Generation of Mesenchymal Cell Lines Derived from Aged Donors
Abstract
1. Introduction
2. Results
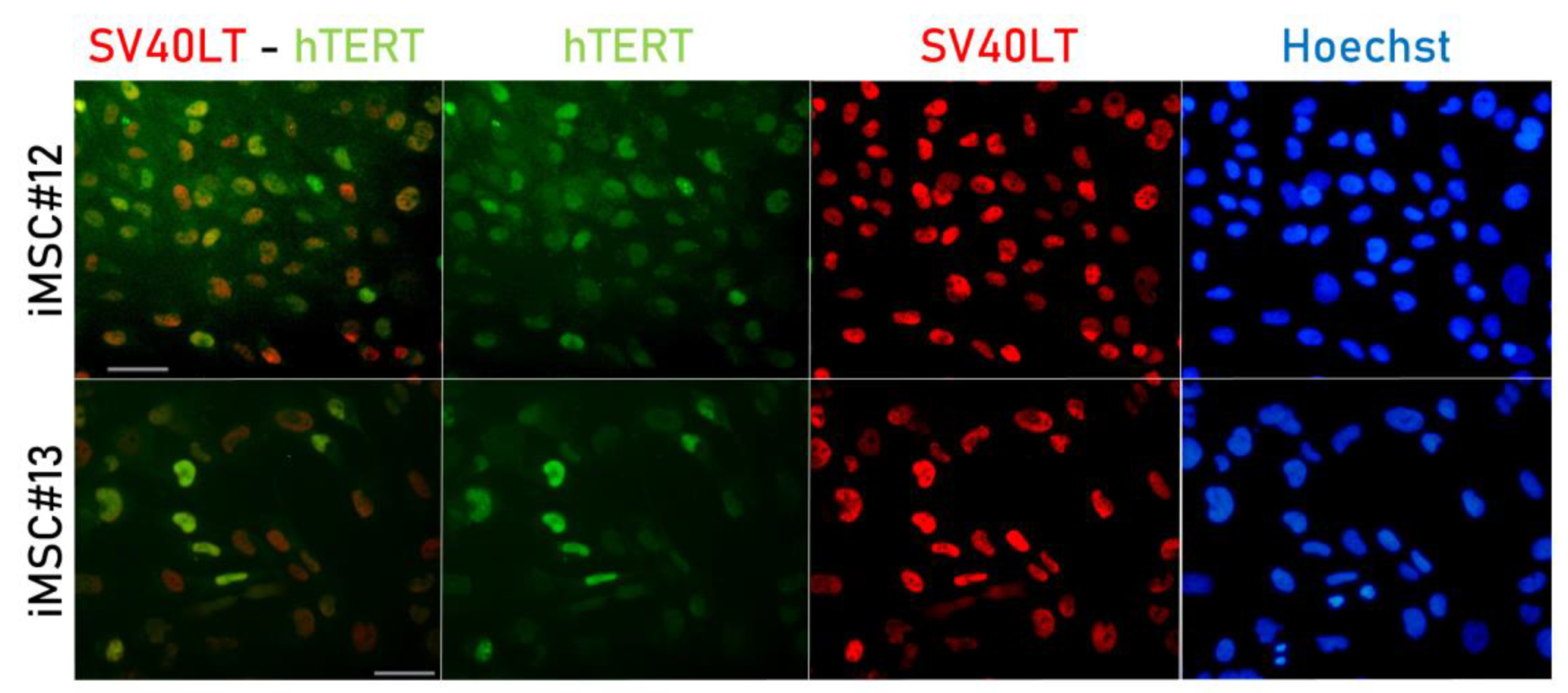
2.1. SV40LT and hTERT Expression in Transduced MSCs
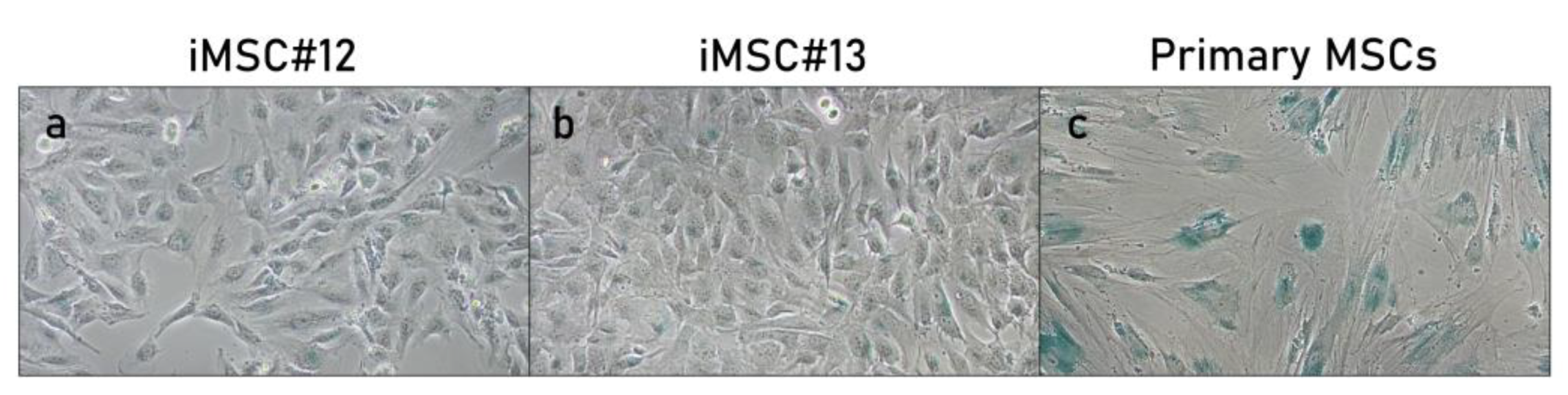
2.2. Senescence-Associated ß-Gal Activity of Transduced MSCs
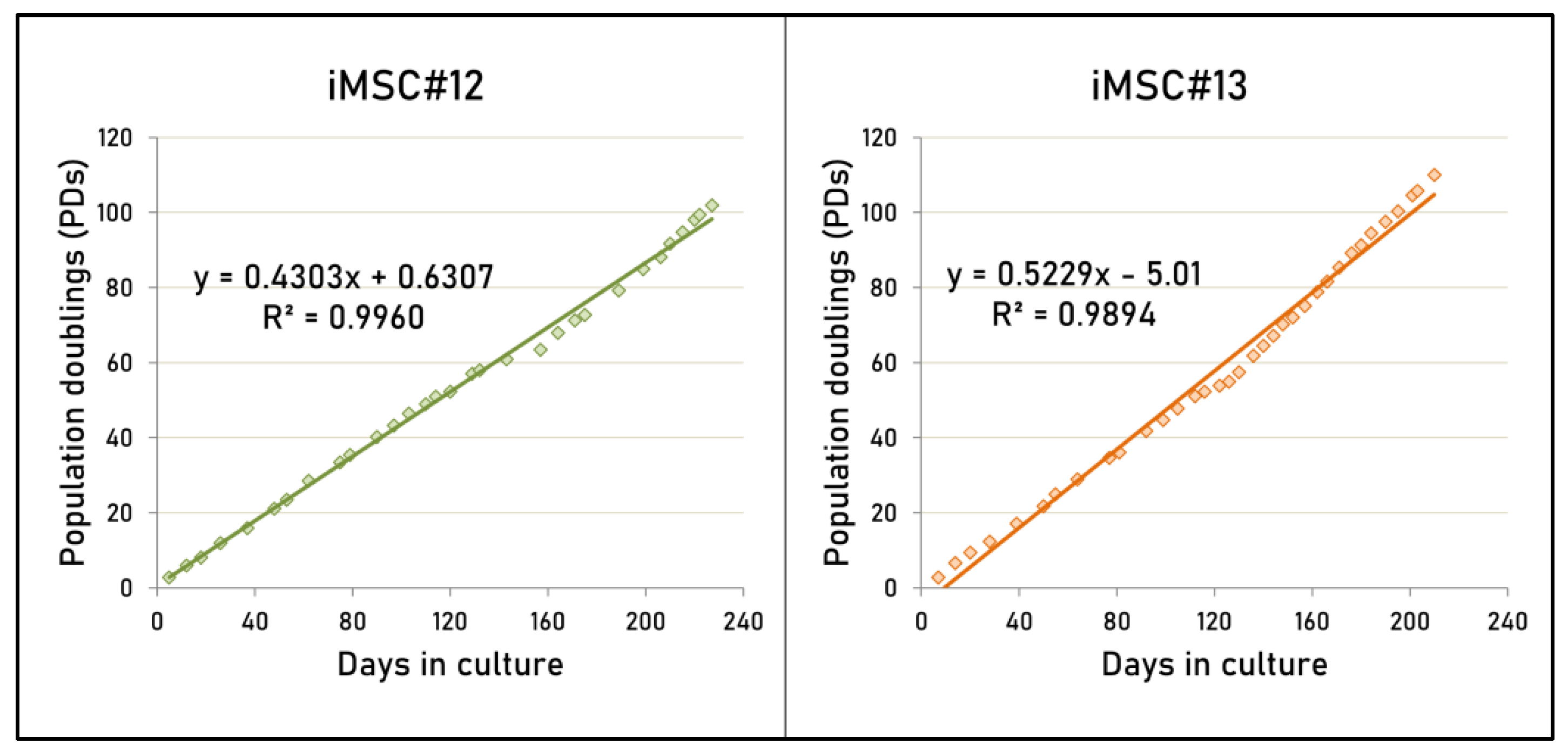
2.3. Proliferative Capacity of Transduced MSCs
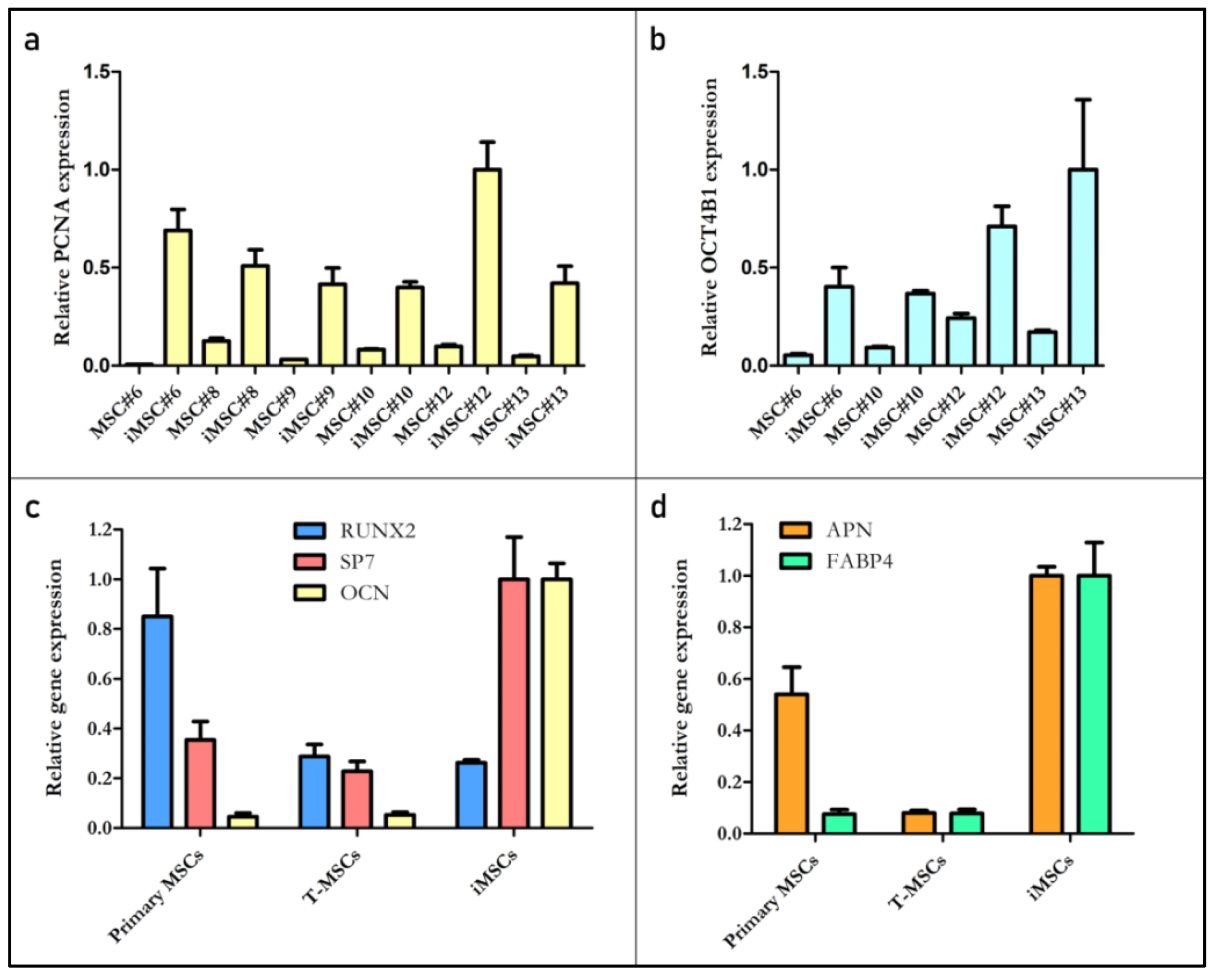
2.4. Mesenchymal Surface Marker Expression in Transduced MSCs
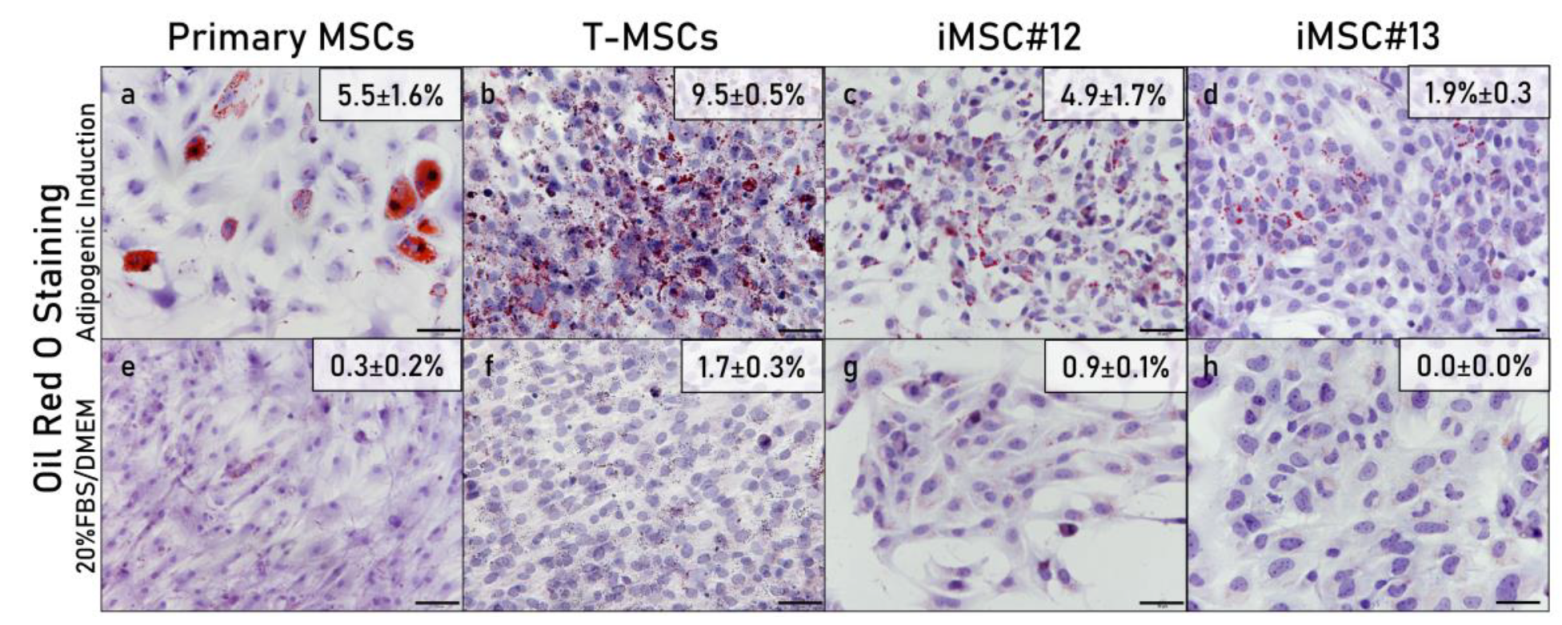
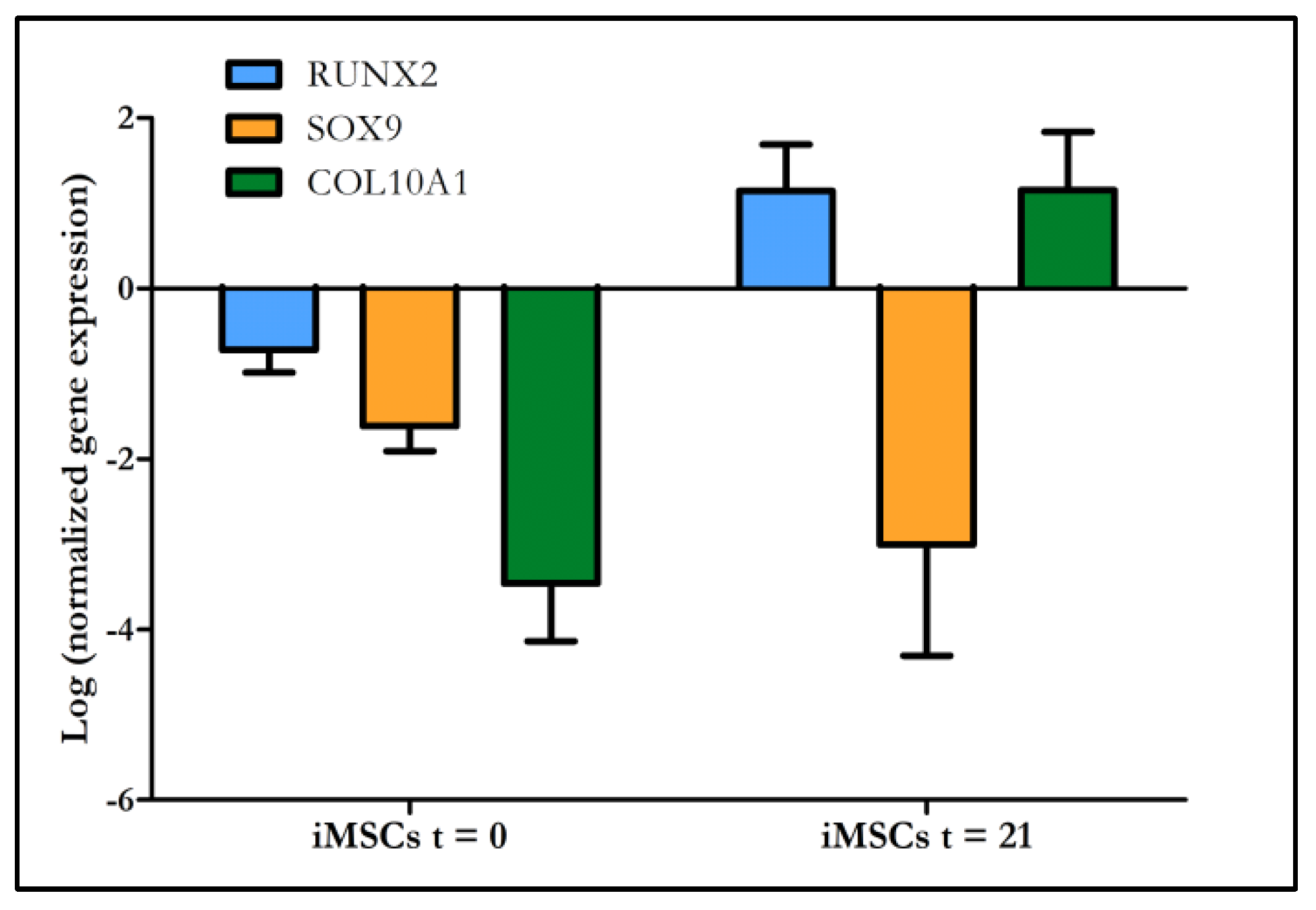
2.5. Multipotency of Transduced MSCs
2.5.1. Histological Analysis
2.5.2. Molecular Analysis
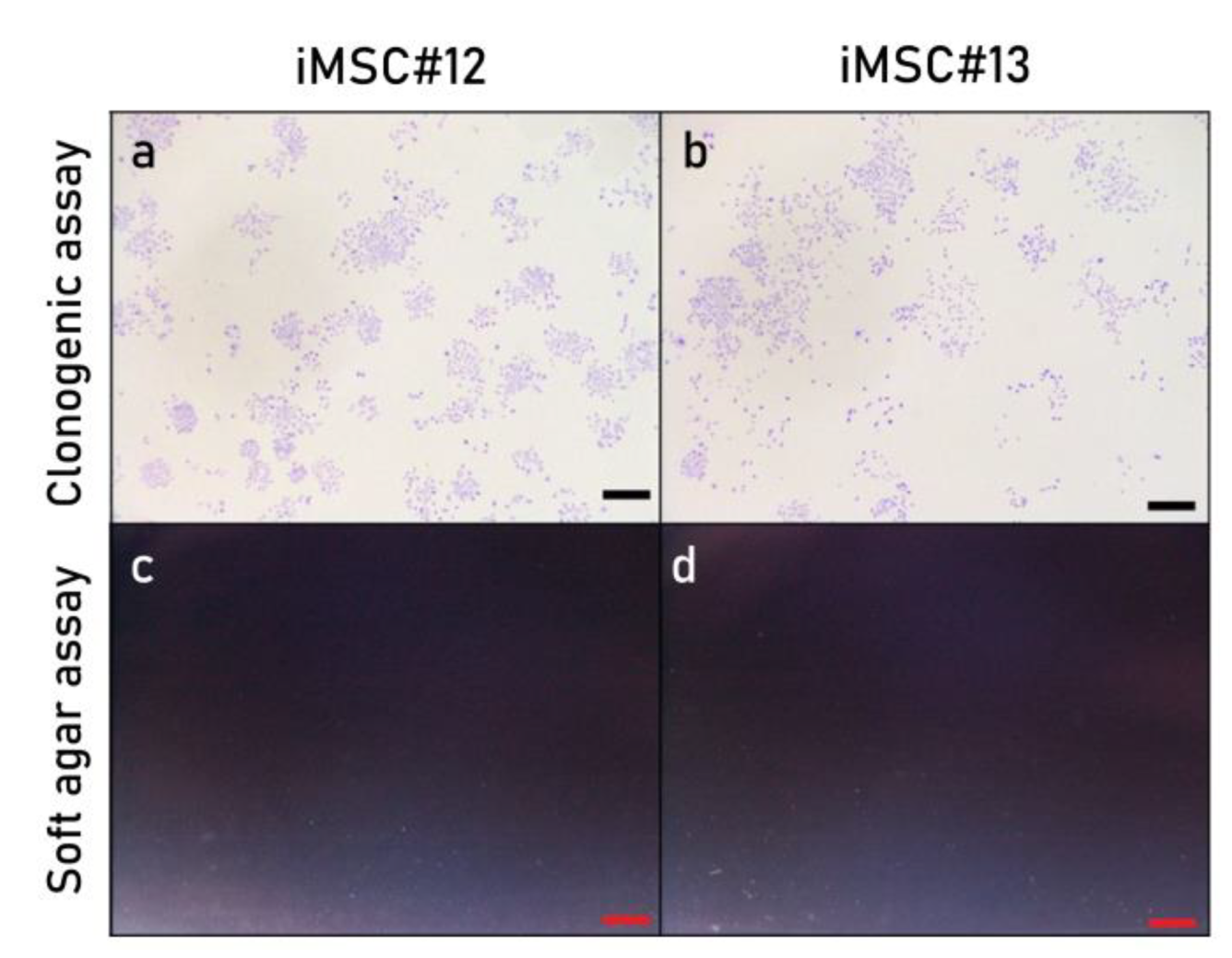
2.6. Colony-Forming Ability and Oncogenic Potential of Immortalized MSCs
3. Discussion
4. Materials and Methods
4.1. Primary MSCs Isolation and Culture
4.2. Primary MSCs Immortalization
4.3. Immunofluorescence of SV40LT and hTERT
4.4. Senescence Activity
4.5. Proliferation Analysis
4.6. Flow Cytometric Analysis
4.7. Cell Differentiatin Induction
4.8. Histological Analysis
4.9. Molecular Analysis
4.10. Colony Formation Assay
4.11. Soft Agar Assay
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bomer, N.; den Hollander, W.; Ramos, Y.F.M.; Meulenbelt, I. Translating genomics into mechanisms of disease: Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 683–691. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Piñeiro-Ramil, M.; Castro-Viñuelas, R.; Sanjurjo-Rodríguez, C.; Hermida-Gómez, T.; Fuentes-Boquete, I.; de Toro-Santos, F.J.; Blanco-García, F.J.; Díaz-Prado, S.M. Cell Therapy and Tissue Engineering for Cartilage Repair. In Cartilage Repair and Regeneration; InTech: London, UK, 2018. [Google Scholar]
- Akter, F.; Ibanez, J. Bone and Cartilage Tissue Engineering. In Tissue Engineering Made Easy; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 77–97. ISBN 9780128092286. [Google Scholar]
- Bianco, P.; Robey, P.G. Skeletal stem cells. Development 2015, 142, 1023–1027. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem. Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Stoddart, M.J.; D’Amora, U.; Ambrosio, L.; Alini, M.; Musumeci, G. Mesenchymal Stem Cell-Based Cartilage Regeneration Approach and Cell Senescence: Can We Manipulate Cell Aging and Function? Tissue Eng. Part B Rev. 2017, 23, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tsai, T.-L.; Li, W.