Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)–NF-κB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats
Abstract
1. Introduction
2. Results
2.1. Effect of Pantoprazole on Different Biochemical Parameters
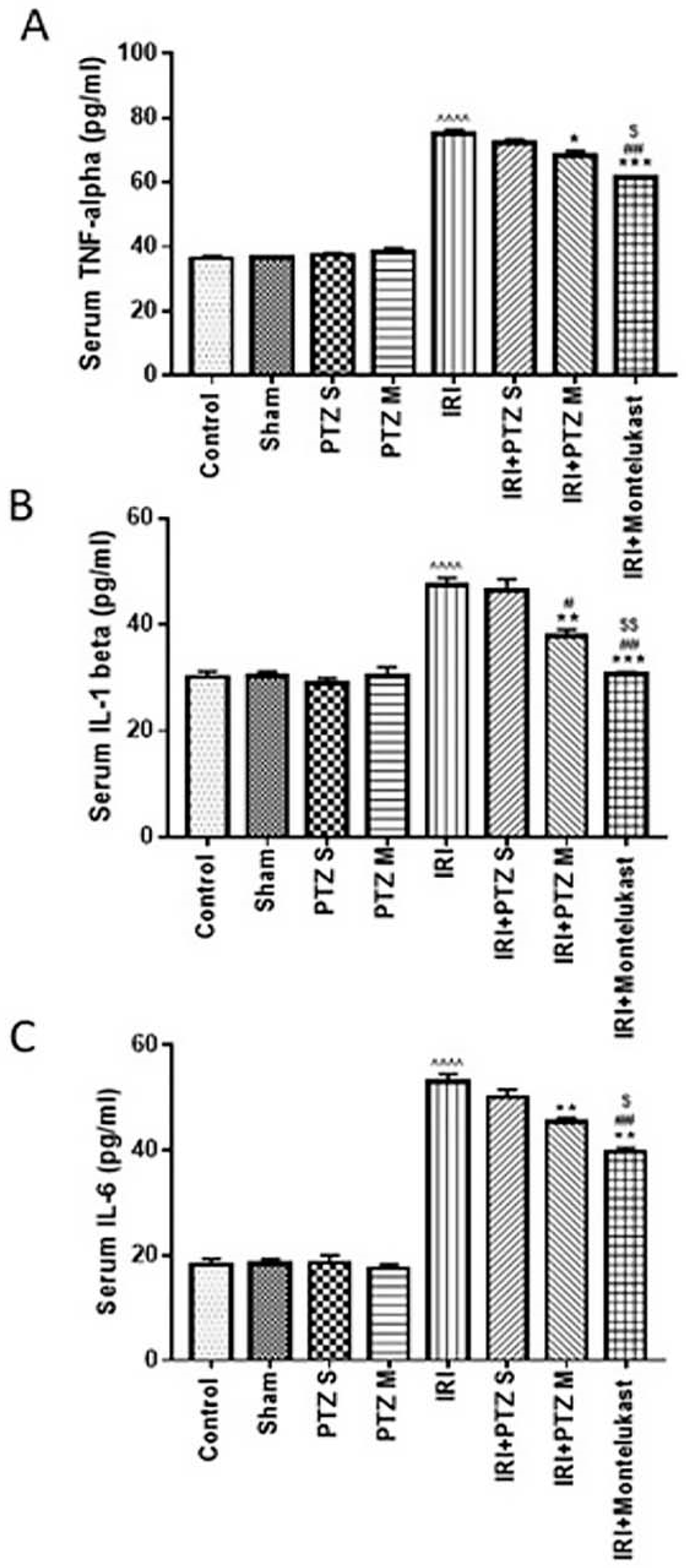
2.2. Serum Levels of the Inflammatory Cytokines (TNF-α, IL-1β, and IL-6)
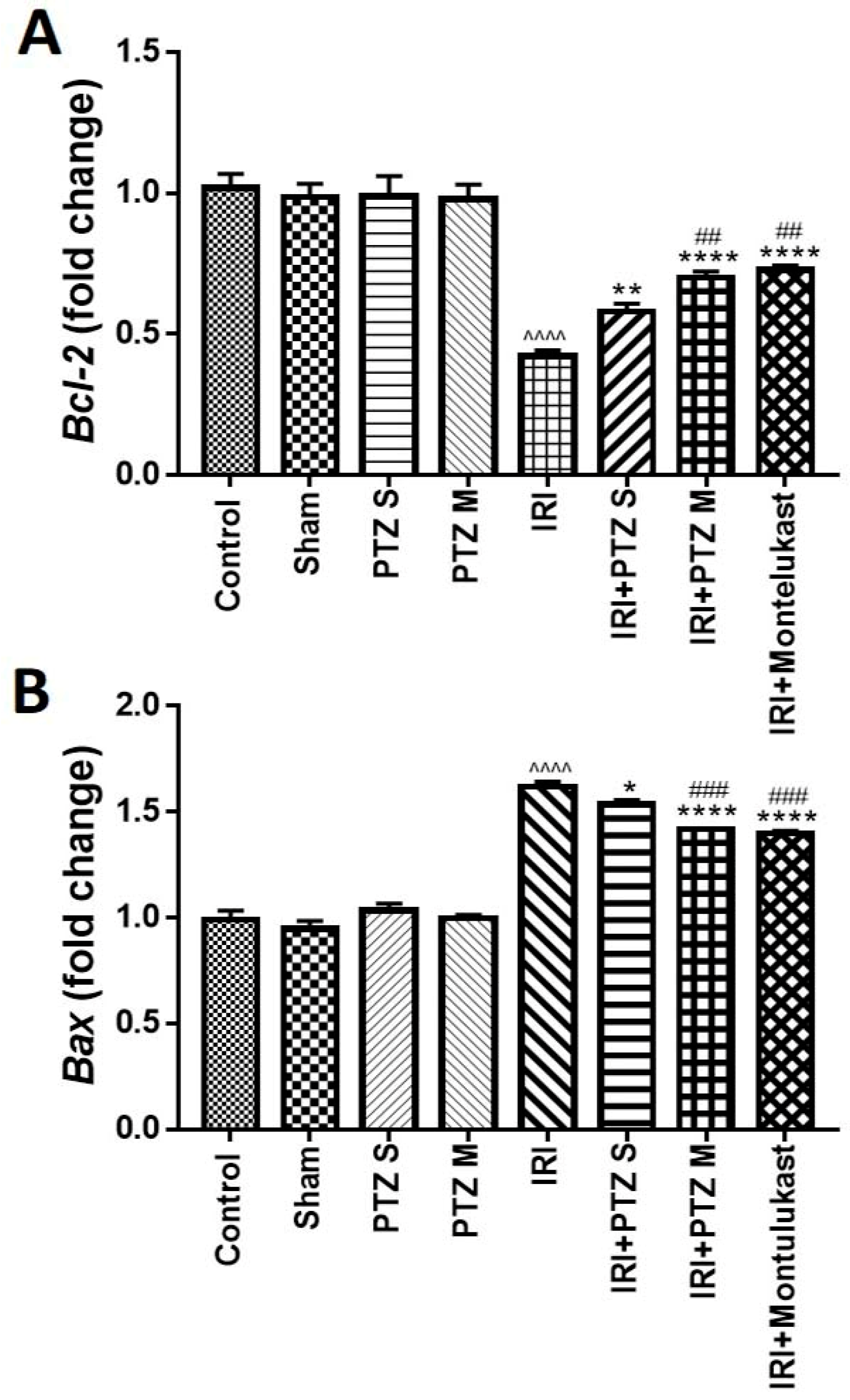
2.3. Expression of B-Cell Lymphoma 2 (Bcl-2) and Bcl-2 Associated X-Protein (Bax) Genes
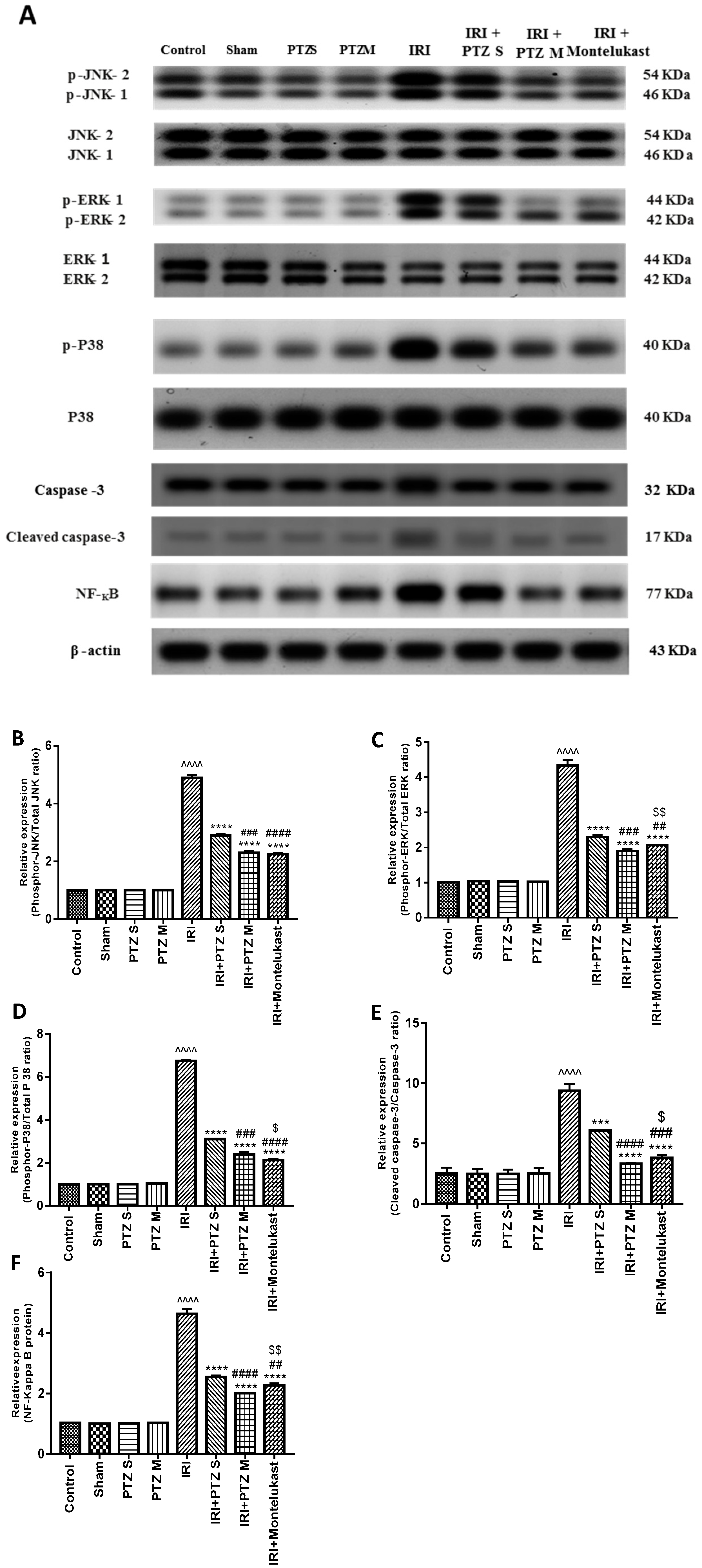
2.4. Expression of p-JNK1/2, p-ERK1/2, p-P38, Cleaved Caspase-3, and NF-κB Proteins
2.5. Histopathological Examination for the Renal Tissues of Different Groups
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals and Care
4.3. Experimental Design
4.4. Biochemical Analysis
4.5. Measurement of Serum Levels of Inflammatory Cytokines
4.6. Quantitative Real-Time Polymerase Chain Reaction
4.7. Western Blotting Analysis
4.8. Histological Examination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ichimaru, N.; Yazawa, K.; Takahara, S. Kidney transplantation: How shall we deal with marginal cases? Future prospects from basic research. Hinyokika Kiyo. Acta Urol. Jpn. 2010, 56, 481–484. [Google Scholar]
- Versteilen, A.; Di Maggio, F.; Leemreis, J.; Groeneveld, A.; Musters, R.; Sipkema, P. Molecular mechanisms of acute renal failure following ischemia/reperfusion. Int. J. Artif. Organs 2004, 27, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M. Triggers of inflammation after renal ischemia/reperfusion. Clin. Immunol. 2007, 123, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol exerts apoptotic effect and modulates the sensitivity of HeLa cells to cisplatin and radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Nikaido, T. In vivo modulation of iNOS pathway in hepatocellular carcinoma by Nigella sativa. Environ. Health Prev. Med. 2013, 18, 377–385. [Google Scholar] [CrossRef]
- Fathy, M.; Nikaido, T. In vivo attenuation of angiogenesis in hepatocellular carcinoma by Nigella sativa. Turk J. Med. Sci. 2018, 48, 178–186. [Google Scholar] [CrossRef]
- Fathy, M.; Awale, S.; Nikaido, T. Phosphorylated Akt Protein at Ser473 Enables HeLa Cells to Tolerate Nutrient-Deprived Conditions. Asian Pac. J. Cancer Prev. 2017, 18, 3255–3260. [Google Scholar] [CrossRef]
