Immunogenetic, Molecular and Microbiotic Determinants of Eosinophilic Esophagitis and Clinical Practice—A New Perspective of an Old Disease
Abstract
1. Introduction
2. Pathogenesis of Eosinophilic Esophagitis
2.1. Environmental Factors
2.2. Immunogenetics
2.3. Microbiotic Factors
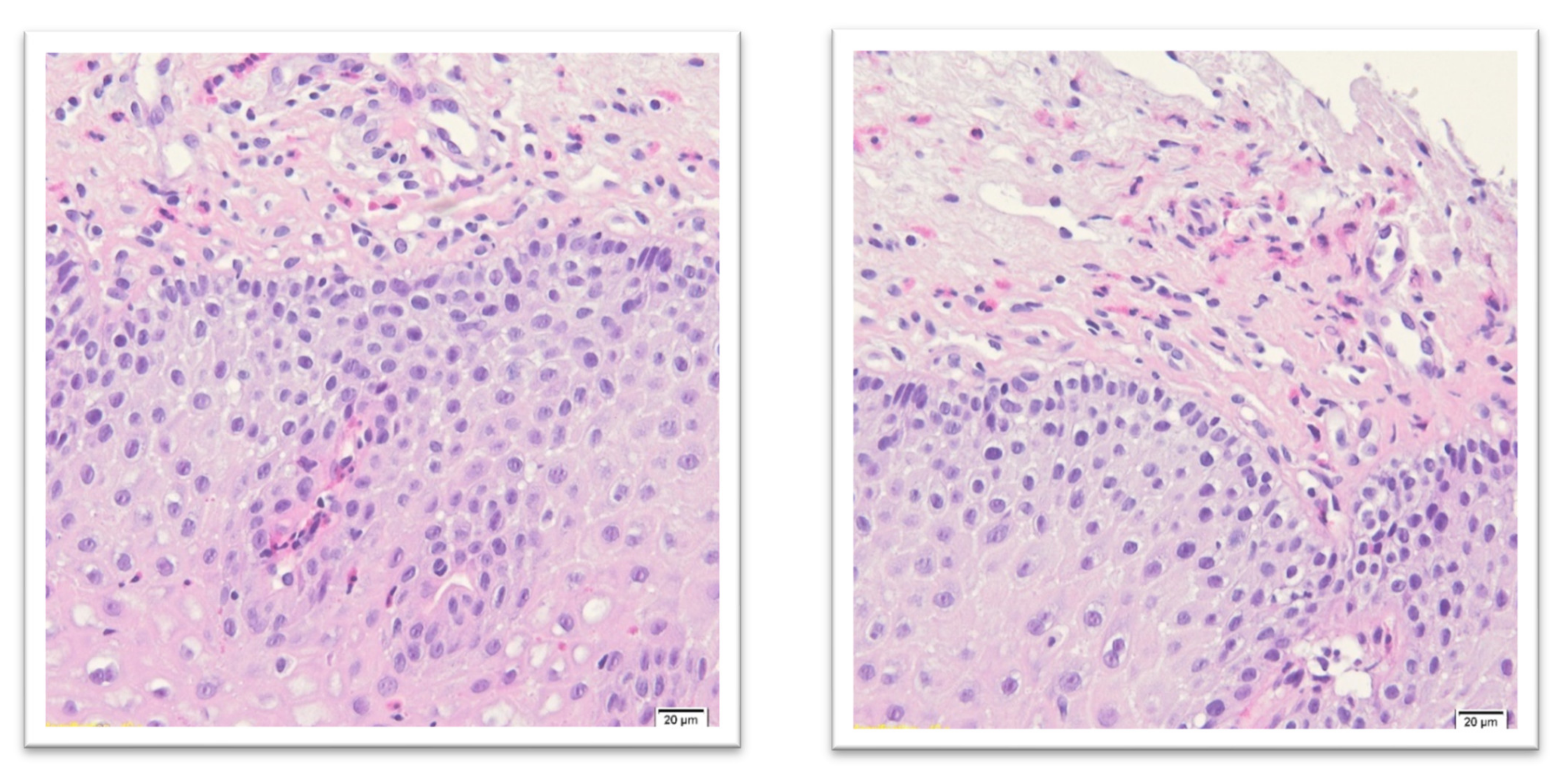
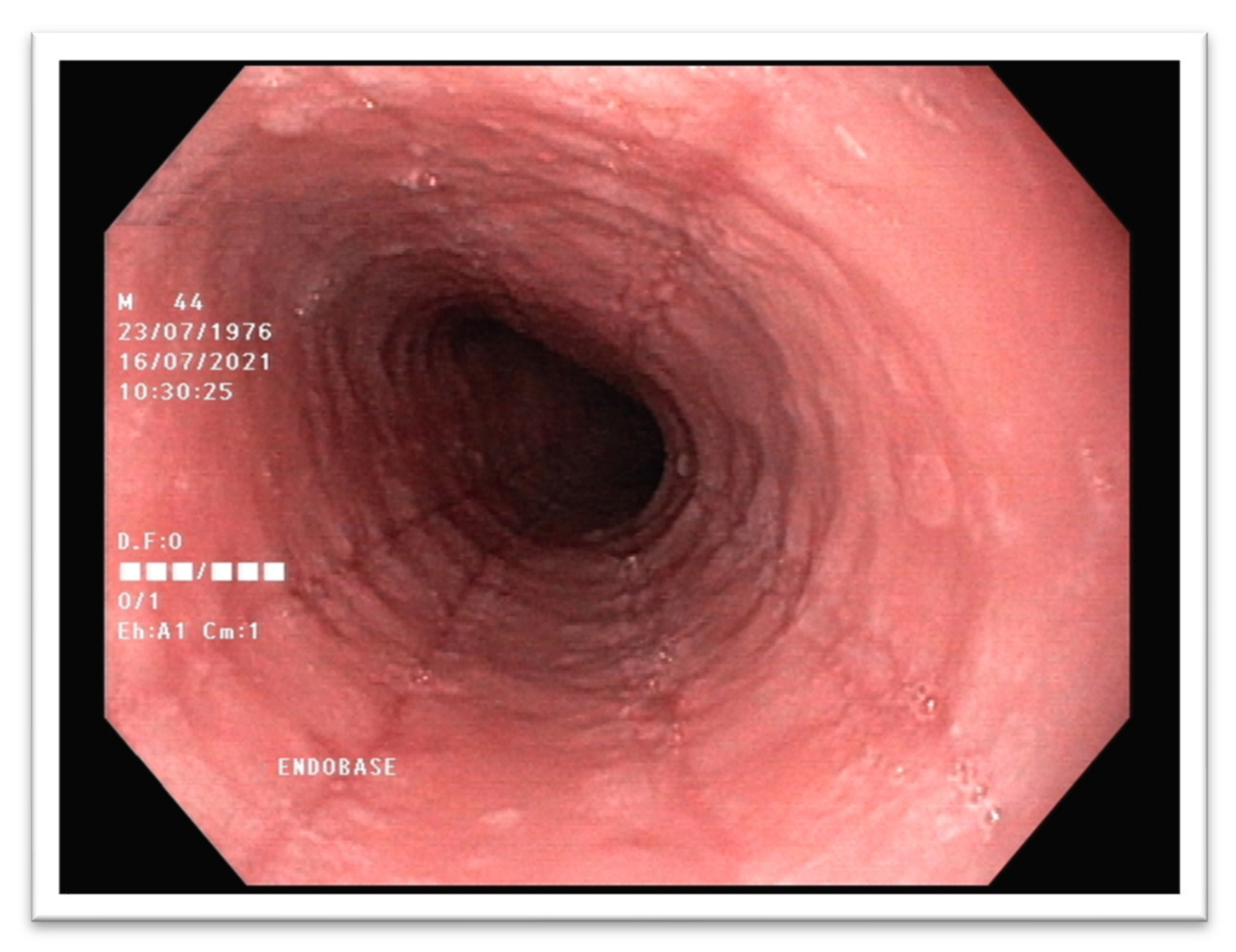
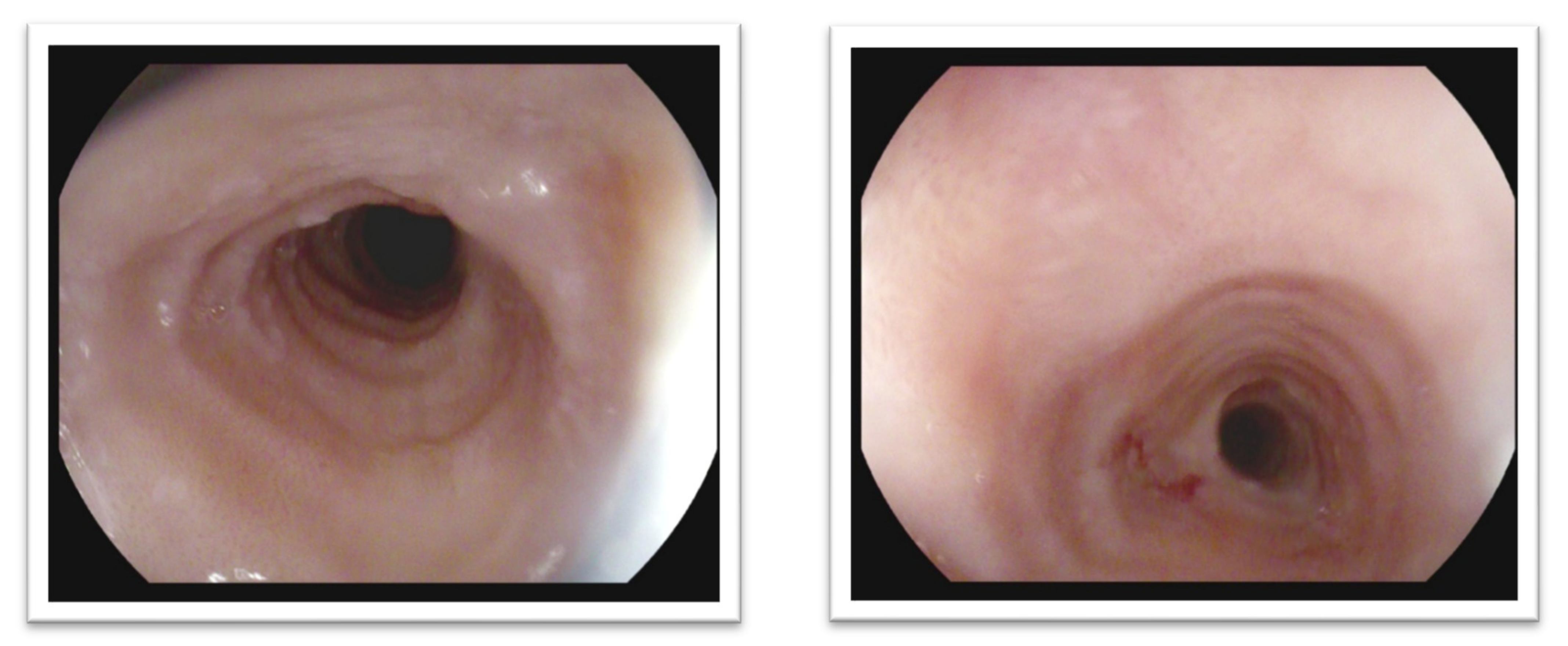
3. Diagnostic Standards of Eosinophilic Esophagitis
- Epithelial layer expanded and pale;
- Squamous cells vacuolization and spongiosis with visible desmosomes;
- Basal layer expansion;
- Papillary elongation;
- Surface disrupted with squamous cell necrosis (dense eosinophilic band);
- Eosinophils concentration (usually 30 and more/HPF) near the epithelial surface;
- In superficial epithelium eosinophilic microabscesses (more than four contiguous eosinophils);
- Eosinophil degranulation with intracellular eosinophilic dust;
- Variable submucosal hyaline fibrosis;
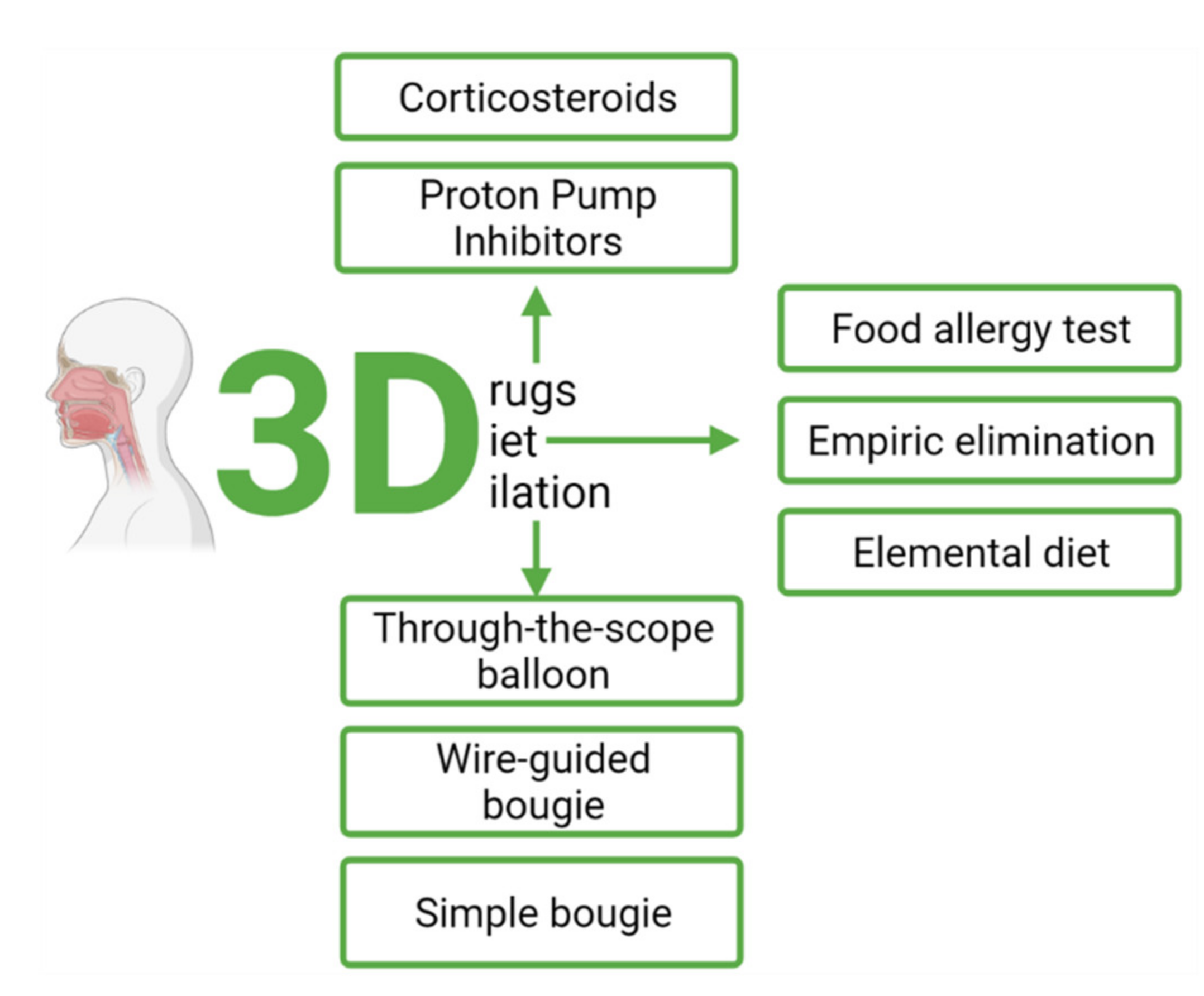
4. Therapy of Eosinophilic Esophagitis
4.1. Pharmacological Treatment
4.1.1. Proton Pump Inhibitors (PPI)
4.1.2. Corticosteroids
4.1.3. Maintenance Therapy
4.1.4. Emerging Therapies
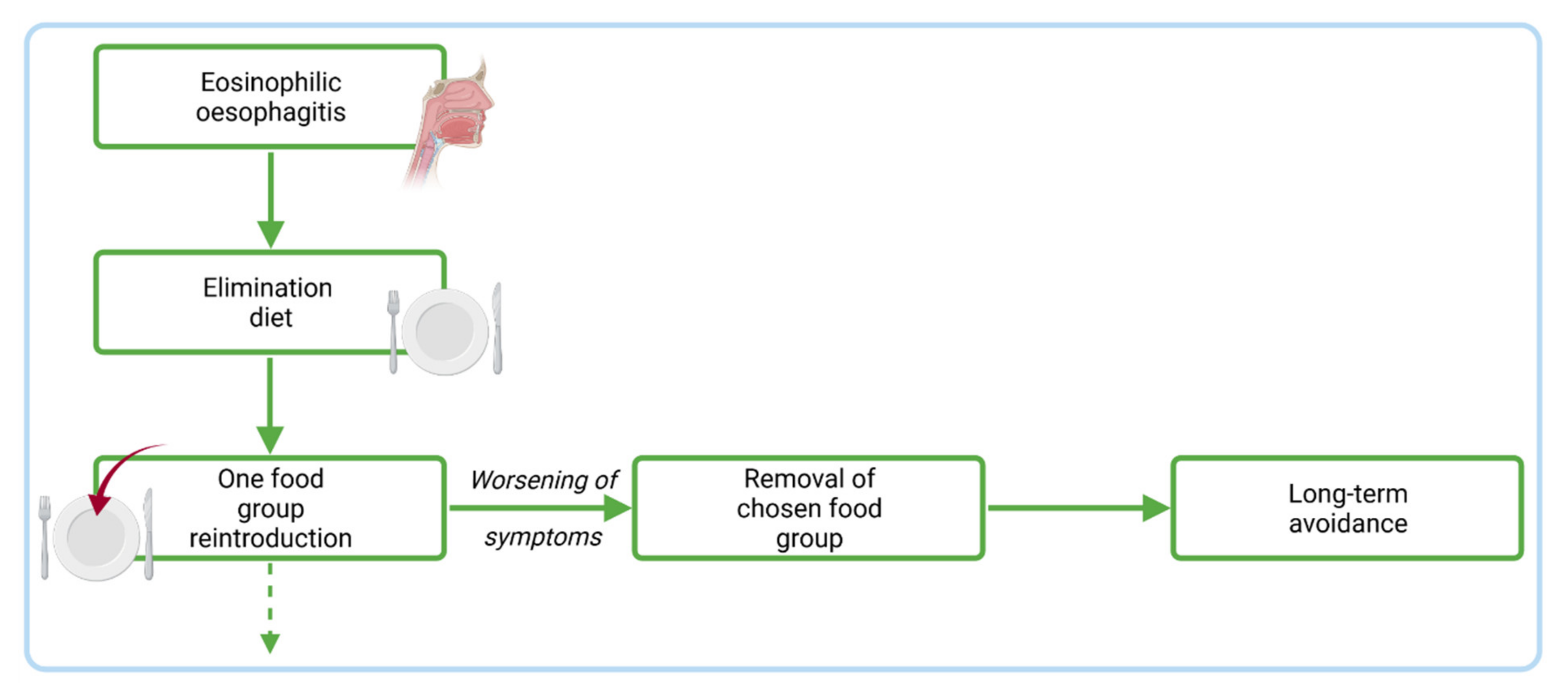
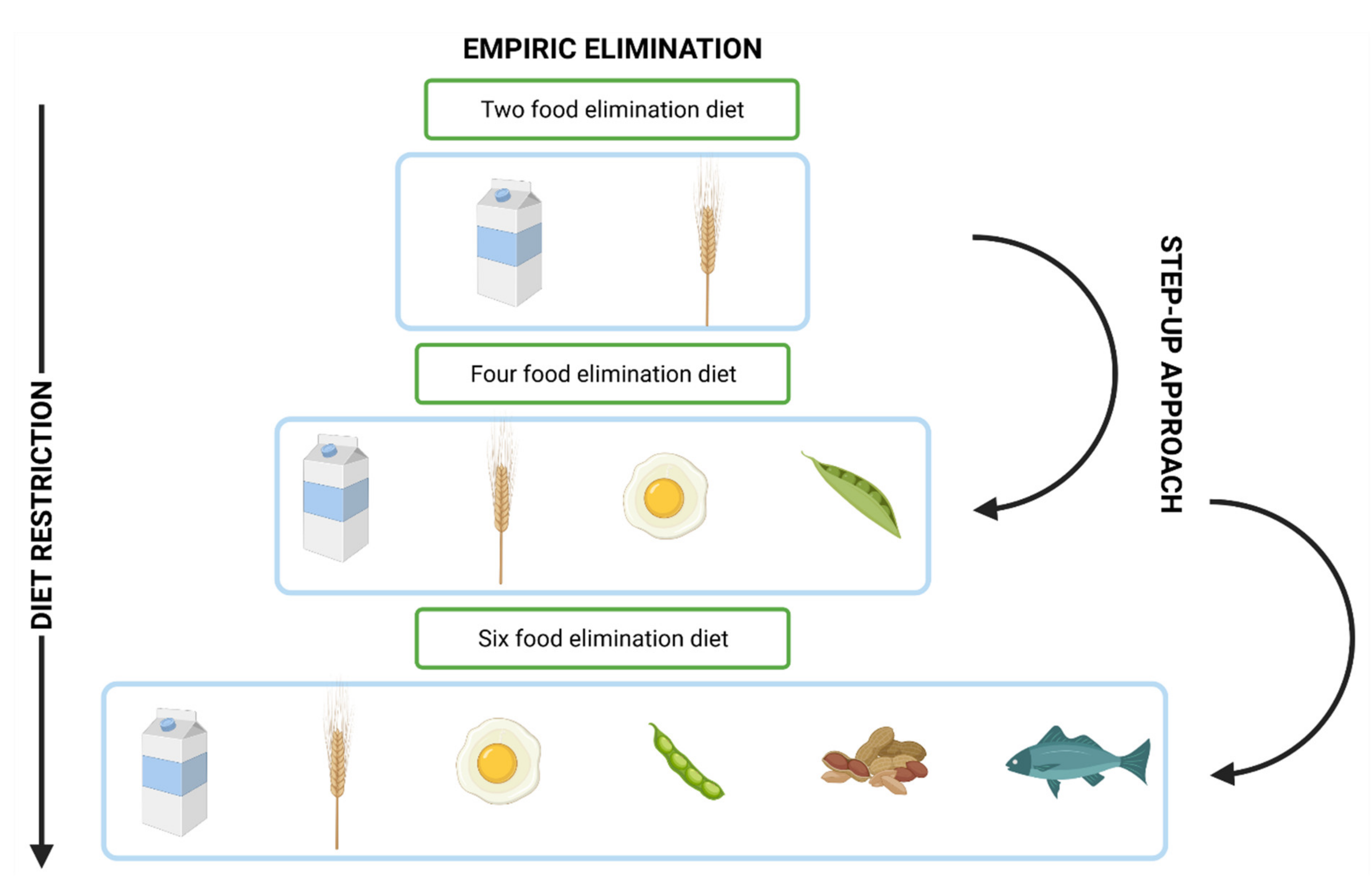
4.2. Dietary Management
4.3. Endoscopic Treatment
5. Summary—New Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Jensen, E.T.; Martin, C.F.; Shaheen, N.J.; Kappelman, M.D. Prevalence of Eosinophilic Esophagitis in the United States. Clin. Gastroenterol. Hepatol. 2014, 12, 589–596. [Google Scholar] [CrossRef]
- Kapel, R.C.; Miller, J.K.; Torres, C.; Aksoy, S.; Lash, R.; Katzka, D.A. Eosinophilic Esophagitis: A Prevalent Disease in the United States That Affects All Age Groups. Gastroenterology 2008, 134, 1316–1321. [Google Scholar] [CrossRef]
- Philpott, H.; Nandurkar, S.; Royce, S.G.; Thien, F.; Gibson, P.R. Risk Factors for Eosinophilic Esophagitis. Clin. Exp. Allergy 2014, 44, 1012–1019. [Google Scholar] [CrossRef]
- Lynch, K.L.; Dhalla, S.; Chedid, V.; Ravich, W.J.; Stein, E.M.; Montgomery, E.A.; Bochner, B.S.; Clarke, J.O. Gender Is a Determinative Factor in the Initial Clinical Presentation of Eosinophilic Esophagitis. Dis. Esophagus 2016, 29, 174–178. [Google Scholar] [CrossRef]
- Votto, M.; Marseglia, G.L.; De Filippo, M.; Brambilla, I.; Caimmi, S.M.E.; Licari, A. Early Life Risk Factors in Pediatric EoE: Could We Prevent This Modern Disease? Front. Pediatr. 2020, 8, 263. [Google Scholar] [CrossRef]
- Guajardo, J.R.; Zegarra-Bustamante, M.A.; Brooks, E.G. Does Aeroallergen Sensitization Cause or Contribute to Eosinophilic Esophagitis? Clin. Rev. Allergy Immunol. 2018, 55, 65–69. [Google Scholar] [CrossRef] [PubMed]
- González-Cervera, J.; Arias, Á.; Redondo-González, O.; Cano-Mollinedo, M.M.; Terreehorst, I.; Lucendo, A.J. Association between Atopic Manifestations and Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis. Ann. Allergy Asthma Immunol. 2017, 118, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Katzka, D.A. Eosinophilic Esophagitis. Ann. Intern. Med. 2020, 172, ITC65–ITC80. [Google Scholar] [CrossRef]
- Dowling, P.J.; Neuhaus, H.; Polk, B.I. The Role of the Environment in Eosinophilic Esophagitis. Clin. Rev. Allergy Immunol. 2019, 57, 330–339. [Google Scholar] [CrossRef]
- Silva, F.M.; Oliveira, E.E.; Ambrósio, M.G.; Ayupe, M.C.; Souza, V.P.; Gameiro, J.; Reis, D.R.; Machado, M.A.; Macedo, G.C.; Mattes, J.; et al. High-Fat Diet-Induced Obesity Worsens TH2 Immune Response and Immunopathologic Characteristics in Murine Model of Eosinophilic Oesophagitis. Clin. Exp. Allergy 2020, 50, 244–255. [Google Scholar] [CrossRef]
- Radano, M.C.; Yuan, Q.; Katz, A.; Fleming, J.T.; Kubala, S.; Shreffler, W.; Keet, C.A. Cesarean Section and Antibiotic Use Found to Be Associated with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. Pract. 2014, 2, 475–477.e1. [Google Scholar] [CrossRef]
- Leung, J.; Beukema, K.R.; Shen, A.H. Allergic Mechanisms of Eosinophilic Oesophagitis. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Žaja Franulović, O.; Lesar, T.; Busic, N.; Tešović, G. Herpes Simplex Primo-Infection in an Immunocompetent Host with Eosinophilic Esophagitis. Pediatr. Int. 2013, 55, e38–e41. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Gutierrez-Junquera, C.; Savarino, E.; Penagini, R.; Modolell, I.; Bartolo, O.; Prieto-García, A.; Mauro, A.; Alcedo, J.; Perelló, A.; et al. Helicobacter Pylori Infection Does Not Protect Against Eosinophilic Esophagitis: Results From a Large Multicenter Case-Control Study. Off. J. Am. Coll. Gastroenterol.|ACG 2018, 113, 972–979. [Google Scholar] [CrossRef]
