Ionic Silicon Protects Oxidative Damage and Promotes Skeletal Muscle Cell Regeneration
Abstract
1. Introduction
2. Results
2.1. Effects of Si-Ions Dose on C2C12 Myoblasts
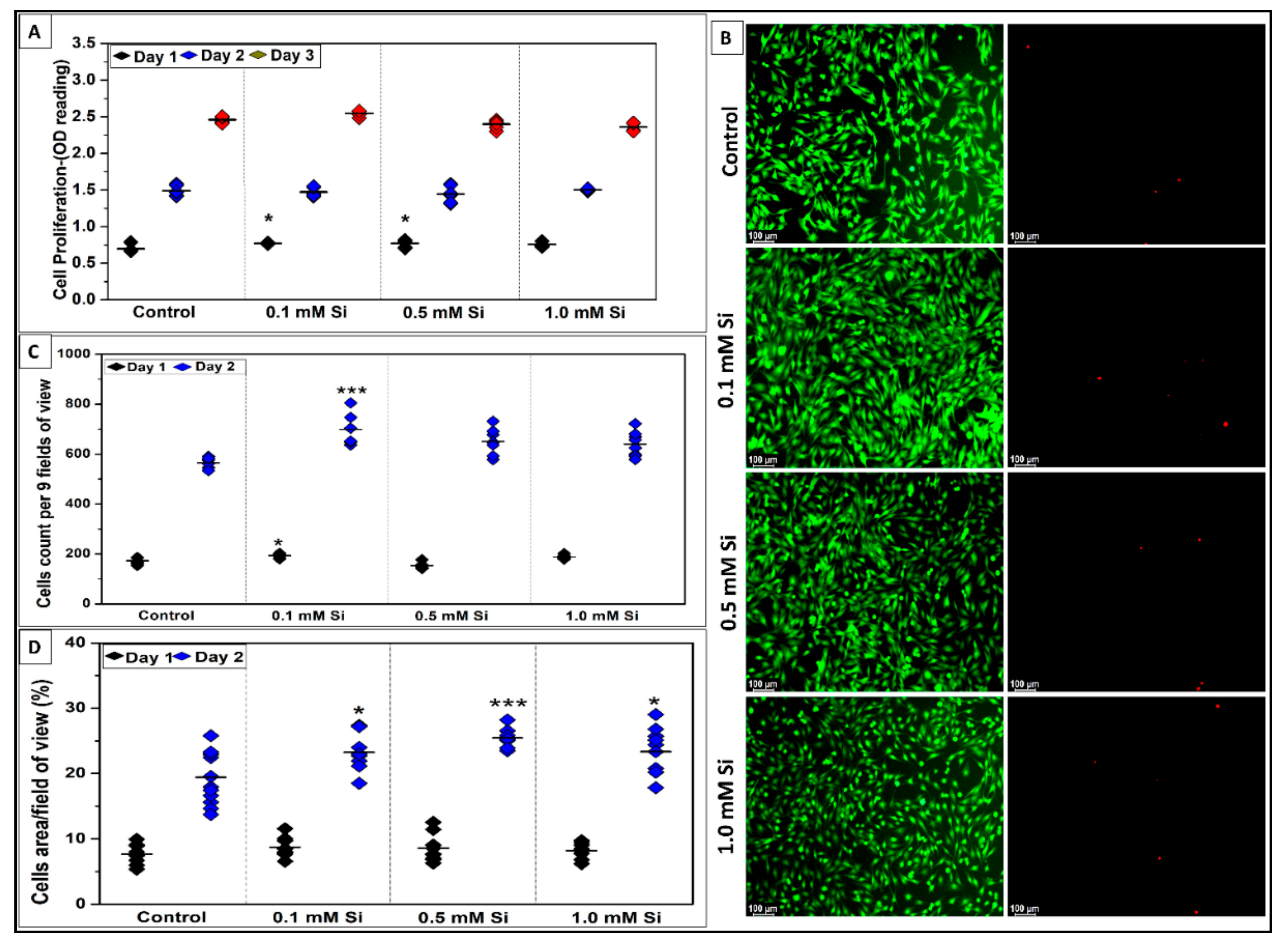
2.1.1. Si-Ions Enhance C2C12 Cell Viability
2.1.2. Si-Ions Promote C2C12 Cell Proliferation
2.1.3. Si-Ions Promote C2C12 Cell Differentiation
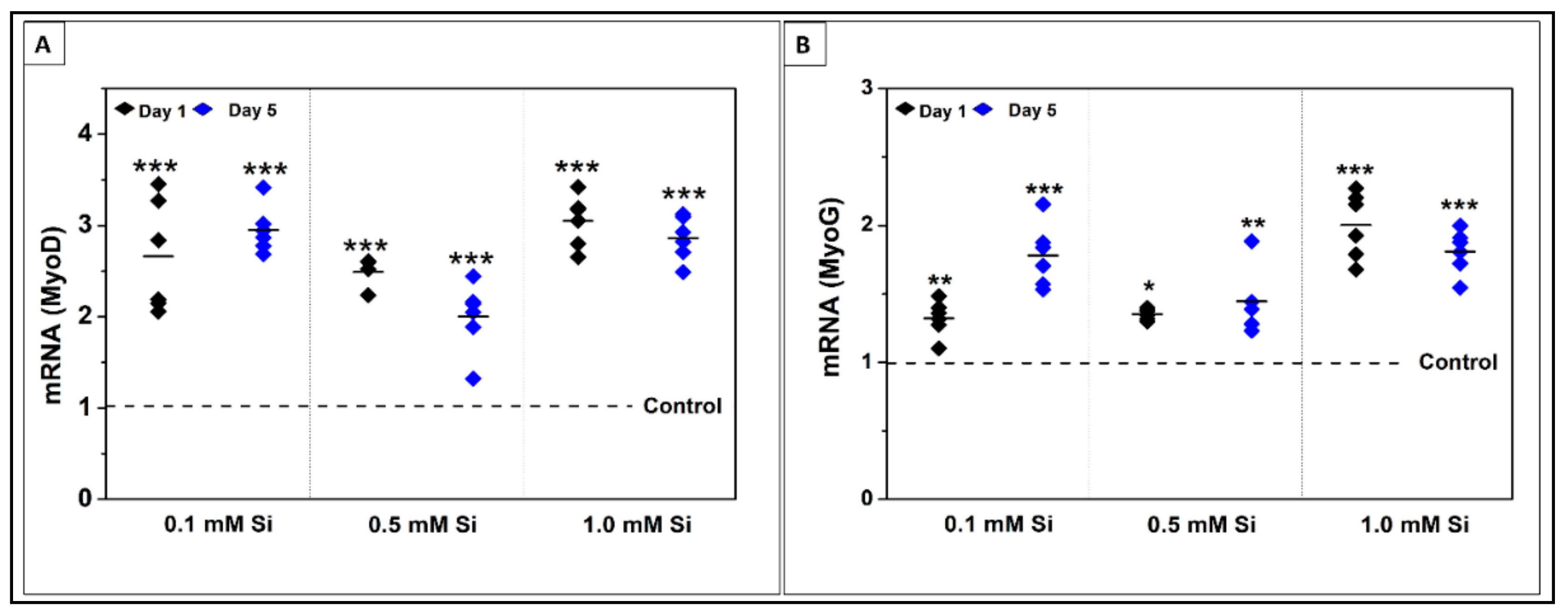
2.1.4. Si-Ions Enhance MyoG and MyoD Gene Expression
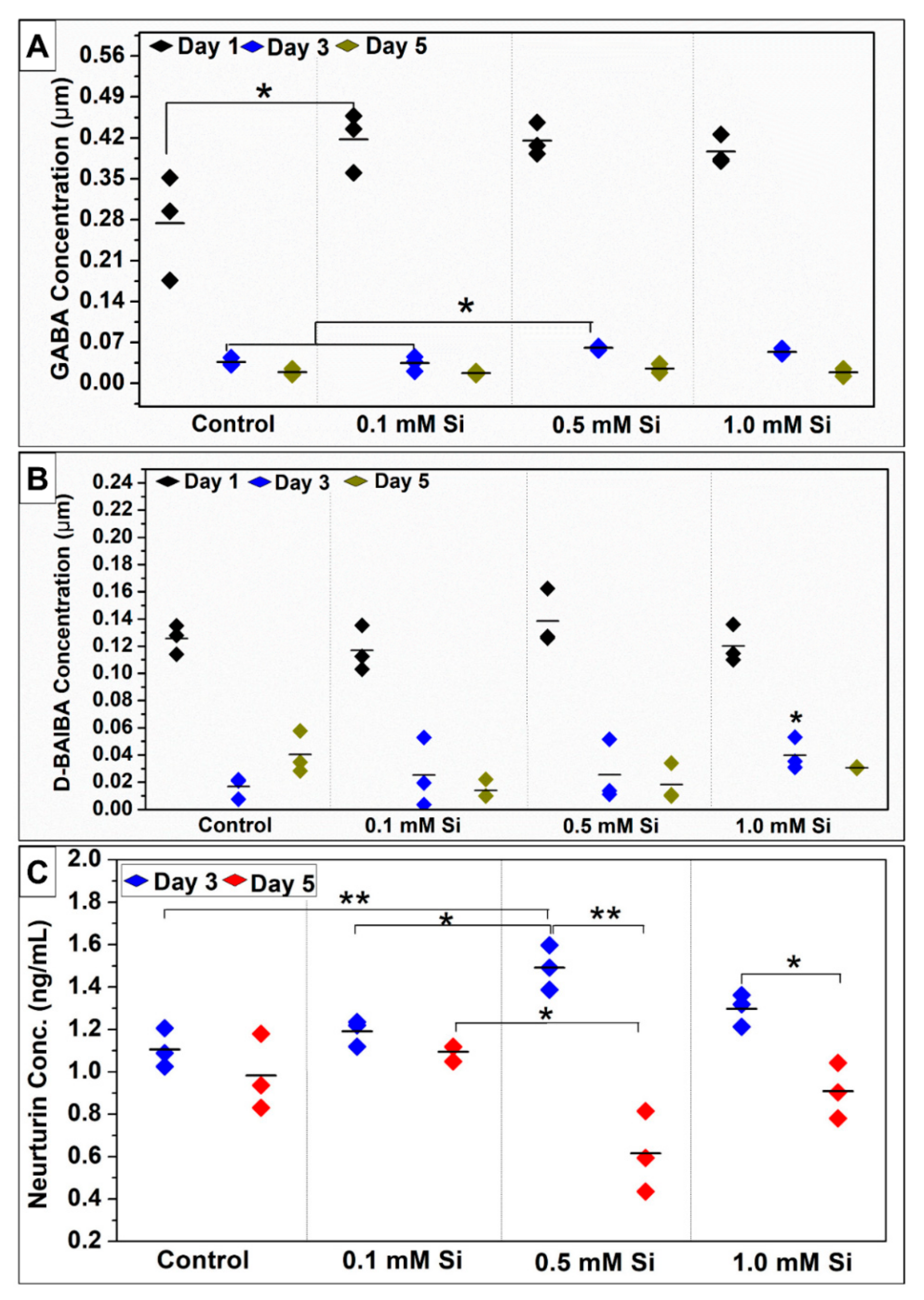
2.1.5. Si-Ions Enhance Myokines Expression
2.2. Effects of Different H2O2 Concentrations and Si-Ions on C2C12 Cells
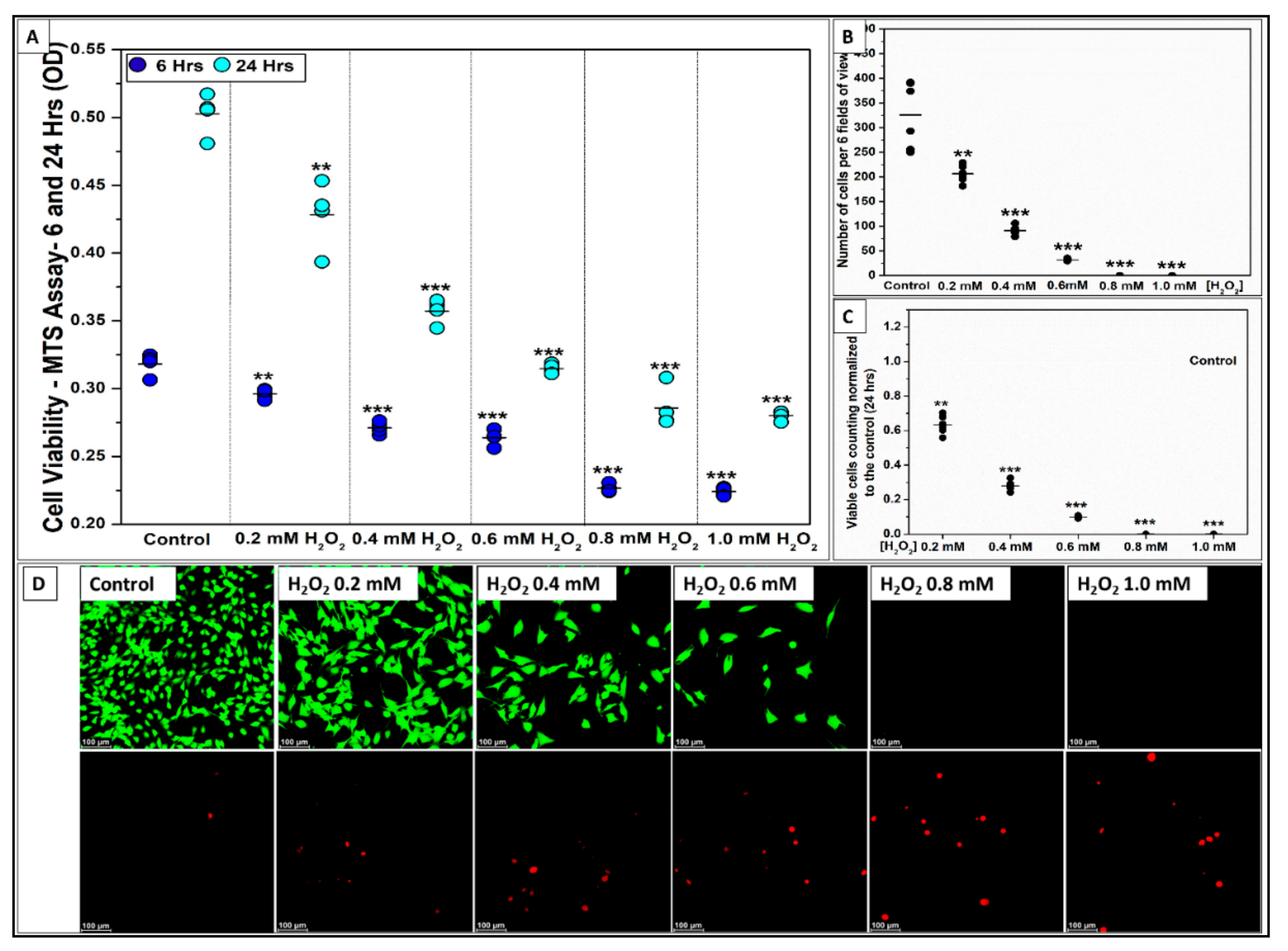
2.2.1. H2O2 Impairs C2C12 Myoblast Cell Viability
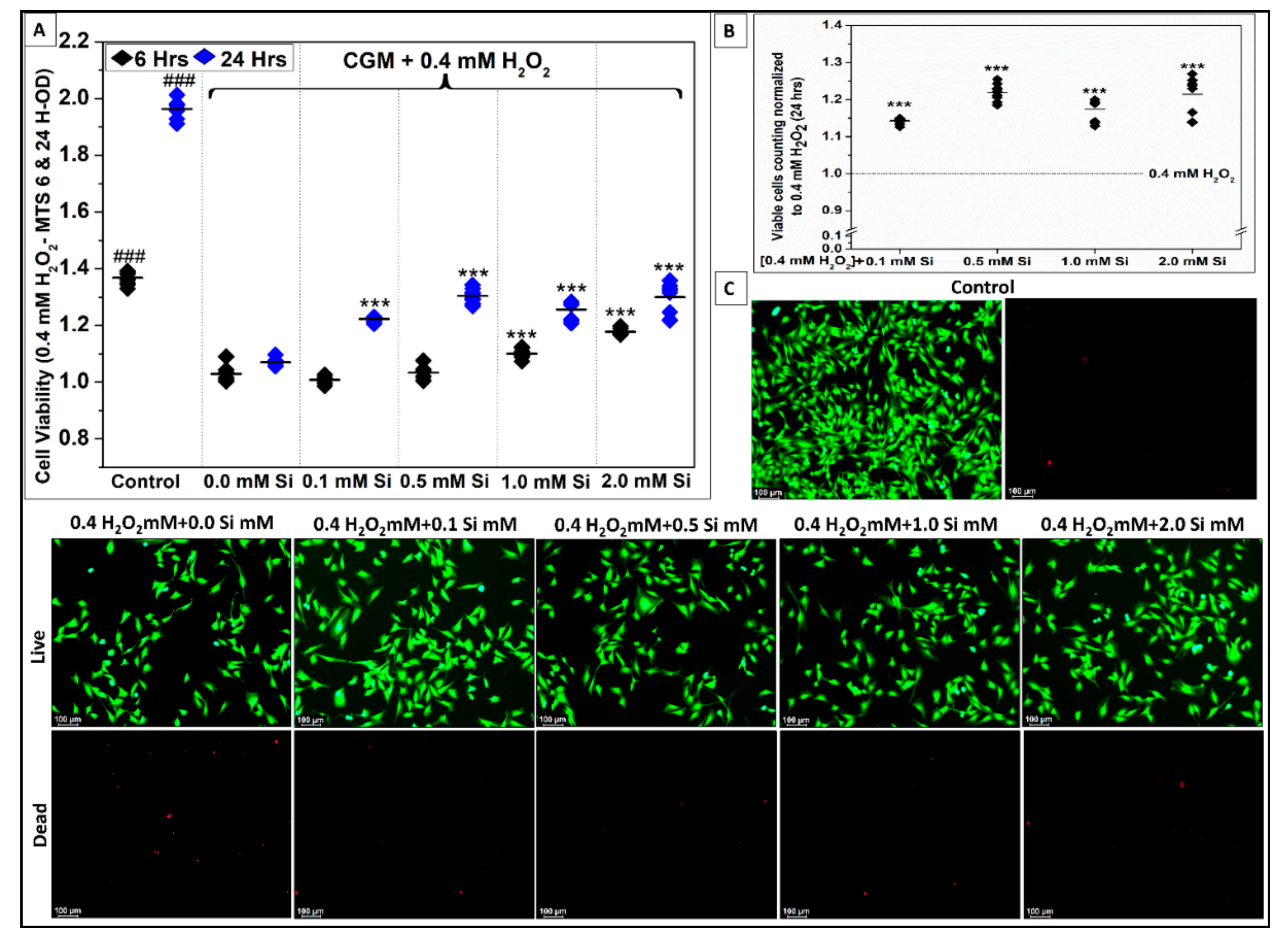
2.2.2. Si-Ions Protect C2C12 Myoblast Cells against Oxidative Damage
2.2.3. Si-Ions Enhance Cell Differentiation under Toxic Oxidative Stress
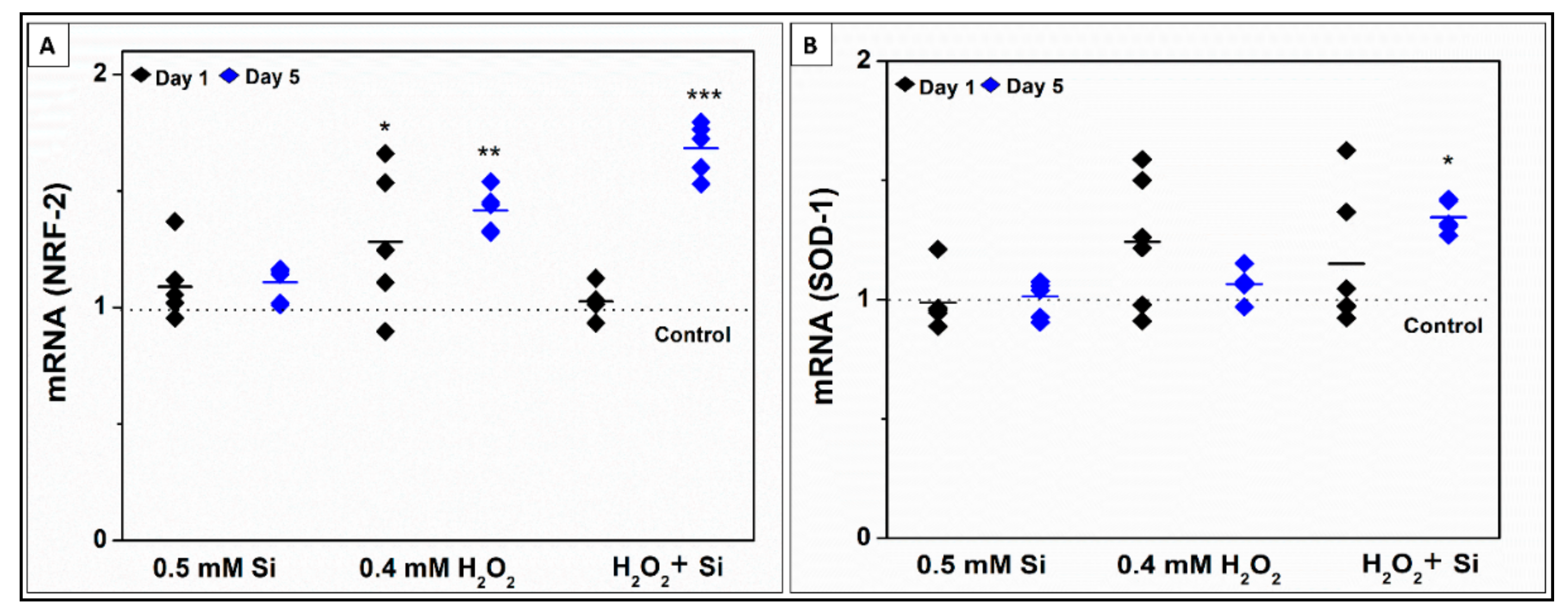
2.2.4. Si-Ions Enhance Antioxidant Marker Expression in the Presence of Toxic Oxidative Stress
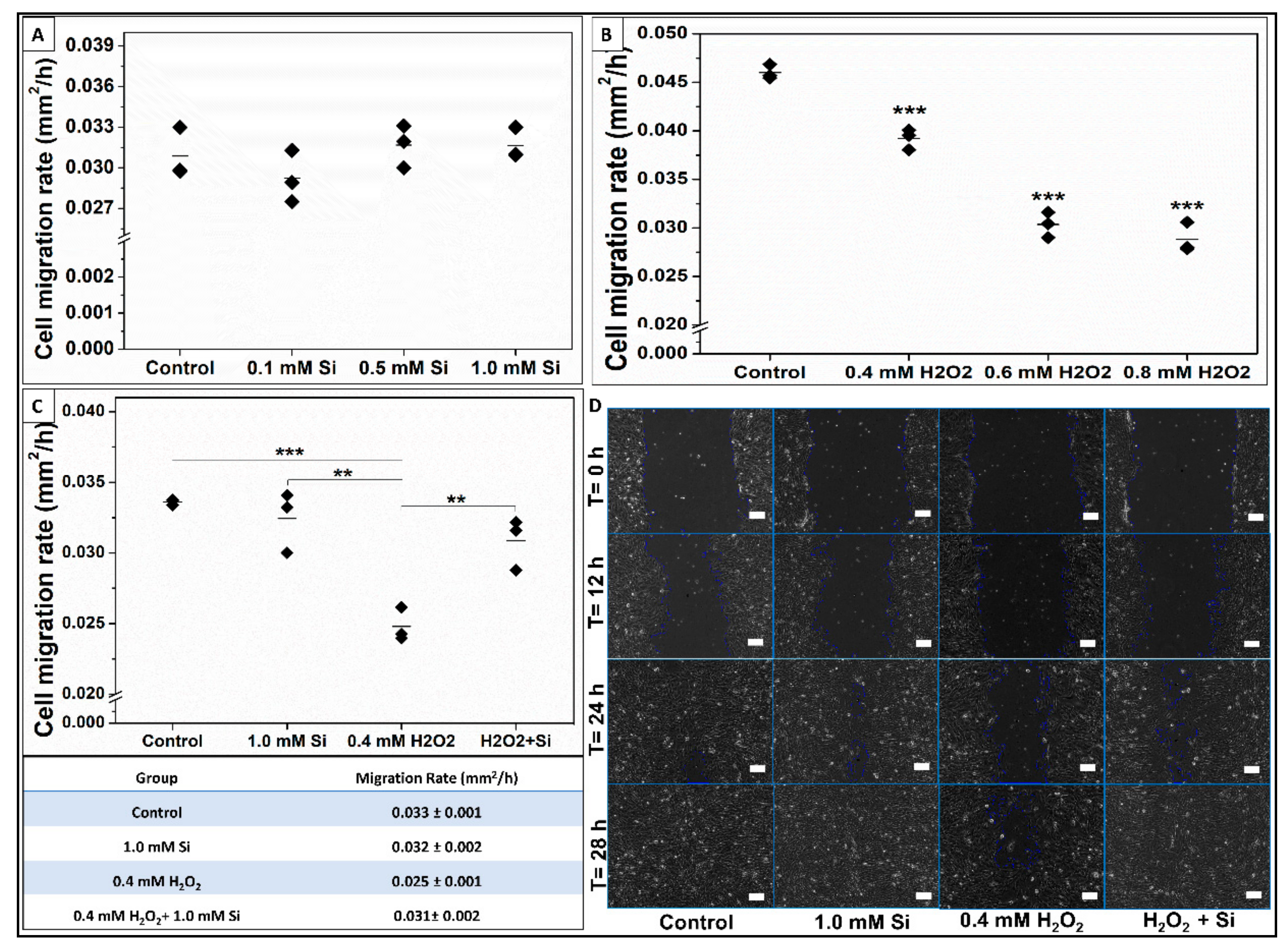
2.2.5. Si-Ions Enhance Wound Healing and Cell Migration Rate under Toxic Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Experimental Study Design
4.2. Materials
4.3. Preparation of Si-Ions and Hydrogen Peroxide Solutions
4.4. Cell Culture Studies
4.4.1. Effects of Different Ionic Si-Ions Concentrations on C2C12 Cells Functional Capacity
4.4.2. Different H2O2 Concentrations on C2C12 Cell Functional Capacity
4.5. Scratch-Wound Healing Assay Using Live Imaging Microscopy (LIM)
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Biomarker Expression
4.8. Statistical Data Analysis and Reporting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakayama, K.H.; Quarta, M.; Paine, P.; Alcazar, C.; Karakikes, I.; Garcia, V.; Abilez, O.J.; Calvo, N.S.; Simmons, C.S.; Rando, T.A.; et al. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun. Biol. 2019, 2, 170. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.M.; Henderson, B.E.P.; Walters, T.J.; Corona, B.T. Co-delivery of a laminin-111 supplemented hyaluronic acid based hydrogel with minced muscle graft in the treatment of volumetric muscle loss injury. PLoS ONE 2018, 13, e0191245. [Google Scholar] [CrossRef] [PubMed]