-J. Strategies to retain properties of bone marrow-derived mesenchymal stem cells ex vivo. Ann. N. Y. Acad. Sci. 2017, 1409, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kouroupis, D.; Sanjurjo-Rodriguez, C.; Jones, E.; Correa, D. MSC functionalization for enhanced therapeutic applications. Tissue Eng. Part B Rev. 2018, 25, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Stölzel, K.; Schulze-Tanzil, G.; Olze, H.; Schwarz, S.; Feldmann, E.M.; Rotter, N. Immortalised human mesenchymal stem cells undergo chondrogenic differentiation in alginate and PGA/PLLA scaffolds. Cell Tissue Bank. 2015, 16, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Akmammedov, R.; Huysal, M.; Isik, S.; Senel, M. Preparation and characterization of novel chitosan/zeolite scaffolds for bone tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 110–118. [Google Scholar] [CrossRef]
- Ringe, J.; Sittinger, M. Tissue engineering in the rheumatic diseases. Arthritis Res. Ther. 2009, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.E.; Yoon, S.H.; Hur, Y.S.; Park, H.W.; Ha, J.Y.; Kim, K.-H.; Shim, J.H.; Yoo, S.H.; Son, J.H.; Paek, S.L.; et al. Mitochondrial Dysfunction of Immortalized Human Adipose Tissue-Derived Mesenchymal Stromal Cells from Patients with Parkinson’s Disease. Exp. Neurobiol. 2013, 22, 283–300. [Google Scholar] [CrossRef]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Shim, J.S.; Paik, M.J.; Joo, W.H.; Kim, S.H.; Lee, G.; Kim, D.W. Characterization of a growth-elevated cell line of human bone marrow-derived mesenchymal stem cells by SV40 T-antigen. Biotechnol. Bioprocess Eng. 2015, 20, 498–505. [Google Scholar] [CrossRef]
- Skårn, M.; Noordhuis, P.; Wang, M.-Y.; Veuger, M.; Kresse, S.H.; Egeland, E.V.; Micci, F.; Namløs, H.M.; Håkelien, A.-M.; Olafsrud, S.M.; et al. Generation and Characterization of an Immortalized Human Mesenchymal Stromal Cell Line. Stem Cells Dev. 2014, 23, 2377–2389. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chen, C.-L.; Liu, H.-C.; Lee, Y.-T.; Wang, H.-W.; Hou, L.-T.; Hung, S.-C. Overexpression of hTERT increases stem-like properties and decreases spontaneous differentiation in human mesenchymal stem cell lines. J. Biomed. Sci. 2010, 17, 64. [Google Scholar] [CrossRef]
- Jayasuriya, C.T.; Hu, N.; Li, J.; Lemme, N.; Terek, R.; Ehrlich, M.G.; Chen, Q. Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci. Rep. 2018, 8, 7044. [Google Scholar] [CrossRef]
- Mori, T.; Kiyono, T.; Imabayashi, H.; Takeda, Y.; Tsuchiya, K.; Miyoshi, S.; Makino, H.; Matsumoto, K.; Saito, H.; Ogawa, S.; et al. Combination of hTERT and bmi-1, E6, or E7 Induces Prolongation of the Life Span of Bone Marrow Stromal Cells from an Elderly Donor without Affecting Their Neurogenic Potential. Mol. Cell. Biol. 2005, 25, 5183–5195. [Google Scholar] [CrossRef]
- Bourgine, P.; Le Magnen, C.; Pigeot, S.; Geurts, J.; Scherberich, A.; Martin, I. Combination of immortalization and inducible death strategies to generate a human mesenchymal stromal cell line with controlled survival. Stem Cell Res. 2014, 12, 584–598. [Google Scholar] [CrossRef]
- Koch, C.M.; Reck, K.; Shao, K.; Lin, Q.; Joussen, S.; Ziegler, P.; Walenda, G.; Drescher, W.; Opalka, B.; May, T.; et al. Pluripotent stem cells escape from senescence-associated DNA methylation changes. Genome Res. 2013, 23, 248–259. [Google Scholar] [CrossRef]