- Shin, J.S.; Noh, Y.S.; Lee, Y.S.; Cho, Y.W.; Baek, N.I.; Choi, M.S.; Jeong, T.S.; Kang, E.; Chung, H.G.; Lee, K.T. Arvelexin from Brassica rapa suppresses NF-κB-regulated pro-inflammatory gene expression by inhibiting activation of IκB kinase. Br. J. Pharmacol. 2011, 164, 145–158. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.-M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef]
- Abd El-Baky, R.M.; Hetta, H.F.; Koneru, G.; Ammar, M.; Shafik, E.A.; Mohareb, D.A.; Abbas El-Masry, M.; Ramadan, H.K.; Abu Rahma, M.Z.; Fawzy, M.A.; et al. Impact of interleukin IL-6 rs-1474347 and IL-10 rs-1800896 genetic polymorphisms on the susceptibility of HCV-infected Egyptian patients to hepatocellular carcinoma. Immunol. Res. 2020, 68, 118–125. [Google Scholar] [CrossRef]
- Fathy, M.; Okabe, M.; Saad Eldien, H.M.; Yoshida, T. AT-MSCs Antifibrotic Activity is Improved by Eugenol through Modulation of TGF-beta/Smad Signaling Pathway in Rats. Molecules 2020, 25, 348. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Othman, E.M.; Fathy, M.; Iqbal, J.; Howari, F.M.; AlRemeithi, F.A.; Kodandaraman, G.; Stopper, H.; Bencurova, E.; Vlachakis, D.; et al. Integrated structural and functional analysis of the protective effects of kinetin against oxidative stress in mammalian cellular systems. Sci. Rep. 2020, 10, 13330. [Google Scholar] [CrossRef] [PubMed]
- Eldafashi, N.; Darlay, R.; Shukla, R.; McCain, M.V.; Watson, R.; Liu, Y.L.; McStraw, N.; Fathy, M.; Fawzy, M.A.; Zaki, M.Y.W.; et al. A PDCD1 Role in the Genetic Predisposition to NAFLD-HCC? Cancers (Basel) 2021, 13, 1412. [Google Scholar] [CrossRef]
- Dardenne, M.; Bach, J.-F. Rationale for the mechanism of zinc interaction in the immune system. In Nutrient Modulation of the Immune Response; CRC Press: Boca Raton, FL, USA, 2020; pp. 501–510. [Google Scholar]
- Franzin, R.; Stasi, A.; Fiorentino, M.; Stallone, G.; Cantaluppi, V.; Gesualdo, L.; Castellano, G. Inflammaging and complement system: A link between acute kidney injury and chronic graft damage. Front. Immunol. 2020, 11, 734. [Google Scholar] [CrossRef]
- Cho, S.O.; Lim, J.W.; Kim, H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J. Ethnopharmacol. 2013, 150, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Li, J.-r.; Wang, Y.; Lei, L.-s.; Yu, C.-l.; Chen, N.-n. Cyclovirobuxinum D suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages in vitro by blocking JAK-STAT signaling pathway. Acta Pharmacol. Sin. 2014, 35, 770–778. [Google Scholar] [CrossRef]