- Kasukonis, B.; Kim, J.; Brown, L.; Jones, J.; Ahmadi, S.; Washington, T.; Wolchok, J. Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng. Part A 2016, 22, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.T.; Garg, K.; Ward, C.L.; McDaniel, J.S.; Walters, T.J.; Rathbone, C.R. Autologous minced muscle grafts: A tissue engineering therapy for the volumetric loss of skeletal muscle. Am. J. Physiol. Physiol. 2013, 305, C761–C775. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Ward, C.L.; Leopold Wager, C.M.; Garg, K.; Goldman, S.M.; Henderson, B.E.P.; McKinley, T.O.; Greising, S.M.; Wenke, J.C.; Corona, B.T. Autologous minced muscle grafts improve endogenous fracture healing and muscle strength after musculoskeletal trauma. Physiol. Rep. 2017, 5, e13362. [Google Scholar] [CrossRef]
- Corona, B.T.; Rivera, J.C.; Wenke, J.C.; Greising, S.M. Tacrolimus as an adjunct to autologous minced muscle grafts for the repair of a volumetric muscle loss injury. J. Exp. Orthop. 2017, 4, 36. [Google Scholar] [CrossRef]
- Corona, B.T.; Henderson, B.E.P.; Ward, C.L.; Greising, S.M. Contribution of minced muscle graft progenitor cells to muscle fiber formation after volumetric muscle loss injury in wild-type and immune deficient mice. Physiol. Rep. 2017, 5, e13249. [Google Scholar] [CrossRef]
- Turner, N.J.; Yates, A.J.; Weber, D.J.; Qureshi, I.R.; Stolz, D.B.; Gilbert, T.W.; Badylak, S.F. Xenogeneic Extracellular Matrix as an Inductive Scaffold for Regeneration of a Functioning Musculotendinous Junction. Tissue Eng. Part A 2010, 16, 3309–3317. [Google Scholar] [CrossRef]
- Valentin, J.E.; Turner, N.J.; Gilbert, T.W.; Badylak, S.F. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 2010, 31, 7475–7484. [Google Scholar] [CrossRef]
- Corona, B.T.; Machingal, M.A.; Criswell, T.; Vadhavkar, M.; Dannahower, A.C.; Bergman, C.; Zhao, W.; Christ, G.J. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng. Part A 2012, 18, 1213–1228. [Google Scholar] [CrossRef]
- Merritt, E.K.; Cannon, M.V.; Hammers, D.W.; Le, L.N.; Gokhale, R.; Sarathy, A.; Song, T.J.; Tierney, M.T.; Suggs, L.J.; Walters, T.J.; et al. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng. Part A 2010, 16, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.T.; Rivera, J.C.; Dalske, K.A.; Wenke, J.C.; Greising, S.M. Pharmacological Mitigation of Fibrosis in a Porcine Model of Volumetric Muscle Loss Injury. Tissue Eng. Part A 2019, 26, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Quintero, A.J.; Wright, V.J.; Fu, F.H.; Huard, J. Stem cells for