- An, P.; Sáenz Robles, M.T.; Pipas, J.M. Large T Antigens of Polyomaviruses: Amazing Molecular Machines. Annu. Rev. Microbiol. 2012, 66, 213–236. [Google Scholar] [CrossRef]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Heidenreich, B.; Kumar, R. TERT promoter mutations in telomere biology. Mutat. Res. Rev. Mutat. Res. 2017, 771, 15–31. [Google Scholar] [CrossRef]
- Dale, T.P.; de Castro, A.; Kuiper, N.J.; Parkinson, E.K.; Forsyth, N.R. Immortalisation with hTERT Impacts on Sulphated Glycosaminoglycan Secretion and Immunophenotype in a Variable and Cell Specific Manner. PLoS ONE 2015, 10, e0133745. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Mori, T.; Imabayashi, H.; Kiyono, T.; Gojo, S.; Miyoshi, S.; Hida, N.; Ita, M.; Segawa, K.; Ogawa, S.; et al. Can the life span of human marrow stromal cells be prolonged by bmi-1, E6, E7, and/or telomerase without affecting cardiomyogenic differentiation? J. Gene Med. 2004, 6, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Aoyama, T.; Nakayama, T.; Nakamata, T.; Hosaka, T.; Nishijo, K.; Nakamura, T.; Kiyono, T.; Toguchida, J. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2002, 295, 354–361. [Google Scholar] [CrossRef]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Coccè, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res. Ther. 2014, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Ramil, M.; Castro-Viñuelas, R.; Sanjurjo-Rodríguez, C.; Rodríguez-Fernández, S.; Hermida-Gómez, T.; Blanco-García, F.J.; Fuentes-Boquete, I.; Díaz-Prado, S. Immortalizing Mesenchymal Stromal Cells from Aged Donors while Keeping Their Essential Features. Stem Cells Int. 2020, 2020, 5726947. [Google Scholar] [CrossRef]
- Carnero, A.; Blanco-Aparicio, C.; Kondoh, H.; Lleonart, M.E.; Martinez-Leal, J.F.; Mondello, C.; Scovassi, A.I.; Bisson, W.H.; Amedei, A.; Roy, R.; et al. Disruptive chemicals, senescence and immortality. Carcinogenesis 2015, 36, S19–S37. [Google Scholar] [CrossRef]
- Liu, T.M.; Ng, W.M.; Tan, H.S.; Vinitha, D.; Yang, Z.; Fan, J.B.; Zou, Y.; Hui, J.H.; Lee, E.H.; Lim, B. Molecular Basis of Immortalization of Human Mesenchymal Stem Cells by Combination of p53 Knockdown and Human Telomerase Reverse Transcriptase Overexpression. Stem Cells Dev. 2013, 22, 268–278. [Google Scholar] [CrossRef]
- Harting, M.T.; Jimenez, F.; Pati, S.; Baumgartner, J.; Cox, C.S. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy 2008, 10, 243–253. [Google Scholar] [CrossRef]
- Abarrategi, A.; Gambera, S.; Alfranca, A.; Rodriguez-Milla, M.A.; Perez-Tavarez, R.; Rouault-Pierre, K.; Waclawiczek, A.; Chakravarty, P.; Mulero, F.; Trigueros, C.; et al. c-Fos induces chondrogenic tumor formation in immortalized human mesenchymal progenitor cells. Sci. Rep. 2018, 8, 15615. [Google Scholar] [CrossRef]