- Jensen, E.T.; Hoffman, K.; Shaheen, N.J.; Genta, R.M.; Dellon, E.S. Esophageal Eosinophilia Is Increased in Rural Areas with Low Population Density: Results from a National Pathology Database. Am. J. Gastroenterol. 2014, 109, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Dudley, J.W.; Spergel, J.M. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J. Allergy Clin. Immunol. Pract. 2017, 5, 369–375. [Google Scholar] [CrossRef]
- Fahey, L.; Robinson, G.; Weinberger, K.; Giambrone, A.E.; Solomon, A.B. Correlation Between Aeroallergen Levels and New Diagnosis of Eosinophilic Esophagitis in New York City. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 22–25. [Google Scholar] [CrossRef]
- Suryawala, K.; Palle, S.; Altaf, M.A. Epidemiology, Clinical Presentation, and Seasonal Variation in the Diagnosis of Children with Eosinophilic Esophagitis in Oklahoma. South Med. J. 2020, 113, 37–41. [Google Scholar] [CrossRef]
- Mishra, A.; Hogan, S.P.; Brandt, E.B.; Rothenberg, M.E. An Etiological Role for Aeroallergens and Eosinophils in Experimental Esophagitis. J. Clin. Investig. 2001, 107, 83–90. [Google Scholar] [CrossRef]
- Béné, J.; Ley, D.; Roboubi, R.; Gottrand, F.; Gautier, S. Eosinophilic Esophagitis after Desensitization to Dust Mites with Sublingual Immunotherapy. Ann. Allergy Asthma Immunol. 2016, 116, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Ishihara, S.; Masuhara, M.; Mikami, H.; Okimoto, E.; Oshima, N.; Ishimura, N.; Araki, A.; Maruyama, R.; Kinoshita, Y. Development of Eosinophilic Esophagitis Following Sublingual Immunotherapy with Cedar Pollen Extract: A Case Report. Allergol. Int. 2018, 67, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Miehlke, S.; Alpan, O.; Schröder, S.; Straumann, A. Induction of Eosinophilic Esophagitis by Sublingual Pollen Immunotherapy. Case Rep. Gastroenterol. 2013, 7, 363–368. [Google Scholar] [CrossRef]
- Koutlas, N.T.; Eluri, S.; Rusin, S.; Perjar, I.; Hollyfield, J.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Impact of Smoking, Alcohol Consumption, and NSAID Use on Risk for and Phenotypes of Eosinophilic Esophagitis. Dis. Esophagus 2018, 31, dox111. [Google Scholar] [CrossRef]
- Lipka, S.; Kumar, A.; Richter, J.E. Impact of Diagnostic Delay and Other Risk Factors on Eosinophilic Esophagitis Phenotype and Esophageal Diameter. J. Clin. Gastroenterol. 2016, 50, 134–140. [Google Scholar] [CrossRef]
- Remedios, M.; Campbell, C.; Jones, D.M.; Kerlin, P. Eosinophilic Esophagitis in Adults: Clinical, Endoscopic, Histologic Findings, and Response to Treatment with Fluticasone Propionate. Gastrointest. Endosc. 2006, 63, 3–12. [Google Scholar] [CrossRef]
- Slae, M.; Persad, R.; Leung, A.J.-T.; Gabr, R.; Brocks, D.; Huynh, H.Q. Role of Environmental Factors in the Development of Pediatric Eosinophilic Esophagitis. Dig. Dis. Sci. 2015, 60, 3364–3372. [Google Scholar] [CrossRef] [PubMed]
- Ridolo, E.; Martignago, I.; Pellicelli, I.; Incorvaia, C. Assessing the Risk Factors for Refractory Eosinophilic Esophagitis in Children and Adults. Gastroenterol. Res. Pract. 2019, 2019, 1654543. [Google Scholar] [CrossRef] [PubMed]
- Elitsur, Y. Confounding Factors Affect the Pathophysiology of Eosinophilic Esophagitis. WJG 2012, 18, 4466. [Google Scholar] [CrossRef]
- Gomez Torrijos, E.; Gonzalez-Mendiola, R.; Alvarado, M.; Avila, R.; Prieto-Garcia, A.; Valbuena, T.; Borja, J.; Infante, S.; Lopez, M.P.; Marchan, E.; et al. Eosinophilic Esophagitis: Review and Update. Front. Med. 2018, 5, 247. [Google Scholar] [CrossRef]
- Straumann, A.; Bauer, M.; Fischer, B.; Blaser, K.; Simon, H.U. Idiopathic Eosinophilic Esophagitis Is Associated with a T(H)2-Type Allergic Inflammatory Response. J. Allergy Clin. Immunol. 2001, 108, 954–961. [Google Scholar] [CrossRef]
- Kottyan, L.C.; Rothenberg, M.E. Genetics of Eosinophilic Esophagitis. Mucosal Immunol. 2017, 10, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.P. Pathophysiology of Eosinophilic Esophagitis. Clin. Rev. Allergy Immunol. 2018, 55, 19–42. [Google Scholar] [CrossRef]
- Davis, B.P.; Stucke, E.M.; Khorki, M.E.; Litosh, V.A.; Rymer, J.K.; Rochman, M.; Travers, J.; Kottyan, L.C.; Rothenberg, M.E. Eosinophilic Esophagitis-Linked Calpain 14 Is an IL-13-Induced Protease That Mediates Esophageal Epithelial Barrier Impairment. JCI Insight 2016, 1, e86355. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.M.; Aceves, S.S.; Dellon, E.S.; Gupta, S.K.; Spergel, J.M.; Furuta, G.T.; Rothenberg, M.E. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldana, A.; Jaramillo-Santos, M.; Delgado, A.; Jaramillo, C.; Lúquez-Mindiola, A. Eosinophilic Esophagitis: Current Concepts in Diagnosis and Treatment. World J. Gastroenterol. 2019, 25, 4598–4613. [Google Scholar] [CrossRef]