the treatment of skeletal muscle injury. Clin. Sports Med. 2009, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stilhano, R.S.; Martins, L.; Ingham, S.J.M.; Pesquero, J.B.; Huard, J. Gene and cell therapy for muscle regeneration. Curr. Rev. Musculoskelet. Med. 2015, 8, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, R.; Bearzi, C.; Mauretti, A.; Bernardini, S.; Cannata, S.; Gargioli, C. Tissue engineering for skeletal muscle regeneration. Muscles Ligaments Tendons J. 2012, 2, 230–234. [Google Scholar] [PubMed]
- Patel, A.; Mukundan, S.; Wang, W.; Karumuri, A.; Sant, V.; Mukhopadhyay, S.M.; Sant, S. Carbon-based hierarchical scaffolds for myoblast differentiation: Synergy between nano-functionalization and alignment. Acta Biomater. 2016, 32, 77–88. [Google Scholar] [CrossRef]
- Greising, S.M.; Corona, B.T.; McGann, C.; Frankum, J.K.; Warren, G.L. Therapeutic Approaches for Volumetric Muscle Loss Injury: A Systematic Review and Meta-Analysis. Tissue Eng. Part B Rev. 2019, 25, 510–525. [Google Scholar] [CrossRef]
- Poussard, S.; Decossas, M.; Le Bihan, O.; Mornet, S.; Naudin, G.; Lambert, O. Internalization and fate of silica nanoparticles in C2C12 skeletal muscle cells: Evidence of a beneficial effect on myoblast fusion. Int. J. Nanomed. 2015, 10, 1479–1492. [Google Scholar] [CrossRef]
- Quiroga, H.P.O.; Goto, K.; Zammit, P.S. Skeletal Muscle Regeneration in the Mouse. Methods Mol. Biol. 2016, 1460, 85–100. [Google Scholar] [CrossRef]
- Gurevich, D.; Siegel, A.; Currie, P.D. Vertebrate Myogenesis. Results Probl. Cell Differ. 2015, 56, 191–213. [Google Scholar] [CrossRef]
- Shintaku, J.; Peterson, J.M.; Talbert, E.E.; Gu, J.-M.; Ladner, K.J.; Williams, D.R.; Mousavi, K.; Wang, R.; Sartorelli, V.; Guttridge, D.C. MyoD Regulates Skeletal Muscle Oxidative Metabolism Cooperatively with Alternative NF-κB. Cell Rep. 2016, 17, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Jin, Y.-R.; Seto, M.; Yoon, J.K. A WNT/beta-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J. Biol. Chem. 2011, 286, 10649–10659. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Demontis, F. Systemic Nutrient and Stress Signaling via Myokines and Myometabolites. Annu. Rev. Physiol. 2016, 78, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jähn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. β-aminoisobutyric Acid, L-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.; Taylor-Weiner, H.; Correia, J.C.; Agudelo, L.Z.; Allodi, I.; Kolonelou, C.; Martinez-Redondo, V.; Ferreira, D.M.S.; Nichterwitz, S.; Comley, L.H.; et al. Neurturin is a PGC-1α1-controlled myokine that promotes motor neuron recruitment and neuromuscular junction formation. Mol. Metab. 2018, 7, 12–22. [Google Scholar] [CrossRef]