- Alexander, D.; Biller, R.; Rieger, M.; Ardjomandi, N.; Reinert, S. Phenotypic Characterization of a Human Immortalized Cranial Periosteal Cell Line. Cell. Physiol. Biochem. 2015, 35, 2244–2254. [Google Scholar] [CrossRef]
- Takeuchi, M.; Higashino, A.; Takeuchi, K.; Hori, Y.; Koshiba-Takeuchi, K.; Makino, H.; Monobe, Y.; Kishida, M.; Adachi, J.; Takeuchi, J.; et al. Transcriptional Dynamics of Immortalized Human Mesenchymal Stem Cells during Transformation. PLoS ONE 2015, 10, e0126562. [Google Scholar]
- Schoonderwoerd, M.J.A.; Goumans, M.J.T.H.; Hawinkels, L.J.A.C. Endoglin: Beyond the endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.A.; Johnson, D.A.; Sarrió, D.; Lee, S.; Quinlan, P.R.; Crook, T.; Thompson, A.M.; Reis-Filho, J.S.; Isacke, C.M. Endoglin expression in breast tumor cells suppresses invasion and metastasis and correlates with improved clinical outcome. Oncogene 2011, 30, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, E.; Eleno, N.; López-Novoa, J.M.; Ramirez, J.R.; Velasco, B.; Letarte, M.; Bernabéu, C.; Quintanilla, M. Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene 2005, 24, 4450–4461. [Google Scholar] [CrossRef] [PubMed]
- Gambera, S.; Abarrategi, A.; Rodríguez-Milla, M.A.; Mulero, F.; Menéndez, S.T.; Rodriguez, R.; Navarro, S.; García-Castro, J. Role of Activator Protein-1 Complex on the Phenotype of Human Osteosarcomas Generated from Mesenchymal Stem Cells. Stem Cells 2018, 36, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Q.; Kusuma, G.D.; Al-Sowayan, B.; Pace, R.A.; Isenmann, S.; Pertile, M.D.; Gronthos, S.; Abumaree, M.H.; Brennecke, S.P.; Kalionis, B. Establishment and characterization of fetal and maternal mesenchymal stem/stromal cell lines from the human term placenta. Placenta 2016, 39, 134–146. [Google Scholar] [CrossRef]
- Atlasi, Y.; Mowla, S.J.; Ziaee, S.A.M.; Gokhale, P.J.; Andrews, P.W. OCT4 Spliced Variants Are Differentially Expressed in Human Pluripotent and Nonpluripotent Cells. Stem Cells 2008, 26, 3068–3074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Hu, S.Q.; Jiang, H.; Chen, Y.L.; Feng, J.H.; Chen, Z.Q.; Wen, K.M. OCT4B1 Promoted EMT and Regulated the Self-Renewal of CSCs in CRC: Effects Associated with the Balance of miR-8064/PLK1. Mol. Ther. Oncolytics 2019, 15, 7–20. [Google Scholar] [CrossRef]
- Wang, X.; Dai, J. Concise review: Isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells 2010, 28, 885–893. [Google Scholar]
- Han, S.M.; Han, S.H.; Coh, Y.R.; Jang, G.; Ra, J.C.; Kang, S.K.; Lee, H.W.; Youn, H.Y. Enhanced proliferation and differentiation of Oct4- And Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp. Mol. Med. 2014, 46, e101. [Google Scholar] [CrossRef]
