JAK Inhibition Prevents DNA Damage and Apoptosis in Testicular Ischemia-Reperfusion Injury via Modulation of the ATM/ATR/Chk Pathway
Abstract
1. Introduction
2. Results
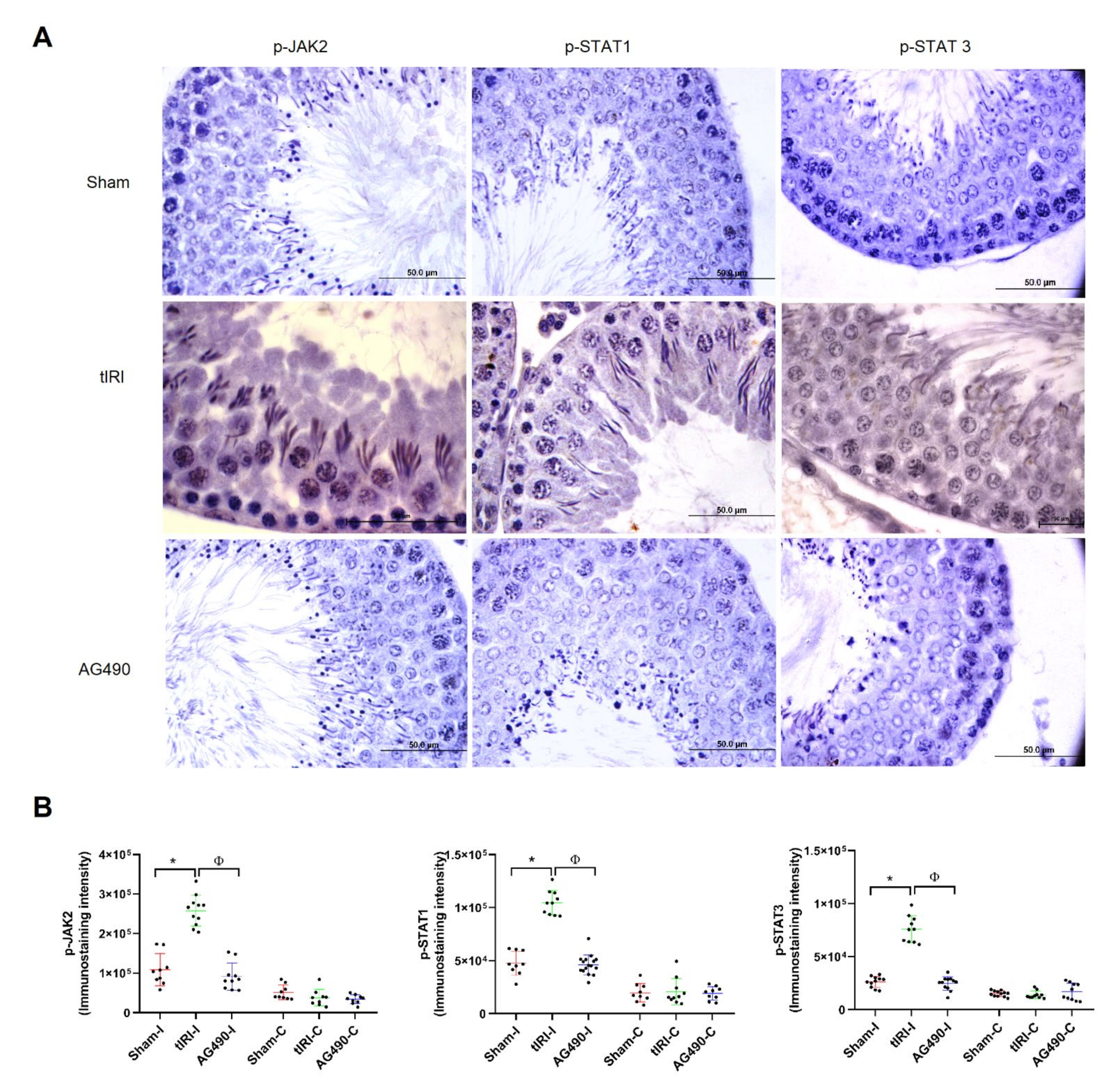
2.1. The JAK2/STAT3/STAT1 Pathway Is Directly Involved in tIRI
2.2. JAK2 Inhibition Prevents tIRI-Induced Spermatogenic Arrest
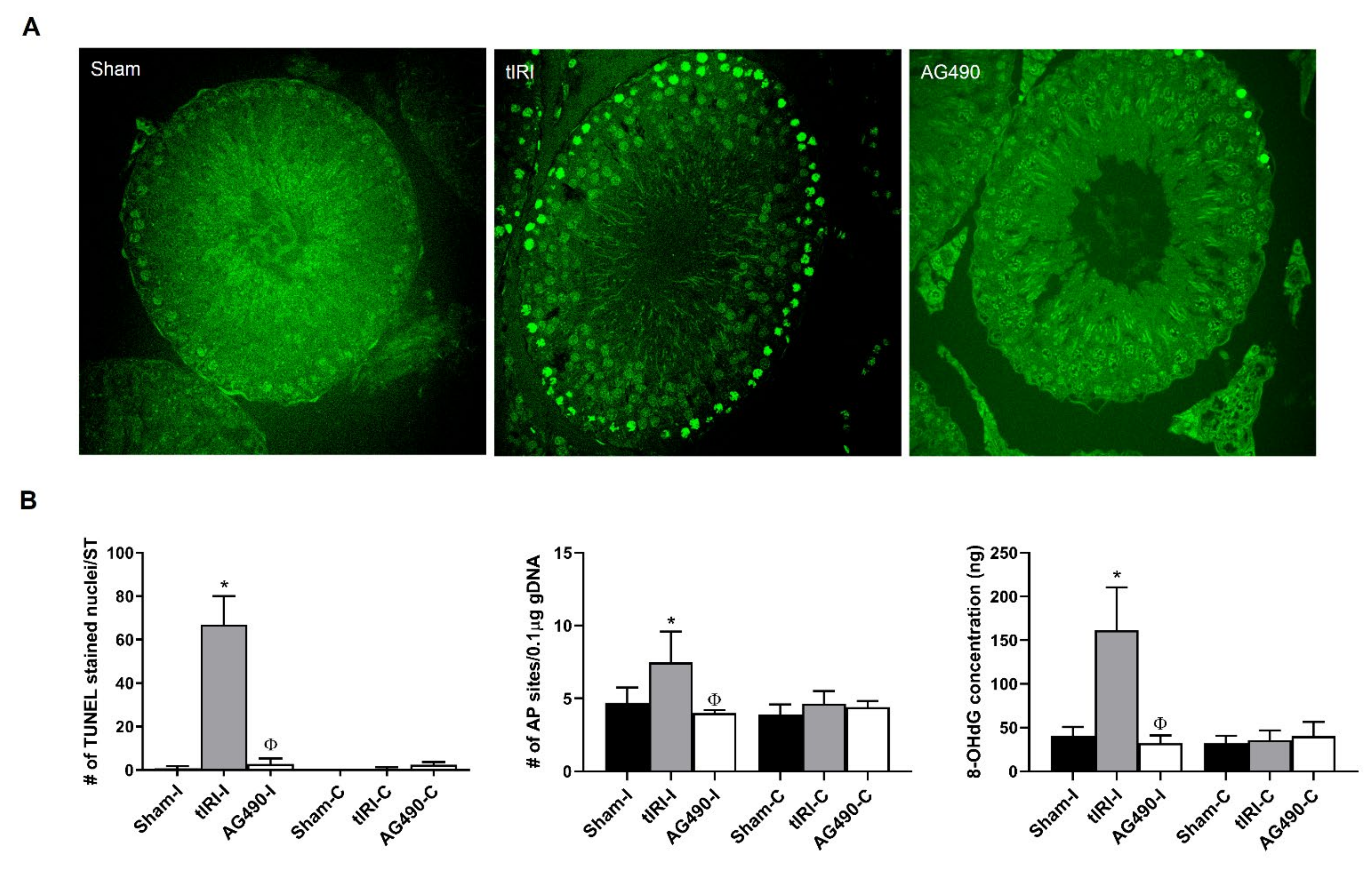
2.3. JAK2 Inhibition Prevents Oxidative DNA Damage during tIRI
2.4. JAK2/STAT1/STAT3 Activation Promotes Apoptosis during tIRI
2.5. PARP Activity Is Regulated by JAK2
2.6. Modulation of the DDR Pathways by JAK2 Signaling
3. Discussion
4. Materials and Methods
4.1. AG490 and Primary Antibodies
4.2. Testicular Ischemia Reperfusion Injury (tIRI) Model and AG490 Treatment
4.3. Histological Analyses
4.4. Protein Extraction and Western Blot
4.5. Caspase Activity
4.6. Genomic DNA (gDNA) Extraction and Oxidative DNA Damage Assays
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filho, D.W.; Torres, M.A.; Bordin, A.L.; Crezcynski-Pasa, T.B.; Boveris, A. Spermatic cord torsion, reactive oxygen and nitro-gen species and ischemia-reperfusion injury. Mol. Asp. Med. 2004, 25, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Tsounapi, P.; Dimitriadis, F.; Higashi, Y.; Shimizu, T.; Saito, M. Testicular torsion-detorsion and potential therapeutic treatments: A possible role for ischemic postconditioning. Int. J. Urol. 2016, 23, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Pancreat. ß-Cell Biol. Health Dis. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Simon, A.R.; Rai, U.; Fanburg, B.L.; Cochran, B. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. Physiol. 1998, 275, C1640–C1652. [Google Scholar] [CrossRef]
- Lachance, C.; Leclerc, P. Mediators of the JAK/STAT Signaling Pathway in Human Spermatozoa1. Biol. Reprod. 2011, 85, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zuo, Q.-S.; Li, D.; Lian, C.; Ahmed, E.K.; Tang, B.-B.; Song, J.-Z.; Zhang, Y.-N.; Li, B.-C. Study on the role of JAK/STAT signaling pathway during chicken spermatogonial stem cells generation based on RNA-Seq. J. Integr. Agric. 2015, 14, 939–948. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, B. Correlative study on the JAK-STAT/PSMβ3 signal transduction pathway in asthenozoospermia. Exp. Ther. Med. 2016, 13, 127–130. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Sharma, V.; Collins, L.B.; Chen, T.-H.; Herr, N.; Takeda, S.; Sun, W.; Swenberg, J.A.; Nakamura, J. Oxidative stress at low levels can induce clustered DNA lesions leading to NHEJ mediated mutations. Oncotarget 2016, 7, 25377–25390. [Google Scholar] [CrossRef]