- Giannetti, M.; Schroeder, H.A.; Zalewski, A.; Gonsalves, N.; Bryce, P.J. Dysregulation of the Wnt Pathway in Adult Eosinophilic Esophagitis. Dis. Esophagus 2015, 28, 705–710. [Google Scholar] [CrossRef]
- Blanchard, C.; Wang, N.; Stringer, K.F.; Mishra, A.; Fulkerson, P.C.; Abonia, J.P.; Jameson, S.C.; Kirby, C.; Konikoff, M.R.; Collins, M.H.; et al. Eotaxin-3 and a Uniquely Conserved Gene-Expression Profile in Eosinophilic Esophagitis. J. Clin. Investig. 2006, 116, 536–547. [Google Scholar] [CrossRef]
- Farh, K.K.-H.; Marson, A.; Zhu, J.; Kleinewietfeld, M.; Housley, W.J.; Beik, S.; Shoresh, N.; Whitton, H.; Ryan, R.J.H.; Shishkin, A.A.; et al. Genetic and Epigenetic Fine Mapping of Causal Autoimmune Disease Variants. Nature 2015, 518, 337–343. [Google Scholar] [CrossRef]
- Maurano, M.T.; Humbert, R.; Rynes, E.; Thurman, R.E.; Haugen, E.; Wang, H.; Reynolds, A.P.; Sandstrom, R.; Qu, H.; Brody, J.; et al. Systematic Localization of Common Disease-Associated Variation in Regulatory DNA. Science 2012, 337, 1190–1195. [Google Scholar] [CrossRef]
- Ortiz, R.A.; Barnes, K.C. Genetics of Allergic Diseases. Immunol. Allergy Clin. N. Am. 2015, 35, 19–44. [Google Scholar] [CrossRef]
- Sleiman, P.M.A.; Wang, M.-L.; Cianferoni, A.; Aceves, S.; Gonsalves, N.; Nadeau, K.; Bredenoord, A.J.; Furuta, G.T.; Spergel, J.M.; Hakonarson, H. GWAS Identifies Four Novel Eosinophilic Esophagitis Loci. Nat. Commun. 2014, 5, 5593. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Spergel, J.M.; Sherrill, J.D.; Annaiah, K.; Martin, L.J.; Cianferoni, A.; Gober, L.; Kim, C.; Glessner, J.; Frackelton, E.; et al. Common Variants at 5q22 Associate with Pediatric Eosinophilic Esophagitis. Nat. Genet. 2010, 42, 289–291. [Google Scholar] [CrossRef]
- Sherrill, J.D.; Gao, P.-S.; Stucke, E.M.; Blanchard, C.; Collins, M.H.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Assa’ad, A.H.; et al. Variants of Thymic Stromal Lymphopoietin and Its Receptor Associate with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2010, 126, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Fulkerson, P.C.; Finkelman, F.D.; Mingler, M.; Fischetti, C.A.; Blanchard, C.; Rothenberg, M.E. IL-13 Induces Esophageal Remodeling and Gene Expression by an Eosinophil-Independent, IL-13R Alpha 2-Inhibited Pathway. J. Immunol. 2010, 185, 660–669. [Google Scholar] [CrossRef]
- Blanchard, C.; Stucke, E.M.; Burwinkel, K.; Caldwell, J.M.; Collins, M.H.; Ahrens, A.; Buckmeier, B.K.; Jameson, S.C.; Greenberg, A.; Kaul, A.; et al. Coordinate Interaction between IL-13 and Epithelial Differentiation Cluster Genes in Eosinophilic Esophagitis. J. Immunol. 2010, 184, 4033–4041. [Google Scholar] [CrossRef]
- McAleer, M.A.; Irvine, A.D. The Multifunctional Role of Filaggrin in Allergic Skin Disease. J. Allergy Clin. Immunol. 2013, 131, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.D.; Kim, L.H.; Park, B.L.; Jung, J.H.; Kim, J.Y.; Chung, I.-Y.; Kim, J.S.; Lee, J.H.; Chung, S.H.; Kim, Y.H.; et al. Association of Eotaxin Gene Family with Asthma and Serum Total IgE. Hum. Mol. Genet. 2003, 12, 1279–1285. [Google Scholar] [CrossRef][Green Version]
- Kottyan, L.C.; Davis, B.P.; Sherrill, J.D.; Liu, K.; Rochman, M.; Kaufman, K.; Weirauch, M.T.; Vaughn, S.; Lazaro, S.; Rupert, A.M.; et al. Genome-Wide Association Analysis of Eosinophilic Esophagitis Provides Insight into the Tissue Specificity of This Allergic Disease. Nat. Genet. 2014, 46, 895–900. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Nossa, C.W.; Francois, F.; Peek, R.M.; Pei, Z. Inflammation and Intestinal Metaplasia of the Distal Esophagus Are Associated With Alterations in the Microbiome. Gastroenterology 2009, 137, 588–597. [Google Scholar] [CrossRef]
- Benitez, A.J.; Hoffmann, C.; Muir, A.B.; Dods, K.K.; Spergel, J.M.; Bushman, F.D.; Wang, M.-L. Inflammation-Associated Microbiota in Pediatric Eosinophilic Esophagitis. Microbiome 2015, 3, 23. [Google Scholar] [CrossRef]
- Hill, D.A.; Siracusa, M.C.; Abt, M.C.; Kim, B.S.; Kobuley, D.; Kubo, M.; Kambayashi, T.; LaRosa, D.F.; Renner, E.D.; Orange, J.S.; et al. Commensal Bacteria–Derived Signals Regulate Basophil Hematopoiesis and Allergic Inflammation. Nat. Med. 2012, 18, 538–546. [Google Scholar] [CrossRef]
