset, L.; Owada, Y.; Fukunaga, K. Crucial Role of FABP3 in αSyn-Induced Reduction of Septal GABAergic Neurons and Cognitive Decline in Mice. Int. J. Mol. Sci. 2021, 22, 400. https://doi.org/10.3390/ijms22010400
Matsuo K, Yabuki Y, Melki R, Bousset L, Owada Y, Fukunaga K. Crucial Role of FABP3 in αSyn-Induced Reduction of Septal GABAergic Neurons and Cognitive Decline in Mice. International Journal of Molecular Sciences. 2021; 22(1):400. https://doi.org/10.3390/ijms22010400
Chicago/Turabian StyleMatsuo, Kazuya, Yasushi Yabuki, Ronald Melki, Luc Bousset, Yuji Owada, and Kohji Fukunaga. 2021. "Crucial Role of FABP3 in αSyn-Induced Reduction of Septal GABAergic Neurons and Cognitive Decline in Mice" International Journal of Molecular Sciences 22, no. 1: 400. https://doi.org/10.3390/ijms22010400
APA StyleMatsuo, K., Yabuki, Y., Melki, R., Bousset, L., Owada, Y., & Fukunaga, K. (2021). Crucial Role of FABP3 in αSyn-Induced Reduction of Septal GABAergic Neurons and Cognitive Decline in Mice. International Journal of Molecular Sciences, 22(1), 400. https://doi.org/10.3390/ijms22010400