- Hamer, G.; Kal, H.B.; Westphal, C.H.; Ashley, T.; De Rooij, D.G. Ataxia Telangiectasia Mutated Expression and Activation in the Testis1. Biol. Reprod. 2004, 70, 1206–1212. [Google Scholar] [CrossRef]
- Pacheco, S.; Maldonado-Linares, A.; Marcet-Ortega, M.; Rojas, C.; Martínez-Marchal, A.; Lazaro, J.F.; Lange, J.; Jasin, M.; Keeney, S.; Fernandez-Capetillo, O.; et al. ATR is required to complete meiotic recombination in mice. Nat. Commun. 2018, 9, e2622. [Google Scholar] [CrossRef]
- Barry, S.P.; Townsend, P.A.; Knight, R.A.; Scarabelli, T.M.; Latchman, D.S.; Stephanou, A. STAT3 modulates the DNA damage response pathway. Int. J. Exp. Pathol. 2010, 91, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Laimins, L.A. The JAK-STAT Transcriptional Regulator, STAT-5, Activates the ATM DNA Damage Pathway to Induce HPV 31 Genome Amplification upon Epithelial Differentiation. PLOS Pathog. 2013, 9, e1003295. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, A.; Zou, L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Yan, S.; Sorrell, M.; Berman, Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell. Mol. Life Sci. 2014, 71, 3951–3967. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.; Foiani, M.; Kumar, A. ATM and ATR signaling at a glance. J. Cell Sci. 2015, 128, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Bakkenist, C.J.; Meyts, E.R.-D.; Skakkebæk, N.E.; Sehested, M.; Lukas, J.; Kastan, M.B.; Bartek, J. ATM Activation in Normal Human Tissues and Testicular Cancer. Cell Cycle 2005, 4, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Royo, H.; Prosser, H.; Ruzankina, Y.; Mahadevaiah, S.K.; Cloutier, J.M.; Baumann, M.; Fukuda, T.; Höög, C.; Tóth, A.; de Rooij, D.G.; et al. ATR acts stage specifically to regulate multiple aspects of mammalian meiotic silencing. Genes Dev. 2013, 27, 1484–1494. [Google Scholar] [CrossRef]