- Jensen, E.T.; Kuhl, J.T.; Martin, L.J.; Rothenberg, M.E.; Dellon, E.S. Prenatal, Intrapartum, and Postnatal Factors Are Associated with Pediatric Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2018, 141, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.T.; Kuhl, J.T.; Martin, L.J.; Langefeld, C.D.; Dellon, E.S.; Rothenberg, M.E. Early-Life Environmental Exposures Interact with Genetic Susceptibility Variants in Pediatric Patients with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2018, 141, 632–637. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like Release of Mitochondrial DNA by Eosinophils Contributes to Antibacterial Defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef]
- Driss, V.; Legrand, F.; Hermann, E.; Loiseau, S.; Guerardel, Y.; Kremer, L.; Adam, E.; Woerly, G.; Dombrowicz, D.; Capron, M. TLR2-Dependent Eosinophil Interactions with Mycobacteria: Role of α-Defensins. Blood 2009, 113, 3235–3244. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What Role Do Proteobacteria Play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Musavian, H.S.; Butt, T.M.; Ingvorsen, C.; Thysen, A.H.; Brix, S. Chronic Obstructive Pulmonary Disease and Asthma-Associated Proteobacteria, but Not Commensal Prevotella Spp., Promote Toll-like Receptor 2-Independent Lung Inflammation and Pathology. Immunology 2015, 144, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Kassis, A.; Major, G.; Chou, C.J. Is the Gut Microbiota a New Factor Contributing to Obesity and Its Metabolic Disorders? J. Obes. 2012, 2012, 879151. [Google Scholar] [CrossRef]
- Hiremath, G.; Shilts, M.H.; Boone, H.H.; Correa, H.; Acra, S.; Tovchigrechko, A.; Rajagopala, S.V.; Das, S.R. The Salivary Microbiome Is Altered in Children With Eosinophilic Esophagitis and Correlates With Disease Activity. Clin. Transl. Gastroenterol. 2019, 10, e00039. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Johnson, S.; Geno, D.M.; Lekatz, H.R.; Lavey, C.; Alexander, J.A.; Chen, J.; Katzka, D.A. A Decreased Abundance of Clostridia Characterizes the Gut Microbiota in Eosinophilic Esophagitis. Physiol. Rep. 2019, 7, e14261. [Google Scholar] [CrossRef] [PubMed]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal Bacteria Protect against Food Allergen Sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X.-F.; Zhuang, Y.; Zhang, J.-Y.; Liu, T.; Yin, Z.; Wu, C.; Mao, X.-H.; Jia, K.-R.; Wang, F.-J.; et al. Helicobacter Pylori -Induced Th17 Responses Modulate Th1 Cell Responses, Benefit Bacterial Growth, and Contribute to Pathology in Mice. J. Immunol. 2010, 184, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Amedei, A. The Neutrophil-Activating Protein of Helicobacter Pylori Promotes Th1 Immune Responses. J. Clin. Investig. 2006, 116, 1092–1101. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Oouchi, S.; Fujisawa, T. Eosinophilic gastrointestinal diseases - Pathogenesis, diagnosis, and treatment. Allergol. Int. 2019, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.; Adachi, K.; Aimi, M.; Ishimura, N.; Sato, S.; Ishihara, S.; Kinoshita, Y. Case-Control Study of Association of Eosinophilic Gastrointestinal Disorders with Helicobacter Pylori Infection in Japan. J. Clin. Biochem. Nutr. 2013, 53, 60–62. [Google Scholar] [CrossRef]
- Oertli, M.; Sundquist, M.; Hitzler, I.; Engler, D.B.; Arnold, I.C.; Reuter, S.; Maxeiner, J.; Hansson, M.; Taube, C.; Quiding-Järbrink, M.; et al. DC-Derived IL-18 Drives Treg Differentiation, Murine Helicobacter Pylori–Specific Immune Tolerance, and Asthma Protection. J. Clin. Investig. 2012, 122, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Dehzad, N.; Reuter, S.; Martin, H.; Becher, B.; Taube, C.; Müller, A. Helicobacter Pylori Infection Prevents Allergic Asthma in Mouse Models through the Induction of Regulatory T Cells. J. Clin. Investig. 2011, 121, 3088–3093. [Google Scholar] [CrossRef]
- Shah, S.C.; Tepler, A.; Peek, R.M., Jr.; Colombel, J.-F.; Hirano, I.; Narula, N. Association Between Helicobacter Pylori Exposure and Decreased Odds of Eosinophilic Esophagitis—A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 2185–2198. [Google Scholar] [CrossRef]
- Holvoet, S.; Zuercher, A.W.; Julien-Javaux, F.; Perrot, M.; Mercenier, A. Characterization of Candidate Anti-Allergic Probiotic Strains in a Model of Th2-Skewed Human Peripheral Blood Mononuclear Cells. Int. Arch. Allergy Immunol. 2013, 161, 142–154. [Google Scholar] [CrossRef]
- Holvoet, S.; Doucet-Ladevèze, R.; Perrot, M.; Barretto, C.; Nutten, S.; Blanchard, C. Beneficial Effect of Lactococcus Lactis NCC 2287 in a Murine Model of Eosinophilic Esophagitis. Allergy 2016, 71, 1753–1761. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Amil Dias, J.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on Eosinophilic Esophagitis: Evidence-Based Statements and Recommendations for Diagnosis and Management in Children and Adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef]