- Ji, L.L. Exercise at Old Age: Does It Increase or Alleviate Oxidative Stress? Ann. N. Y. Acad. Sci. 2001, 928, 236–247. [Google Scholar] [CrossRef]
- Bosutti, A.; Degens, H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci. Rep. 2015, 5, 8093. [Google Scholar] [CrossRef]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y. Do Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef]
- Siu, P.M.; Wang, Y.; Alway, S.E. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009, 84, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G. Basic science: Pathophysiology: Oxidative stress. J. Am. Soc. Hypertens. 2014, 8, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Cheng, A.; Westerblad, H. Antioxidants and Skeletal Muscle Performance: “Common Knowledge” vs. Experimental Evidence. Front. Physiol. 2012, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Leelayuwat, N. Chapter 19—Update of Nutritional Antioxidants and Antinociceptives on Improving Exercise-Induced Muscle Soreness; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 199–208. ISBN 978-0-12-805094-1. [Google Scholar]
- Drowley, L.; Okada, M.; Beckman, S.; Vella, J.; Keller, B.; Tobita, K.; Huard, J. Cellular antioxidant levels influence muscle stem cell therapy. Mol. Ther. 2010, 18, 1865–1873. [Google Scholar] [CrossRef]
- Bennett, B.T.; Mohamed, J.S.; Alway, S.E. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS ONE 2013, 8, e83518. [Google Scholar] [CrossRef]
- Pallafacchina, G.; Blaauw, B.; Schiaffino, S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr. Metab. Cardiovasc. Dis. 2013, 23, S12–S18. [Google Scholar] [CrossRef]
- Henstock, J.R.; Canham, L.T.; Anderson, S.I. Silicon: The evolution of its use in biomaterials. Acta Biomater. 2015, 11, 17–26. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef]
- Do Monte, F.A.; Ahuja, N.; Awad, K.R.; Pan, Z.; Young, S.; Kim, H.K.W.; Aswath, P.; Brotto, M.; Varanasi, V.G. Silicon Oxynitrophosphide Nanoscale-Coating Enhances Antioxidant Marker -Induced Angiogenesis During In Vivo Cranial Bone Defect Healing. JBMR Plus 2020, e10425. [Google Scholar] [CrossRef]