- Turner, T.T.; Lysiak, J.J. Oxidative Stress: A Common Factor in Testicular Dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Song, J.; Gao, X.; Tang, Z.; Li, H.; Ruan, Y.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Jiang, H. Protective effect of Berberine on reproductive function and spermatogenesis in diabetic rats via inhibition of ROS/JAK2/NFκB pathway. Andrology 2020, 8, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, C.; Liu, J.; Chen, J.; Huang, C.; Wang, J.; Liang, Z.; Wen, L.; Yi, J.E.; Yuan, Z. Betulinic Acid Attenuates T-2-Toxin-Induced Testis Oxidative Damage Through Regulation of the JAK2/STAT3 Signaling Pathway in Mice. Biomolecules 2019, 9, 787. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zhai, Q.Q.; Zhu, Y.F.; Liu, B.Y.; Xu, Y. Quercetin ameliorates testosterone secretion disorder by inhibiting endoplasmic reticulum stress through the miR-1306-5p/HSD17B7 axis in diabetic rats. Bosn. J. Basic Med. Sci. 2021, 6299. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, A.; Latchman, D.S. Opposing actions of STAT-1 and STAT-3. Growth Factors 2005, 23, 177–182. [Google Scholar] [CrossRef]
- Townsend, P.; Scarabelli, T.M.; Davidson, S.; Knight, R.A.; Latchman, D.S.; Stephanou, A. STAT-1 Interacts with p53 to Enhance DNA Damage-induced Apoptosis. J. Biol. Chem. 2004, 279, 5811–5820. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Liu, B.; Liao, J.; Rao, X.; Kushner, S.; Chung, C.D.; Chang, D.D.; Shuai, K. Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl. Acad. Sci. USA 1998, 95, 10626–10631. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Egashira, J.; Urase, K.; Kuida, K.; Momoi, T. Caspase-9 processing by caspase-3 via a feedback amplification loop in vivo. Cell Death Differ. 2001, 8, 335–344. [Google Scholar] [CrossRef]
- Alvarez, J.V.; Frank, D.A. Genome-wide analysis of STAT target genes: Elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol. Ther. 2004, 3, 1045–1050. [Google Scholar] [CrossRef]
- Arany, I.; Megyesi, J.; Nelkin, B.; Safirstein, R. STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int. 2006, 70, 669–674. [Google Scholar] [CrossRef]
- Al-Maghrebi, M.; Alnajem, A.S.; Esmaeil, A. Epigallocatechin-3-gallate modulates germ cell apoptosis through the SAFE/Nrf2 signaling pathway. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2019, 393, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, X.; Meng, F.; Deng, S.; Dai, H.; Bao, B.; Feng, J.; Li, H.; Wang, B. Biological Network Model of Effect of Chronic Intermittent Hypoxia on Spermatogenesis in Rats. Med. Sci. Monit. 2020, 26, e925579-1. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.C.D.S.; Uchida, Y.; Zhao, D.; Ke, B.