- Feakins, R.M. Non-Neoplastic Pathology of the Gastrointestinal Tract: A Practical Guide to Biopsy Diagnosis; Feakins, R.M., Ed.; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Yantiss, R.K.; Panarelli, N.C.; Lamps, L.W. Non-Neoplastic Disorders of the Gastrointestinal Tract. American Registry of Pathology: Arlington, VA, USA, 2021. [Google Scholar]
- Arnold, C.; Lam-Himlin, D.; Montgomery, E.A. Atlas of Gastrointestinal Pathology: A Pattern Based Approach to Non-Neoplastic Biopsies, 5th ed.LWW: Philadelphia, PA, USA, 2014. [Google Scholar]
- Warners, M.J.; Ambarus, C.A.; Bredenoord, A.J.; Verheij, J.; Lauwers, G.Y.; Walsh, J.C.; Katzka, D.A.; Nelson, S.; van Viegen, T.; Furuta, G.T.; et al. Reliability of Histologic Assessment in Patients with Eosinophilic Oesophagitis. Aliment. Pharmacol. Ther. 2018, 47, 940–950. [Google Scholar] [CrossRef]
- Collins, M.H.; Martin, L.J.; Alexander, E.S.; Boyd, J.T.; Sheridan, R.; He, H.; Pentiuk, S.; Putnam, P.E.; Abonia, J.P.; Mukkada, V.A.; et al. Newly Developed and Validated Eosinophilic Esophagitis Histology Scoring System and Evidence That It Outperforms Peak Eosinophil Count for Disease Diagnosis and Monitoring. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Protheroe, C.; Woodruff, S.A.; de Petris, G.; Mukkada, V.; Ochkur, S.I.; Janarthanan, S.; Lewis, J.C.; Pasha, S.; Lunsford, T.; Harris, L.; et al. A Novel Histologic Scoring System to Evaluate Mucosal Biopsies from Patients with Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2009, 7, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Cotton, C.C.; Gebhart, J.H.; Higgins, L.L.; Beitia, R.; Woosley, J.T.; Shaheen, N.J. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin. Gastroenterol. Hepatol. 2016, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Eckardt, A.J.; Fisseler-Eckhoff, A.; Haas, S.; Gockel, I.; Wehrmann, T. Endoscopic Findings in Patients with Schatzki Rings: Evidence for an Association with Eosinophilic Esophagitis. World J. Gastroenterol. 2012, 18, 6960–6966. [Google Scholar] [CrossRef] [PubMed]
- Katzka, D.A. Recent Advances in Understanding/Managing Eosinophilic Esophagitis in Adults. F1000Research 2015, 4, 592. [Google Scholar] [CrossRef]
- Furuta, G.T.; Kagalwalla, A.F.; Lee, J.J.; Alumkal, P.; Maybruck, B.T.; Fillon, S.; Masterson, J.C.; Ochkur, S.; Protheroe, C.; Moore, W.; et al. The Oesophageal String Test: A Novel, Minimally Invasive Method Measures Mucosal Inflammation in Eosinophilic Oesophagitis. Gut 2013, 62, 1395–1405. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Straumann, A.; Panczak, R.; Coslovsky, M.; Kuehni, C.E.; Maurer, E.; Haas, N.A.; Romero, Y.; Hirano, I.; Alexander, J.A.; et al. Development and Validation of a Symptom-Based Activity Index for Adults with Eosinophilic Esophagitis. Gastroenterology 2014, 147, 1255–1266. [Google Scholar] [CrossRef]
- Warners, M.J.; Hindryckx, P.; Levesque, B.G.; Parker, C.E.; Shackelton, L.M.; Khanna, R.; Sandborn, W.J.; D’Haens, G.R.; Feagan, B.G.; Bredenoord, A.J.; et al. Systematic Review: Disease Activity Indices in Eosinophilic Esophagitis. Am. J. Gastroenterol. 2017, 112, 1658–1669. [Google Scholar] [CrossRef]
- Dellon, E.S.; Irani, A.-M.; Hill, M.R.; Hirano, I. Development and Field Testing of a Novel Patient-Reported Outcome Measure of Dysphagia in Patients with Eosinophilic Esophagitis. Aliment. Pharmacol. Ther. 2013, 38, 634–642. [Google Scholar] [CrossRef]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Katzka, D.A. Proton-Pump Inhibitor-Responsive Esophageal Eosinophilia. Curr. Opin. Gastroenterol. 2014, 30, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Sachs, G. Pharmacology of Proton Pump Inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, E.; Huo, X.; Yu, C.; Zhang, Q.; Pham, T.H.; Wang, D.H.; Spechler, S.J.; Souza, R.F. Omeprazole Blocks STAT6 Binding to the Eotaxin-3 Promoter in Eosinophilic Esophagitis Cells. PLoS ONE 2012, 7, e50037. [Google Scholar] [CrossRef]
- Cheng, E.; Zhang, X.; Huo, X.; Yu, C.; Zhang, Q.; Wang, D.H.; Spechler, S.J.; Souza, R.F. Omeprazole Blocks Eotaxin-3 Expression by Oesophageal Squamous Cells from Patients with Eosinophilic Oesophagitis and GORD. Gut 2013, 62, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A.; Thomas, K.L.; Hilden, K.; Emerson, L.L.; Wills, J.C.; Fang, J.C. Comparison of Esomeprazole to Aerosolized, Swallowed Fluticasone for Eosinophilic Esophagitis. Dig. Dis. Sci. 2010, 55, 1313–1319. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Arias, Á.; Molina-Infante, J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 13–22. [Google Scholar] [CrossRef]