- Uder, C.; Brückner, S.; Winkler, S.; Tautenhahn, H.-M.; Christ, B. Mammalian MSC from selected species: Features and applications. Cytom. Part A 2018, 93, 32–49. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Harkness, L.; Zaher, W.; Ditzel, N.; Isa, A.; Kassem, M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res. Ther. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, S.; Han, L.; Zhang, C. β-catenin signaling induces the osteoblastogenic differentiation of human pre-osteoblastic and bone marrow stromal cells mainly through the upregulation of osterix expression. Int. J. Mol. Med. 2015, 36, 1572–1582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, X.; Glowacki, J.; Hahne, J.; Xie, L.; LeBoff, M.S.; Zhou, S. Dehydroepiandrosterone Stimulation of Osteoblastogenesis in Human MSCs Requires IGF-I Signaling. J. Cell. Biochem. 2016, 117, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.S.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef]
- Larsen, K.H.; Frederiksen, C.M.; Burns, J.S.; Abdallah, B.M.; Kassem, M. Identifying A Molecular Phenotype for Bone Marrow Stromal Cells With In Vivo Bone Forming Capacity. J. Bone Miner. Res. 2009, 25, 796–808. [Google Scholar]
- Böcker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef]
- Armbruster, N.; Krieg, J.; Weißenberger, M.; Scheller, C.; Steinert, A.F. Rescued Chondrogenesis of Mesenchymal Stem Cells under Interleukin 1 Challenge by Foamyviral Interleukin 1 Receptor Antagonist Gene Transfer. Front. Pharmacol. 2017, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Alajez, N.M. Epigenetic Library Screen Identifies Abexinostat as Novel Regulator of Adipocytic and Osteoblastic Differentiation of Human Skeletal (Mesenchymal) Stem Cells. Stem Cells Transl. Med. 2016, 5, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Fayyad, A.; Khan, A.; Abdallah, S.; Alomran, S.; Bajou, K.; Khattak, M. Rosiglitazone Enhances Browning Adipocytes in Association with MAPK and PI3-K Pathways During the Differentiation of Telomerase-Transformed Mesenchymal Stromal Cells into Adipocytes. Int. J. Mol. Sci. 2019, 20, 1618. [Google Scholar] [CrossRef]
- Galarza Torre, A.; Shaw, J.E.; Wood, A.; Gilbert, H.T.J.; Dobre, O.; Genever, P.; Brennan, K.; Richardson, S.M.; Swift, J. An immortalised mesenchymal stem cell line maintains mechano-responsive behaviour and can be used as a reporter of substrate stiffness. Sci. Rep. 2018, 8, 8981. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, G.; Song, J.; Zhang, Y.; Yu, Y.; Huang, L.; Zheng, L.; Deng, F. TrAmplification of Human Dental Follicle Cells by piggyBac Transposon—Mediated Reversible Immortalization System. PLoS ONE 2015, 10, e0130937. [Google Scholar] [CrossRef][Green Version]