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Blockade of Janus kinase-2 signaling ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transplant. 2010, 16, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Deng, J.; Wang, B.; Cheng, D.; Yang, Q.; Dong, H.; Xiong, L. Reactive Oxygen Species Scavenger Inhibits STAT3 Activation after Transient Focal Cerebral Ischemia–Reperfusion Injury in Rats. Anesthesia Analg. 2011, 113, 153–159. [Google Scholar] [CrossRef]
- Takagi, Y.; Harada, J.; Chiarugi, A.; Moskowitz, M.A. STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. J. Cereb. Blood Flow Metab. 2002, 22, 1311–1318. [Google Scholar] [CrossRef]
- Si, Y.; Bao, H.; Han, L.; Shi, H.; Zhang, Y.; Xu, L.; Liu, C.; Wang, J.; Yang, X.; Vohra, A.; et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J. Transl. Med. 2013, 11, 141. [Google Scholar] [CrossRef]
- Wen, S.-H.; Li, Y.; Li, C.; Xia, Z.-Q.; Liu, W.-F.; Zhang, X.-Y.; Lei, W.-L.; Huang, W.-Q.; Liu, K.-X. Ischemic Postconditioning During Reperfusion Attenuates Intestinal Injury and Mucosal Cell Apoptosis by Inhibiting JAK/STAT Signaling Activation. Shock 2012, 38, 411–419. [Google Scholar] [CrossRef]
- Vilenchik, M.M.; Knudson, A.G. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 12871–12876. [Google Scholar] [CrossRef]
- Al-Saleh, F.; Khashab, F.; Fadel, F.; Al-Kandari, N.; Al-Maghrebi, M. Inhibition of NADPH oxidase alleviates germ cell apoptosis and ER stress during testicular ischemia reperfusion injury. Saudi J. Biol. Sci. 2020, 27, 2174–2184. [Google Scholar] [CrossRef]
- LeDuc, F.; Nkoma, G.B.; Boissonneault, G. Spermiogenesis and DNA Repair: A Possible Etiology of Human Infertility and Genetic Disorders. Syst. Biol. Reprod. Med. 2008, 54, 3–10. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R.I. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Bronson, R.; Smith, T.B.; De Iuliis, G. The source and significance of DNA damage in human spermatozoa; A commentary on diagnostic strategies and straw man fallacies. Mol. Hum. Reprod. 2013, 19, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, R.K.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Alshahrani, S.; Durairajanayagam, D.; Sabanegh, E. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod. Biol. Endocrinol. 2014, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Li, Z.; Pearlman, A.H.; Hsieh, P. DNA mismatch repair and the DNA damage response. DNA Repair 2016, 38, 94–101. [Google Scholar] [CrossRef]