- Rank, M.A.; Sharaf, R.N.; Furuta, G.T.; Aceves, S.S.; Greenhawt, M.; Spergel, J.M.; Falck-Ytter, Y.T.; Dellon, E.S.; AGA Institute; Joint Task Force on Allergy-Immunology Practice Parameters collaborators; et al. Technical Review on the Management of Eosinophilic Esophagitis: A Report from the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Ann. Allergy Asthma Immunol. 2020, 124, 424–440. [Google Scholar] [CrossRef]
- Podboy, A.; Katzka, D.A.; Enders, F.; Larson, J.J.; Geno, D.; Kryzer, L.; Alexander, J. Oesophageal Narrowing on Barium Oesophagram Is More Common in Adult Patients with Eosinophilic Oesophagitis than PPI-Responsive Oesophageal Eosinophilia. Aliment. Pharmacol. Ther. 2016, 43, 1168–1177. [Google Scholar] [CrossRef]
- Dellon, E.S.; Sheikh, A.; Speck, O.; Woodward, K.; Whitlow, A.B.; Hores, J.M.; Ivanovic, M.; Chau, A.; Woosley, J.T.; Madanick, R.D.; et al. Viscous Topical Is More Effective than Nebulized Steroid Therapy for Patients with Eosinophilic Esophagitis. Gastroenterology 2012, 143, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Butz, B.K.; Wen, T.; Gleich, G.J.; Furuta, G.T.; Spergel, J.; King, E.; Kramer, R.E.; Collins, M.H.; Stucke, E.; Mangeot, C.; et al. Efficacy, Dose Reduction, and Resistance to High-Dose Fluticasone in Patients with Eosinophilic Esophagitis. Gastroenterology 2014, 147, 324–333. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Miehlke, S.; Schlag, C.; Vieth, M.; von Arnim, U.; Molina-Infante, J.; Hartmann, D.; Bredenoord, A.J.; Ciriza de Los Rios, C.; Schubert, S.; et al. Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology 2019, 157, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Ferrando-Lamana, L.; Ripoll, C.; Hernandez-Alonso, M.; Mateos, J.M.; Fernandez-Bermejo, M.; Dueñas, C.; Fernandez-Gonzalez, N.; Quintana, E.M.; Gonzalez-Nuñez, M.A. Esophageal Eosinophilic Infiltration Responds to Proton Pump Inhibition in Most Adults. Clin. Gastroenterol. Hepatol. 2011, 9, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Junquera, C.; Fernández-Fernández, S.; Cilleruelo, M.L.; Rayo, A.; Echeverría, L.; Quevedo, S.; Bracamonte, T.; Román, E. High Prevalence of Response to Proton-Pump Inhibitor Treatment in Children With Esophageal Eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torrijos, E.; García-Rodríguez, R.; Castro-Jiménez, A.; Rodríguez-Sanchez, J.; Méndez Díaz, Y.; Molina-Infante, J. The Efficacy of Step-down Therapy in Adult Patients with Proton Pump Inhibitor-Responsive Oesophageal Eosinophilia. Aliment. Pharmacol. Ther. 2016, 43, 534–540. [Google Scholar] [CrossRef]
- Andreae, D.A.; Hanna, M.G.; Magid, M.S.; Malerba, S.; Andreae, M.H.; Bagiella, E.; Chehade, M. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children With Eosinophilic Esophagitis. Am. J. Gastroenterol. 2016, 111, 1187–1197. [Google Scholar] [CrossRef]
- Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Schlag, C.; Biedermann, L.; Vaquero, C.S.; Ciriza de Los Rios, C.; Schmoecker, C.; Madisch, A.; et al. Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients With Eosinophilic Esophagitis. Gastroenterology 2020, 159, 1672–1685. [Google Scholar] [CrossRef]
- Schreiner, P.; Biedermann, L.; Greuter, T.; Wright, B.L.; Straumann, A. How to Approach Adult Patients with Asymptomatic Esophageal Eosinophilia. Dis. Esophagus 2021, 34, doaa105. [Google Scholar] [CrossRef]
- Hirano, I.; Collins, M.H.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.M.; Straumann, A.; Safroneeva, E.; Grimm, M.; Smith, H.; et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology 2019, 156, 592–603. [Google Scholar] [CrossRef]
- Hirano, I.; Dellon, E.S.; Hamilton, J.D.; Collins, M.H.; Peterson, K.; Chehade, M.; Schoepfer, A.M.; Safroneeva, E.; Rothenberg, M.E.; Falk, G.W.; et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults with Active Eosinophilic Esophagitis. Gastroenterology 2020, 158, 111–122.e2. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.A.; Ravi, K.; Enders, F.T.; Geno, D.M.; Kryzer, L.A.; Mara, K.C.; Smyrk, T.C.; Katzka, D.A. Montelukast Does Not Maintain Symptom Remission After Topical Steroid Therapy for Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2017, 15, 214–221. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Lu, X.L.; Mounmin, F.A. Diagnosis and Treatment of Esophageal Candidiasis: Current Updates. Can. J. Gastroenterol. Hepatol. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Greuter, T.; Hirano, I.; Dellon, E.S. Emerging Therapies for Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2020, 145, 38–45. [Google Scholar] [CrossRef]