- James, S.; Fox, J.; Afsari, F.; Lee, J.; Clough, S.; Knight, C.; Ashmore, J.; Ashton, P.; Preham, O.; Hoogduijn, M.; et al. Multiparameter Analysis of Human Bone Marrow Stromal Cells Identifies Distinct Immunomodulatory and Differentiation-Competent Subtypes. Stem Cell Rep. 2015, 4, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Funes, J.M.; Quintero, M.; Henderson, S.; Martinez, D.; Qureshi, U.; Westwood, C.; Clements, M.O.; Bourboulia, D.; Pedley, R.B.; Moncada, S.; et al. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc. Natl. Acad. Sci. USA. 2007, 104, 6223–6228. [Google Scholar] [CrossRef]
- Dale, T.P.; Forsyth, N.R. Ectopic Telomerase Expression Fails to Maintain Chondrogenic Capacity in Three-Dimensional Cultures of Clinically Relevant Cell Types. Biores. Open Access 2018, 7, biores.2018.0008. [Google Scholar] [CrossRef]
- Rashid, H.; Ma, C.; Chen, H.; Wang, H.; Hassan, M.Q.; Sinha, K.; De Crombrugghe, B.; Javed, A. Sp7 and Runx2 molecular complex synergistically regulate expression of target genes. Connect. Tissue Res. 2014, 55 (Suppl. 1), 83–87. [Google Scholar]
- Ok, J.S.; Song, S.B.; Hwang, E.S. Enhancement of replication and differentiation potential of human bone marrow stem cells by nicotinamide treatment. Int. J. Stem Cells 2018, 11, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, S.; Schneider, C.; van Osch, G.V.M.; Keibl, C.; Rieder, B.; Monforte, X.; Teuschl, A.H.; Mühleder, S.; Holnthoner, W.; Schädl, B.; et al. Repopulation of an auricular cartilage scaffold, AuriScaff, perforated with an enzyme combination. Acta Biomater. 2019, 86, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Lui, P.P.Y.; Rui, Y.F. Effect of In Vitro Passaging on the Stem Cell-Related Properties of Tendon-Derived Stem Cells—Implications in Tissue Engineering. Stem Cells Dev. 2012, 21, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef]
- Sanjurjo-Rodríguez, C.; Martínez-Sánchez, A.H.; Hermida-Gómez, T.; Fuentes-Boquete, I.; Díaz-Prado, S.; Blanco, F.J. Human Cartilage Tissue Engineering Using Type I Collagen/Heparan Sulfate Scaffolds. J. Regen. Med. 2014, 3. [Google Scholar] [CrossRef]
- Durgam, S.; Schuster, B.; Cymerman, A.; Stewart, A.; Stewart, M. Differential Adhesion Selection for Enrichment of Tendon-Derived Progenitor Cells During In Vitro Culture. Tissue Eng. Part C Methods 2016, 22, 801–808. [Google Scholar] [CrossRef]
- Swift, S.; Lorens, J.; Achacoso, P.; Nolan, G.P. Rapid Production of Retroviruses for Efficient Gene Delivery to Mammalian Cells Using 293T Cell-Based Systems. In Current Protocols in Immunology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Volume 31, Chapter 10; pp. 10–17. [Google Scholar]
- Wong, J.M.Y.; Kusdra, L.; Collins, K. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat. Cell Biol. 2002, 4, 731–736. [Google Scholar] [CrossRef]