- McCormick, J.; Barry, S.P.; Sivarajah, A.; Stefanutti, G.; Townsend, P.A.; Lawrence, K.M.; Eaton, S.; Knight, R.A.; Thiemermann, C.; Latchman, D.S.; et al. Free radical scavenging inhibits STAT phosphorylation following in vivo ischemia/reperfusion injury. FASEB J. 2006, 20, 2115–2117. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Liang, Z. STAT1-p53-p21axis-dependent stress-induced progression of chronic nephrosis in adriamycin-induced mouse model. Ann. Transl. Med. 2020, 8, 1002. [Google Scholar] [CrossRef]
- Marchenko, N.D.; Moll, U.M. Mitochondrial death functions of p53. Mol. Cell. Oncol. 2014, 1, e955995. [Google Scholar] [CrossRef]
- Valenzuela, M.T.; Guerrero, R.; Nuñez, M.I.; De Almodóvar, J.M.R.; Sarker, M.; De Murcia, G.; Oliver, F.J. PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene 2002, 21, 1108–1116. [Google Scholar] [CrossRef]
- Kedar, P.S.; Stefanick, D.F.; Horton, J.K.; Wilson, S.H. Interaction between PARP-1 and ATR in mouse fibroblasts is blocked by PARP inhibition. DNA Repair 2008, 7, 1787–1798. [Google Scholar] [CrossRef]
- Crawford, R.S.; Albadawi, H.; Atkins, M.D.; Jones, J.E.; Yoo, H.-J.; Conrad, M.F.; Austen, W.G.; Watkins, M.T. Postischemic poly (ADP-ribose) polymerase (PARP) inhibition reduces ischemia reperfusion injury in a hind-limb ischemia model. Surgery 2010, 148, 110–118. [Google Scholar] [CrossRef][Green Version]
- Oliver, F.J.; Menissier-de Murcia, J.; Nacci, C.; Decker, P.; Andriantsitohaina, R.; Muller, S.; de la Rubia, G.; Stoclet, J.C.; de Murcia, G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. Embo J. 1999, 18, 4446–4454. [Google Scholar] [CrossRef]
- Wang, C.; Xu, W.; An, J.; Liang, M.; Li, Y.; Zhang, F.; Tong, Q.; Huang, K. Poly(ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cho, Y.-Y.; Petersen, B.L.; Bode, A.M.; Zhu, F.; Dong, Z. Ataxia Telangiectasia Mutated Proteins, MAPKs, and RSK2 Are Involved in the Phosphorylation of STAT3. J. Biol. Chem. 2003, 278, 12650–12659. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharma, Y.; Viswanathan, P.; Gupta, S. Cellular cytokine receptor signaling and ATM pathway intersections affect hepatic DNA repair. Cytokine 2020, 127, 154946. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C.; Hirotsune, S.; Paylor, R.; Liyanage, M.; Eckhaus, M.; Collins, F.; Shiloh, Y.; Crawley, J.N.; Ried, T.; Tagle, D.; et al. Atm-Deficient Mice: A Paradigm of Ataxia Telangiectasia. Cell 1996, 86, 159–171. [Google Scholar] [CrossRef]