- De Rooij, W.E.; Dellon, E.S.; Parker, C.E.; Feagan, B.G.; Jairath, V.; Ma, C.; Bredenoord, A.J. Pharmacotherapies for the Treatment of Eosinophilic Esophagitis: State of the Art Review. Drugs 2019, 79, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.E. Current Management of Eosinophilic Esophagitis 2015. J. Clin. Gastroenterol. 2016, 50, 99–110. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Nakayama, A.; Abe, J.; Matsuo, M. Efficacy of a Short-Term Six-Food Elimination Diet and Reintroduction Therapy in Pediatric Eosinophilic Gastroenteritis. Intern. Med. 2020, 59, 1379–1385. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Gonzalez-Cordero, P.L.; Arias, A.; Lucendo, A.J. Update on Dietary Therapy for Eosinophilic Esophagitis in Children and Adults. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; González-Cervera, J.; Tenias, J.M.; Lucendo, A.J. Efficacy of Dietary Interventions for Inducing Histologic Remission in Patients with Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis. Gastroenterology 2014, 146, 1639–1648. [Google Scholar] [CrossRef]
- Chen, J.W.; Kao, J.Y. Eosinophilic Esophagitis: Update on Management and Controversies. BMJ 2017, 359, j4482. [Google Scholar] [CrossRef]
- Biedermann, L.; Straumann, A. Medical and Dietary Treatments in Eosinophilic Esophagitis. Curr. Opin. Pharmacol. 2018, 43, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I. Future Directions in Eosinophilic Esophagitis. Gastrointest. Endosc. Clin. N. Am. 2018, 28, 111–122. [Google Scholar] [CrossRef]
- Cotton, C.C.; Durban, R.; Dellon, E.S. Illuminating Elimination Diets: Controversies Regarding Dietary Treatment of Eosinophilic Esophagitis. Dig. Dis. Sci. 2019, 64, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Singla, M.B.; Moawad, F.J. An Overview of the Diagnosis and Management of Eosinophilic Esophagitis. Clin. Transl. Gastroenterol. 2016, 7, e155. [Google Scholar] [CrossRef]
- Hirano, I.; Chan, E.S.; Rank, M.A.; Sharaf, R.N.; Stollman, N.H.; Stukus, D.R.; Wang, K.; Greenhawt, M.; Falck-Ytter, Y.T.; Chachu, K.A.; et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020, 158, 1776–1786. [Google Scholar] [CrossRef]
- Reed, C.C.; Wolf, W.A.; Cotton, C.C.; Rusin, S.; Perjar, I.; Hollyfield, J.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Optimal Histologic Cutpoints for Treatment Response in Patients With Eosinophilic Esophagitis: Analysis of Data from a Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2018, 16, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J. Eosinophilic Esophagitis: Current Evidence-Based Diagnosis and Treatment in Children and Adults. Minerva Gastroenterol. Dietol. 2018, 64, 62–74. [Google Scholar] [CrossRef]
- Moawad, F.J.; Molina-Infante, J.; Lucendo, A.J.; Cantrell, S.E.; Tmanova, L.; Douglas, K.M. Systematic Review with Meta-Analysis: Endoscopic Dilation Is Highly Effective and Safe in Children and Adults with Eosinophilic Oesophagitis. Aliment. Pharmacol. Ther. 2017, 46, 96–105. [Google Scholar] [CrossRef]
- Dellon, E.S.; Kim, H.P.; Sperry, S.L.W.; Rybnicek, D.A.; Woosley, J.T.; Shaheen, N.J. A Phenotypic Analysis Shows That Eosinophilic Esophagitis Is a Progressive Fibrostenotic Disease. Gastrointest. Endosc. 2014, 79, 577–585.e4. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gibbs, W.B.; Rubinas, T.C.; Fritchie, K.J.; Madanick, R.D.; Woosley, J.T.; Shaheen, N.J. Esophageal Dilation in Eosinophilic Esophagitis: Safety and Predictors of Clinical Response and Complications. Gastrointest. Endosc. 2010, 71, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Runge, T.M.; Eluri, S.; Cotton, C.C.; Burk, C.M.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Outcomes of Esophageal Dilation in Eosinophilic Esophagitis: Safety, Efficacy, and Persistence of the Fibrostenotic Phenotype. Am. J. Gastroenterol. 2016, 111, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Sasaki, Y.; Yagi, M.; Yaoita, T.; Nishise, S.; Ueno, Y. Diagnosis and Treatment of Eosinophilic Esophagitis in Clinical Practice. Clin. J. Gastroenterol. 2017, 10, 87–102. [Google Scholar] [CrossRef] [PubMed]
| Gene | Location | Expression in EoE | Impact on EoE | Cell Type |
|---|---|---|---|---|
| TSLP Thymic Stromal Lymphopoietin | chr5:111,070,062–111,078,026 | ↑ | increased Th2 responses induce Th2 cell development and activate eosinophils and basophils | esophageal epithelial cells |
| CCL26 C-C Motif Chemokine Ligand 26 | chr7:75,769,524–75,791,597 | ↑ | eosinophil recruitment | esophageal epithelial cells |