- Tang, S.; Shen, X.-Y.; Huang, H.-Q.; Xu, S.-W.; Yu, Y.; Zhou, C.-H.; Chen, S.-R.; Le, K.; Wang, Y.-H.; Liu, P.-Q. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW264. 7 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation 2011, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Abdellatef, A.A.; Fathy, M.; Mohammed, A.E.I.; Bakr, M.S.A.; Ahmed, A.H.; Abbass, H.S.; El-Desoky, A.H.; Morita, H.; Nikaido, T.; Hayakawa, Y. Inhibition of cell-intrinsic NF-kappaB activity and metastatic abilities of breast cancer by aloe-emodin and emodic-acid isolated from Asphodelus microcarpus. J. Nat. Med. 2021, 75, 840–853. [Google Scholar] [CrossRef]
- Okabe, M.; Yoshida, T.; Suzuki, M.; Goto, M.; Omori, M.; Taguchi, M.; Toda, A.; Suzuki, T.; Nakagawa, K.; Hiramoto, F.; et al. Hyperdry Human Amniotic Membrane (HD-AM) is Supporting Aciclovir Included Device of Poly-N-p-Vinylbenzyl-D-Lactonamide (PVLA) Sphere for Treatment of HSV-1 Infected Rabbit Keratitis Model. J. Biotechnol. Biomater. 2017, 7, 251. [Google Scholar] [CrossRef]
- Otaka, S.; Nagura, S.; Koike, C.; Okabe, M.; Yoshida, T.; Fathy, M.; Yanagi, K.; Misaki, T.; Nikaido, T. Selective isolation of nanog-positive human amniotic mesenchymal cells and differentiation into cardiomyocytes. Cell. Reprogram. 2013, 15, 80–91. [Google Scholar] [CrossRef]
- Zhou, K.; Koike, C.; Yoshida, T.; Okabe, M.; Fathy, M.; Kyo, S.; Kiyono, T.; Saito, S.; Nikaido, T. Establishment and characterization of immortalized human amniotic epithelial cells. Cell. Reprogram. 2013, 15, 55–67. [Google Scholar] [CrossRef]
- Fathy, M.; Okabe, M.; Othman, E.M.; Saad Eldien, H.M.; Yoshida, T. Preconditioning of Adipose-Derived Mesenchymal Stem-Like Cells with Eugenol Potentiates Their Migration and Proliferation In Vitro and Therapeutic Abilities in Rat Hepatic Fibrosis. Molecules 2020, 25, 2020. [Google Scholar] [CrossRef]
- Fathy, M.; Sun, S.; Zhao, Q.-L.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Awale, S.; Nikaido, T. A New Ciprofloxacin-derivative Inhibits Proliferation and Suppresses the Migration Ability of HeLa Cells. Anticancer Res. 2020, 40, 5025–5033. [Google Scholar] [CrossRef]