- Ilyas, A.; Lavrik, N.V.; Kim, H.K.W.; Aswath, P.B.; Varanasi, V.G. Enhanced Interfacial Adhesion and Osteogenesis for Rapid “Bone-like” Biomineralization by PECVD-Based Silicon Oxynitride Overlays. ACS Appl. Mater. Interfaces 2015, 7, 15368–15379. [Google Scholar] [CrossRef]
- Monte, F.A.D.; Awad, K.R.; Ahuja, N.; Kim, H.K.W.; Aswath, P.; Brotto, M.; Varanasi, V.G. Amorphous Silicon Oxynitrophosphide-Coated Implants Boost Angiogenic Activity of Endothelial Cells. Tissue Eng. Part A 2019, 26, 15–27. [Google Scholar] [CrossRef]
- Heublein, B.; Ozbek, C.; Pethig, K. Silicon carbide-coated stents: Clinical experience in coronary lesions with increased thrombotic risk. J. Endovasc. Surg. 1998, 5, 32–36. [Google Scholar] [CrossRef]
- Awad, K.R.; Ahuja, N.; Shah, A.; Tran, H.; Aswath, P.B.; Brotto, M.; Varanasi, V. Silicon nitride enhances osteoprogenitor cell growth and differentiation via increased surface energy and formation of amide and nanocrystalline HA for craniofacial reconstruction. Med. Devices Sens. 2019, 2, e10032. [Google Scholar] [CrossRef]
- McEntire, B.J.; Lakshminarayanan, R. Processing and Characterization of Silicon Nitride Bioceramics. Bioceram. Dev. Appl. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Ilyas, A.; Velten, M.F.; Shah, A.; Lanford, W.A.; Aswath, P.B. Role of Hydrogen and Nitrogen on the Surface Chemical Structure of Bioactive Amorphous Silicon Oxynitride Films. J. Phys. Chem. B 2017, 121, 8991–9005. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Velton, M.; Shah, A.; Monte, F.; Kim, H.K.W.; Aswath, P.B.; Varanasi, V.G. Rapid Regeneration of Vascularized Bone by Nanofabricated Amorphous Silicon Oxynitrophosphide (SiONP) Overlays. J. Biomed. Nanotechnol. 2019, 15, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Monte, F.; Cebe, T.; Ripperger, D.; Ighani, F.; Kojouharov, H.V.; Chen, B.M.; Kim, H.K.W.; Aswath, P.B.; Varanasi, V.G. Ionic silicon improves endothelial cells’ survival under toxic oxidative stress by overexpressing angiogenic markers and antioxidant enzymes. J. Tissue Eng. Regen. Med. 2018, 12, 2203–2220. [Google Scholar] [CrossRef] [PubMed]
- Cebe, T.; Ahuja, N.; Monte, F.; Awad, K.; Vyavhare, K.; Aswath, P.; Huang, J.; Brotto, M.; Varanasi, V. Novel 3D-printed methacrylated chitosan-laponite nanosilicate composite scaffolds enhance cell growth and biomineral formation in MC3T3 pre-osteoblasts. J. Mater. Res. 2020, 35, 58–75. [Google Scholar] [CrossRef]