- Zhao, J.J.; Gjoerup, O.V.; Subramanian, R.R.; Cheng, Y.; Chen, W.; Roberts, T.M.; Hahn, W.C. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell 2003, 3, 483–495. [Google Scholar] [CrossRef]
- Balducci, L.; Alessandri, G. Isolation, Expansion, and Immortalization of Human Adipose-Derived Mesenchymal Stromal Cells from Biopsies and Liposuction Specimens. In Methods in Molecular Biology (Clifton, N.J.); Humana Press: New York, NY, USA, 2016; Volume 1416, pp. 259–274. [Google Scholar]
- Hildebrandt, C.; Büth, H.; Thielecke, H. A scaffold-free in vitro model for osteogenesis of human mesenchymal stem cells. Tissue Cell 2011, 43, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Prado, S.; Cicione, C.; Muiños-López, E.; Hermida-Gómez, T.; Oreiro, N.; Fernández-López, C.; Blanco, F.J. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet. Disord. 2012, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [PubMed]
- Roca-Lema, D.; Martinez-Iglesias, O.; De Ana Portela, C.F.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In vitro anti-proliferative and anti-invasive effect of polysaccharide-rich extracts from trametes versicolor and grifola frondosa in colon cancer cells. Int. J. Med. Sci. 2019, 16, 231–240. [Google Scholar] [CrossRef] [PubMed]

| Cells | Passage | CD29 | CD44 | CD73 | CD90 | CD105 | CD34 | CD45 |
|---|---|---|---|---|---|---|---|---|
| Primary MSCs | 3 | 93.3% | 95.2% | 71.1% | 98.5% | 69.8% | 0.2% | 1.9% |
| T-MSCs | (3 + 4) | 98.1% | 98.9% | 58.7% | 98.4% | 77.0% | 2.1% | 0.8% |
| iMSC#12 | (3 + 4 + 12) | 97.4% | 96.8% | 85.6% | 99.5% | 80.1% | 0.0% | 0.0% |
| iMSC#12 (PD > 100) | (3 + 4 + 43) | 97.8% | 99.3% | 82.1% | 96.5% | 78.1% | 0.7% | 0.2% |
| iMSC#13 | (3 + 3 + 13) | 99.3% | 99.4% | 98.8% | 99.9% | 97.7% | 0.3% | 0.9% |
| iMSC#13 (PD > 100) | (3 + 3 + 44) | 97.9% | 98.2% | 86.1% | 98.3% | 90.2% | 0.5% | 0.6% |
| Antibody | Specificity | Clone | Source | Dilution |
|---|---|---|---|---|
| FITC Mouse IgG1 Isotype Control | - | ICIG1 | Immunostep | 1:50 |
| PE Mouse IgG1 Isotype Control | - | B11/6 | Immunostep | 1:50 |
| PECy5 Mouse IgG1 Isotype Control | - | 1F8 | Abcam | 2:25 |
| PE Mouse Anti-Human CD29 | Human integrin β1 (ITGB1) | VJ1/14 | Immunostep | 3:50 |
| PE Mouse Anti-Human CD34 | Hematopoietic progenitor cell antigen 1 (HPCA1) | 581 | BD Pharmingen | 2:25 |
| FITC Mouse Anti-Human CD44 | Homing cellular adhesion molecule (HCAM) | IM7 | BD Pharmingen | 1:50 |
| FITC Mouse Anti-Human CD45 | Leukocyte common antigen (LCA) | D3/9 | Immunostep | 3:50 |
| PE Mouse Anti-Human CD73 | Ecto-5′-nucleotidase (NT5E) | AD2 | Immunostep | 3:50 |
| PECy5 Mouse Anti-Human CD90 | Thymocyte differentiation antigen 1 (Thy-1) | 5E10 | Immunostep | 1:50 |
| FITC Mouse Anti-Human CD105 | Human Endoglin (ENG) | SN6 | AbD Serotec | 1:50 |
| Gene | Forward Primer 5′→3′ | Reverse Primer 5′→3′ |
|---|---|---|