- Cheer, S.; Prakash, A.; Faulds, D. Pantoprazole: An update of its pharmacological properties and therapeutic use in the among the proton pump inhibitors in terms of management of acid-related disorders. Drugs 2003, 63, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Swathi, E.; Dhamanigi, S.S.; Asad, M.; Ali Mohzari, Y.; Alrashed, A.A.; Alotaibi, A.S.; Mohammed Alhassan, B.; Nagaraja, S. Role of Daucus carota in Enhancing Antiulcer Profile of Pantoprazole in Experimental Animals. Molecules 2020, 25, 5287. [Google Scholar] [CrossRef] [PubMed]
- Shoman, A.A.; Badwy, A.; Elhammady, M.; Eldesoki, Y. Effect of Proton Pump Inhibitors, Vitamin E and their co-administration on heart function and Oxidative Changes in Isoprenaline Induced Myocardial Infarction in Adult Male Albino Rats. Benha Med. J. 2020, 37, 169–183. [Google Scholar] [CrossRef][Green Version]
- Kohansal, P.; Rajai, N.; Dehpour, A.R.; Rashidian, A.; Shafaroodi, H. The protective effect of acute pantoprazole pretreatment on renal ischemia/reperfusion injury in rats. Fundam. Clin. Pharmacol. 2019, 33, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Şener, G.; Şehirli, Ö.; Velioğlu-Öğünç, A.; Çetinel, Ş.; Gedik, N.; Caner, M.; Sakarcan, A.; Yeğen, B.Ç. Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol. Res. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.; Versteilen, A.M.; Sipkema, P.; van Nieuw Amerongen, G.P.; Musters, R.J.; Groeneveld, A.J. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia–reperfusion-induced endothelial cell apoptosis. Apoptosis 2008, 13, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, O.; Turgut, F.; Karatas, O.F.; Cimentepe, E.; Bayrak, R.; Catal, F.; Atis, O.; Akcay, A.; Unal, D. Oral β-glucan protects kidney against ischemia/reperfusion injury in rats. Am. J. Nephrol. 2008, 28, 190–196. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.; Fathy, M.; Amgad, S.W. Glycoregulatory Enzymes as Early Diagnostic Markers during Premalignant Stage in Hepatocellular Carcinoma. Am. J. Cancer Prev. 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Othman, E.M.; Fathy, M.; Bekhit, A.A.; Abdel-Razik, A.H.; Jamal, A.; Nazzal, Y.; Shams, S.; Dandekar, T.; Naseem, M. Modulatory and Toxicological Perspectives on the Effects of the Small Molecule Kinetin. Molecules 2021, 26, 670. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, R.; Abuo-Rahma, G.E.A.; Zhao, Q.L.; Fathy, M. Modulation of Apoptosis and Epithelial-Mesenchymal Transition E-cadherin/TGF-beta/Snail/TWIST Pathways by a New Ciprofloxacin Chalcone in Breast Cancer Cells. Anticancer Res. 2021, 41, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, R.; Nazmy, M.H.; Abdel-Aziz, M.; Abuo-Rahma, G.E.A.; Fathy, M. Cell Cycle Arrest and Apoptotic Effect of 7-(4-(N-substituted carbamoylmethyl) piperazin-1-yl) Ciprofloxacin-derivative on HCT 116 and A549 Cancer Cells. Anticancer Res. 2020, 40, 2739–2749. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Mustafa, M.; Abuo-Rahma, G.E.A.; Fathy, M. In vitro inhibition and molecular docking of a new ciprofloxacin chalcone against SARS-CoV-2 main protease. Fundam. Clin. Pharm. 2021, 1–11. [Google Scholar] [CrossRef]
- Nagura, S.; Otaka, S.; Koike, C.; Okabe, M.; Yoshida, T.; Fathy, M.; Fukahara, K.; Yoshimura, N.; Misaki, T.; Nikaido, T. Effect of exogenous Oct4 overexpression on cardiomyocyte differentiation of human amniotic mesenchymal cells. Cell. Reprogram. 2013, 15, 471–480. [Google Scholar] [CrossRef]
- Oba, J.; Okabe, M.; Yoshida, T.; Soko, C.; Fathy, M.; Amano, K.; Kobashi, D.; Wakasugi, M.; Okudera, H. Hyperdry human amniotic membrane application as a wound dressing for a full-thickness skin excision after a third-degree burn injury. Burn. Trauma 2020, 8, tkaa014. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, N.; Bedi, P.M.S. Estrogen attenuates renal IRI through PPAR-γ agonism in rats. J. Surg. Res. 2016, 203, 324–330. [Google Scholar] [CrossRef]
- Wever, K.E.; Menting, T.P.; Rovers, M.; Van Der Vliet, J.A.; Rongen, G.A.; Masereeuw, R.; Ritskes-Hoitinga, M.; Hooijmans, C.R.; Warlé, M. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS ONE 2012, 7, e32296. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Su, N.; Zhang, Z.; Zhao, H.; Yu, H.; Xu, Y. Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Mol. Med. Rep. 2016, 13, 1353–1360. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, H.; Li, X.; Zhan, H.; Chen, F.; Han, L.; Xu, Y.; Cao, X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Int. Immunopharmacol. 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Chu, W.; Li, M.; Li, F.; Hu, R.; Chen, Z.; Lin, J.; Feng, H. Immediate splenectomy down-regulates the MAPK–NF-κB signaling pathway in rat brain after severe traumatic brain injury. J. Trauma Acute Care Surg. 2013, 74, 1446–1453. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, S.; Wang, Y.; Cao, J.; Wang, C. Visfatin/PBEF/Nampt induces EMMPRIN and MMP-9 production in macrophages via the NAMPT-MAPK (p38, ERK1/2)-NF-κB signaling pathway. Int. J. Mol. Med. 2011, 27, 607–615. [Google Scholar]