- Bhuyan, M.K.; Rodriguez-Devora, J.I.; Fraser, K.; Tseng, T.L.B. Silicon substrate as a novel cell culture device for myoblast cells. J. Biomed. Sci. 2014, 21, 4–8. [Google Scholar] [CrossRef]
- AWAD, K.R.; Ahuja, N.; Fiedler, M.; Huang, J.; Brotto, L.; Aswath, P.; Brotto, M.; Varanasi, V. Micro-patterned Bioactive Amorphous Silicon Oxynitride Enhances Adhesion, Growth, and Myotubes and Axon Alignment in Muscle and Nerve Cells. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ahuja, N.; Awad, K.; Fiedler, M.; Aswath, P.; Brotto, M.; Varanasi, V. Preliminary study of in-situ 3D bioprinted nano-silicate biopolymer scaffolds for muscle repair in VML defects. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Awad, K.R.; Huang, J.; Brotto, L.; Aswath, P.; Brotto, M.; Varanasi, V. Patterned Silicon Oxynitride (SiONx) Scaffolds Enhance Alignment and Myogenic Differentiation of C2C12 Muscle Cells. FASEB J. 2019, 33, 539.5. [Google Scholar] [CrossRef]
- Cory, G. Scratch-wound assay. Methods Mol. Biol. 2011, 769, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Dirks Naylor, A.J. Cellular effects of resveratrol in skeletal muscle. Life Sci. 2009, 84, 637–640. [Google Scholar] [CrossRef]
- Montesano, A.; Luzi, L.; Senesi, P.; Mazzocchi, N.; Terruzzi, I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J. Transl. Med. 2013, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.; Lançon, A.; Aires, V.; Limagne, E.; Tili, E.; Michaille, J.-J.; Latruffe, N. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochem. Pharmacol. 2012, 84, 1251–1259. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Gilliver, S.F.; Jones, D.A.; Rittweger, J.; Degens, H. Effects of oxidation on the power of chemically skinned rat soleus fibres. J. Musculoskelet. Neuronal Interact. 2010, 10, 267–273. [Google Scholar]
- Jackson, J.R.; Ryan, M.J.; Alway, S.E. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 751–764. [Google Scholar] [CrossRef]
- Rando, T.A. Oxidative Stress and the Pathogenesis of Muscular Dystrophies. Am. J. Phys. Med. Rehabil. 2002, 81, S175–S186. [Google Scholar] [CrossRef]
- Murphy, M.E.; Kehrer, J.P. Oxidative stress and muscular dystrophy. Chem. Biol. Interact. 1989, 69, 101–173. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef] [PubMed]