| TGFB1 Transforming Growth Factor Beta 1 | chr19:41,301,587–41,353,922 | ↑ | response to steroid, eosinophil adhesion, esophageal remodeling | eosinophils and mast cells |
| CRLF2 Cytokine Receptor Like Factor 2 2 | chrX:1,190,437–1,212,762 | Lack of data | increased Th2 responses | eosinophils, mast cells, dendric cells, basophils |
| WDR36 WD Repeat Domain 36 | chr5:111,091,716–111,130,502 | unchanged | induces Th2 cell development and activates eosinophils and basophils | esophageal epithelial cells |
| STAT6 Signal Transducer And Activator Of Transcription 6 | chr12:57,095,408–57,132,139 | ↑ | primary mediator of IL-4 and IL-13 signaling, the downstream signaling mediator of IL-4Ralpha | eosinophils |
| GATA-3 GATA Binding Protein 3 | chr10:8,045,378–8,075,198 | ↑ | transcriptional regulators that drive differentiation of Th0 CD4+ lymphocytes to Th2 lineages | esophageal epithelial cells |
| TBX21 T-Box Transcription Factor 21 | chr17:47,733,236–47,746,122 | ↑ | transcriptional regulators that drive differentiation of Th0 CD4+ lymphocytes to Th1 lineages | esophageal epithelial cells |
| FLG Filaggrin | chr1:152,302,165–152,325,239 | ↓ | Reduced barrier, increased sensitization | unknown |
| Il-13 Interleukin 13 | chr5:132,656,263–132,661,110 | ↑ | responsible for pathophysiological changes in the esophageal epithelium | esophageal epithelial cells |
| Il-5 Interleukin 5 | chr5:132,539,194–132,556,864 | ↑ | major role in eosinophil-related disorders, eosinophil differentiation factor, and activator | esophageal epithelial cells |
| Il-4 Interleukin 4 | chr5:132,673,986–132,682,678 | ↑ | initiating the Th2 response through differentiation of naive T helper cells into Th2 type cells | esophageal epithelial cells |
| MUC5AC Mucin 5AC, Oligomeric Mucus/Gel-Forming | chr11:1,157,953–1,201,138 | ↑ | protects the mucosa from infection and chemical damage | esophageal epithelial cells |
| SMAD2 SMAD Family Member 2 | chr18:47,808,957–47,931,188 and | ↑ | associated with the recruitment of eosinophils | eosinophils, esophageal epithelial cells mast cells |
| SMAD3 SMAD Family Member 3 | chr15:67,063,763–67,195,195 | ↑ | associated with the recruitment of eosinophils | eosinophils, esophageal epithelial cells mast cells |
| VCAM1 vascular cell adhesion molecule | chr1:100,719,742–100,739,045 | ↑ | increased inflammatory cell binding to the endothelium of esophageal vessels, facilitating infiltration of eosinophils into the esophagus | eosinophils, mast cells, endothelial cells |
| CAPN14 Calpain 14 | chr2:31,173,056–31,233,970 | ↑ | Induced by Il-13, involved in epithelial homeostasis and repair, possesses STAT6 binding sites | esophageal epithelial cells |
| ICAM1 intracellular adhesion molecule 1 | chr19:10,271,093–10,286,615 | ↑ | implicated in the tissue recruitment of eosinophils and mast cells | eosinophils, mast cells, endothelial cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanikowska, A.; Hryhorowicz, S.; Rychter, A.M.; Kucharski, M.A.; Zawada, A.; Iwanik, K.; Eder, P.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. Immunogenetic, Molecular and Microbiotic Determinants of Eosinophilic Esophagitis and Clinical Practice—A New Perspective of an Old Disease. Int. J. Mol. Sci. 2021, 22, 10830. https://doi.org/10.3390/ijms221910830
Kanikowska A, Hryhorowicz S, Rychter AM, Kucharski MA, Zawada A, Iwanik K, Eder P, Słomski R, Dobrowolska A, Krela-Kaźmierczak I. Immunogenetic, Molecular and Microbiotic Determinants of Eosinophilic Esophagitis and Clinical Practice—A New Perspective of an Old Disease. International Journal of Molecular Sciences. 2021; 22(19):10830. https://doi.org/10.3390/ijms221910830
Chicago/Turabian StyleKanikowska, Alina, Szymon Hryhorowicz, Anna Maria Rychter, Marcin A. Kucharski, Agnieszka Zawada, Katarzyna Iwanik, Piotr Eder, Ryszard Słomski, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "Immunogenetic, Molecular and Microbiotic Determinants of Eosinophilic Esophagitis and Clinical Practice—A New Perspective of an Old Disease" International Journal of Molecular Sciences 22, no. 19: 10830. https://doi.org/10.3390/ijms221910830
APA StyleKanikowska, A., Hryhorowicz, S., Rychter, A. M., Kucharski, M. A., Zawada, A., Iwanik, K., Eder, P., Słomski, R., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). Immunogenetic, Molecular and Microbiotic Determinants of Eosinophilic Esophagitis and Clinical Practice—A New Perspective of an Old Disease. International Journal of Molecular Sciences, 22(19), 10830. https://doi.org/10.3390/ijms221910830