- Pacheco, S.; Marcet-Ortega, M.; Lange, J.; Jasin, M.; Keeney, S.; Roig, I. The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes. PLoS Genet. 2015, 11, e1005017. [Google Scholar] [CrossRef]
- Cui, J.; Holmes, E.H.; Greene, T.G.; Liu, P.K. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J. 2000, 14, 955–967. [Google Scholar] [CrossRef]
- Kuang, X.; Yan, M.; Ajmo, J.M.; Scofield, V.L.; Stoica, G.; Wong, P.K. Activation of AMP-activated protein kinase in cerebella of Atm−/− mice is attributable to accumulation of reactive oxygen species. Biochem. Biophys. Res. Commun. 2012, 418, 267–272. [Google Scholar] [CrossRef]
- Zahedi, K.; Bissler, J.J.; Wang, Z.; Josyula, A.; Lu, L.; Diegelman, P.; Kisiel, N.; Porter, C.W.; Soleimani, M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am. J. Physiol. Physiol. 2007, 292, C1204–C1215. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.G. Testicular biopsy score count—a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khashab, F.; Al-Saleh, F.; Al-Kandari, N.; Fadel, F.; Al-Maghrebi, M. JAK Inhibition Prevents DNA Damage and Apoptosis in Testicular Ischemia-Reperfusion Injury via Modulation of the ATM/ATR/Chk Pathway. Int. J. Mol. Sci. 2021, 22, 13390. https://doi.org/10.3390/ijms222413390
Khashab F, Al-Saleh F, Al-Kandari N, Fadel F, Al-Maghrebi M. JAK Inhibition Prevents DNA Damage and Apoptosis in Testicular Ischemia-Reperfusion Injury via Modulation of the ATM/ATR/Chk Pathway. International Journal of Molecular Sciences. 2021; 22(24):13390. https://doi.org/10.3390/ijms222413390
Chicago/Turabian StyleKhashab, Farah, Farah Al-Saleh, Nora Al-Kandari, Fatemah Fadel, and May Al-Maghrebi. 2021. "JAK Inhibition Prevents DNA Damage and Apoptosis in Testicular Ischemia-Reperfusion Injury via Modulation of the ATM/ATR/Chk Pathway" International Journal of Molecular Sciences 22, no. 24: 13390. https://doi.org/10.3390/ijms222413390
APA StyleKhashab, F., Al-Saleh, F., Al-Kandari, N., Fadel, F., & Al-Maghrebi, M. (2021). JAK Inhibition Prevents DNA Damage and Apoptosis in Testicular Ischemia-Reperfusion Injury via Modulation of the ATM/ATR/Chk Pathway. International Journal of Molecular Sciences, 22(24), 13390. https://doi.org/10.3390/ijms222413390