- Rando, T.A.; Crowley, R.S.; Carlson, E.J.; Epstein, C.J.M.P. Overexpression of copper/zinc superoxide dismutase: A novel cause of murine muscular dystrophy. Ann. Neurol. 1998, 44, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.C. An Overview of Silica in Biology: Its Chemistry and Recent Technological Advances BT—Biosilica in Evolution, Morphogenesis, and Nanobiotechnology: Case Study Lake Baikal; Müller, W.E.G., Grachev, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 295–313. ISBN 978-3-540-88552-8. [Google Scholar]
- Han, P.; Wu, C.; Xiao, Y. The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomater. Sci. 2013, 1, 379–392. [Google Scholar] [CrossRef]
- Zou, S.; Ireland, D.; Brooks, R.A.; Rushton, N.; Best, S. The effects of silicate ions on human osteoblast adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 123–130. [Google Scholar] [CrossRef]
- Bobadilla, A.V.P.; Arévalo, J.; Sarró, E.; Byrne, H.M.; Maini, P.K.; Carraro, T.; Balocco, S.; Meseguer, A.; Alarcón, T. In vitro cell migration quantification method for scratch assays. J. R. Soc. Interface 2019, 16, 20180709. [Google Scholar] [CrossRef]
- Simerabet, M.; Robin, E.; Aristi, I.; Adamczyk, S.; Tavernier, B.; Vallet, B.; Bordet, R.; Lebuffe, G. Preconditioning by an in situ administration of hydrogen peroxide: Involvement of reactive oxygen species and mitochondrial ATP-dependent potassium channel in a cerebral ischemia–reperfusion model. Brain Res. 2008, 1240, 177–184. [Google Scholar] [CrossRef]
- Lin, M.; Zhou, S.; Sakamoto, K. Alpha Mangostin promotes myogenic differentiation of C2C12 mouse myoblast cells. Biochem. Biophys. Res. Commun. 2020, 528, 193–198. [Google Scholar] [CrossRef]
- Jähn, K.; Lara-Castillo, N.; Brotto, L.; Mo, C.L.; Johnson, M.L.; Brotto, M.; Bonewald, L.F. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin. Eur. Cell. Mater. 2012, 24, 197–210. [Google Scholar] [CrossRef]
- Mo, C.; Romero-Suarez, S.; Bonewald, L.; Johnson, M.; Brotto, M. Prostaglandin E2: From clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat. Biotechnol. 2012, 6, 223–229. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Shiflett, L.A.; Brotto, L.; Bonewald, L.F.; Wacker, M.J.; Dallas, S.L.; Brotto, M. Fibroblast growth factor 9 (FGF9) inhibits myogenic differentiation of C2C12 and human muscle cells. Cell Cycle 2019, 18, 3562–3580. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Romero-Suarez, S.; Lara, N.; Mo, C.; Kaja, S.; Brotto, L.; Dallas, S.L.; Johnson, M.L.; Jähn, K.; Bonewald, L.F.; et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway. JBMR Plus 2017, 1, 86–100. [Google Scholar] [CrossRef]