- Guo, X.; Jiang, H.; Chen, J.; Zhang, B.-F.; Hu, Q.; Yang, S.; Yang, J.; Zhang, J. RP105 ameliorates hypoxia̸reoxygenation injury in cardiac microvascular endothelial cells by suppressing TLR4̸MAPKs̸NF-κB signaling. Int. J. Mol. Med. 2018, 42, 505–513. [Google Scholar] [CrossRef]
- Mehmeti, I.; Lenzen, S.; Lortz, S. Modulation of Bcl-2-related protein expression in pancreatic beta cells by pro-inflammatory cytokines and its dependence on the antioxidative defense status. Mol. Cell. Endocrinol. 2011, 332, 88–96. [Google Scholar] [CrossRef]
- Fathy, M.; Khalifa, E.; Fawzy, M.A. Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sci. 2019, 216, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, Z.; Li, H.; Li, C.; Yang, Z.; Li, Y.; She, D.; Cao, L.; Wang, W.; Liu, C. Perfluorocarbon reduces cell damage from blast injury by inhibiting signal paths of NF-κB, MAPK and Bcl-2/Bax signaling pathway in A549 cells. PLoS ONE 2017, 12, e0173884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Yuan, X.; Ou, Y.; Zhu, X.; Cheng, Z.; Zhang, P.; Wu, X.; Meng, Y.; Zhang, L. The relationship between the Bcl-2/Bax proteins and the mitochondria-mediated apoptosis pathway in the differentiation of adipose-derived stromal cells into neurons. PLoS ONE 2016, 11, e0163327. [Google Scholar] [CrossRef]
- Gown, A.M.; Willingham, M.C. Improved detection of apoptotic cells in archival paraffin sections: Immunohistochemistry using antibodies to cleaved caspase 3. J. Histochem. Cytochem. 2002, 50, 449–454. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F. Stability of pantoprazole sodium in glass vials, polyvinyl chloride minibags, and polypropylene syringes. Can. J. Hosptal Pharm. 2011, 64, 192. [Google Scholar] [CrossRef] [PubMed]
- Ozbilgin, S.; Ozkardesler, S.; Akan, M.; Boztas, N.; Ozbilgin, M.; Ergur, B.U.; Derici, S.; Guneli, M.E.; Meseri, R. Renal ischemia/reperfusion injury in diabetic rats: The role of local ischemic preconditioning. BioMed Res. Int. 2016, 2016, 8580475. [Google Scholar] [CrossRef] [PubMed]