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) | GATCCCCAATGCTTCACAAG | TGCTTGTTGTGACTGATCGAC |
| Homo sapiens runt related transcription factor 2 (RUNX2) | TTACTTACACCCCGCCAGTC | TATGGAGTGCTGCTGGTCTG |
| Homo sapiens Sp7 transcription factor (SP7) | TCCCCTGTTGCCATGGTTAT | CCACCCATTCTTCAGGAGGT |
| Homo sapiens bone gamma-carboxyglutamate protein (OCN) | GGCGCTACCTGTATCAATGG | TCAGCCAACTCGTCACAGTC |
| Homo sapiens adiponectin, C1Q and collagen domain containing (APN) | GGTGAGAAAGGAGATCCAGGT | TGCTGAGCGGTATACATAGGC |
| Homo sapiens fatty acid binding protein 4 (FABP4) | GGATGATAAACTGGTGGTGGA | CACAGAATGTTGTAGAGTTCAATGC |
| Simian virus 40 complete genome (SV40) | TGGGGAGAAGAACATGGAAG | AAATGAGCCTTGGGACTGTG |
| Homo sapiens telomerase reverse transcriptase (hTERT) | GCTAGTGGACCCCGAAGG | CCTCCCTGACGCTATGGTT |
| Homo sapiens proliferating cell nuclear antigen (PCNA) | TAGACTTTCCTCCTTCCCGC | TGCCTCCAACACCTTCTTGA |
| Homo sapiens POU class 5 homeobox 1 (POU5F1), transcript variant 4 (OCT4B1) | AGGGAGAGGGAGAAGATGCT | GAAGCAAAGTGAGGGAGCAC |
| Homo sapiens SRY-box transcription factor 9 (SOX9) | GTACCCGCACTTGCACAAC | TCGCTCTCGTTCAGAAGTCTC |
| Homo sapiens collagen type X alpha 1 chain (COL10A1) | CACCTTCTGCACTGCTCATC | GGCAGCATATTCTCAGATGGA |
| Homo sapiens collagen type II alpha 1 chain (COL2A1) | TGGTGCTAATGGCGAGAAG | CCCAGTCTCTCCACGTTCAC |
| Homo sapiens aggrecan (ACAN) | CGGTCTACCTCTACCCTAACCA | GAGAAGGAACCGCTGAAATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñeiro-Ramil, M.; Sanjurjo-Rodríguez, C.; Rodríguez-Fernández, S.; Castro-Viñuelas, R.; Hermida-Gómez, T.; Blanco-García, F.J.; Fuentes-Boquete, I.; Díaz-Prado, S. Generation of Mesenchymal Cell Lines Derived from Aged Donors. Int. J. Mol. Sci. 2021, 22, 10667. https://doi.org/10.3390/ijms221910667
Piñeiro-Ramil M, Sanjurjo-Rodríguez C, Rodríguez-Fernández S, Castro-Viñuelas R, Hermida-Gómez T, Blanco-García FJ, Fuentes-Boquete I, Díaz-Prado S. Generation of Mesenchymal Cell Lines Derived from Aged Donors. International Journal of Molecular Sciences. 2021; 22(19):10667. https://doi.org/10.3390/ijms221910667
Chicago/Turabian StylePiñeiro-Ramil, María, Clara Sanjurjo-Rodríguez, Silvia Rodríguez-Fernández, Rocío Castro-Viñuelas, Tamara Hermida-Gómez, Francisco J. Blanco-García, Isaac Fuentes-Boquete, and Silvia Díaz-Prado. 2021. "Generation of Mesenchymal Cell Lines Derived from Aged Donors" International Journal of Molecular Sciences 22, no. 19: 10667. https://doi.org/10.3390/ijms221910667
APA StylePiñeiro-Ramil, M., Sanjurjo-Rodríguez, C., Rodríguez-Fernández, S., Castro-Viñuelas, R., Hermida-Gómez, T., Blanco-García, F. J., Fuentes-Boquete, I., & Díaz-Prado, S. (2021). Generation of Mesenchymal Cell Lines Derived from Aged Donors. International Journal of Molecular Sciences, 22(19), 10667. https://doi.org/10.3390/ijms221910667