- Huang, J.; Hsu, Y.-H.; Mo, C.; Abreu, E.; Kiel, D.P.; Bonewald, L.F.; Brotto, M.; Karasik, D. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-κB signaling pathway. J. Bone Miner. Res. 2014, 29, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Silva Nunes, J.P.; Martins Dias, A.A. ImageJ macros for the user-friendly analysis of soft-agar and wound-healing assays. Biotechniques 2017, 62, 175–179. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Saiz, E.; Loomer, P.M.; Ancheta, B.; Uritani, N.; Ho, S.P.; Tomsia, A.P.; Marshall, S.J.; Marshall, G.W. Enhanced osteocalcin expression by osteoblast-like cells (MC3T3-E1) exposed to bioactive coating glass (SiO2–CaO–P2O5–MgO–K2O–Na2O system) ions. Acta Biomater. 2009, 5, 3536–3547. [Google Scholar] [CrossRef]
- Wang, Z.; Bian, L.; Mo, C.; Shen, H.; Zhao, L.J.; Su, K.-J.; Kukula, M.; Lee, J.T.; Armstrong, D.W.; Recker, R.; et al. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun. Biol. 2020, 3, 39. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, K.; Ahuja, N.; Fiedler, M.; Peper, S.; Wang, Z.; Aswath, P.; Brotto, M.; Varanasi, V. Ionic Silicon Protects Oxidative Damage and Promotes Skeletal Muscle Cell Regeneration. Int. J. Mol. Sci. 2021, 22, 497. https://doi.org/10.3390/ijms22020497
Awad K, Ahuja N, Fiedler M, Peper S, Wang Z, Aswath P, Brotto M, Varanasi V. Ionic Silicon Protects Oxidative Damage and Promotes Skeletal Muscle Cell Regeneration. International Journal of Molecular Sciences. 2021; 22(2):497. https://doi.org/10.3390/ijms22020497
Chicago/Turabian StyleAwad, Kamal, Neelam Ahuja, Matthew Fiedler, Sara Peper, Zhiying Wang, Pranesh Aswath, Marco Brotto, and Venu Varanasi. 2021. "Ionic Silicon Protects Oxidative Damage and Promotes Skeletal Muscle Cell Regeneration" International Journal of Molecular Sciences 22, no. 2: 497. https://doi.org/10.3390/ijms22020497
APA StyleAwad, K., Ahuja, N., Fiedler, M., Peper, S., Wang, Z., Aswath, P., Brotto, M., & Varanasi, V. (2021). Ionic Silicon Protects Oxidative Damage and Promotes Skeletal Muscle Cell Regeneration. International Journal of Molecular Sciences, 22(2), 497. https://doi.org/10.3390/ijms22020497