- Fitton, A.; Wiseman, L. Pantoprazole. Drugs 1996, 51, 460–482. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Fathy, M.; Koike, C.; Yoshida, T.; Okabe, M.; Zho, K.; Abouzied, M.; Nikaido, T. Identification of Chemo and Radio-Resistant Sub-Population of Stem Cells in Human Cervical Cancer HeLa Cells. Cancer Investig. 2021, 39, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yoshida, T.; Okabe, M.; Fathy, M.; Sun, Y.; Koike, C.; Salto, S.; Nikaido, T. CD24+SSEA4+cells in Ovarian Carcinoma Cells Demonstrated the Characteristics as Cancer Stem Cells. J. Cancer Sci. Ther. 2017, 9, 343–352. [Google Scholar] [CrossRef]
- Banchrof, J.; Steven, A.; Turner, D. Theory and Practice of Histopathological Techniques; Churchil Livingstone: New York, NY, USA; London, UK; San Francisco, CA, USA; Tokyo, Japan, 1996. [Google Scholar]


| Primer | Sequence of the Primer |
|---|---|
| BAX | Forward: 5′-GGT GTT GAC GGT TCA CTT GC-3′ Reverse: 5′-AAC GCC TGG ATG GGC TTT TA-′. |
| Bcl-2 | Forward: 5′-TGT ATC AAA CCA TGC GGC TG-3′ Reverse: 5′-GGC TGG TTT TAC CGC ACC TT-3′. |
| GAPDH | Forward: 5′-ACC AAC TGC TTA GCC CCC C-3′ Reverse: 5′-GCA TGT CAG ATC CAC AAC GG-3′. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawzy, M.A.; Maher, S.A.; Bakkar, S.M.; El-Rehany, M.A.; Fathy, M. Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)–NF-κB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2021, 22, 10669. https://doi.org/10.3390/ijms221910669
Fawzy MA, Maher SA, Bakkar SM, El-Rehany MA, Fathy M. Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)–NF-κB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats. International Journal of Molecular Sciences. 2021; 22(19):10669. https://doi.org/10.3390/ijms221910669
Chicago/Turabian StyleFawzy, Michael A., Sherif A. Maher, Sally M. Bakkar, Mahmoud A. El-Rehany, and Moustafa Fathy. 2021. "Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)–NF-κB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats" International Journal of Molecular Sciences 22, no. 19: 10669. https://doi.org/10.3390/ijms221910669
APA StyleFawzy, M. A., Maher, S. A., Bakkar, S. M., El-Rehany, M. A., & Fathy, M. (2021). Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)–NF-κB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats. International Journal of Molecular Sciences, 22(19), 10669. https://doi.org/10.3390/ijms221910669