Production and Characterization of Chitooligosaccharides: Evaluation of Acute Toxicity, Healing, and Anti-Inflammatory Actions
Abstract
:1. Introduction
2. Results and Discussion
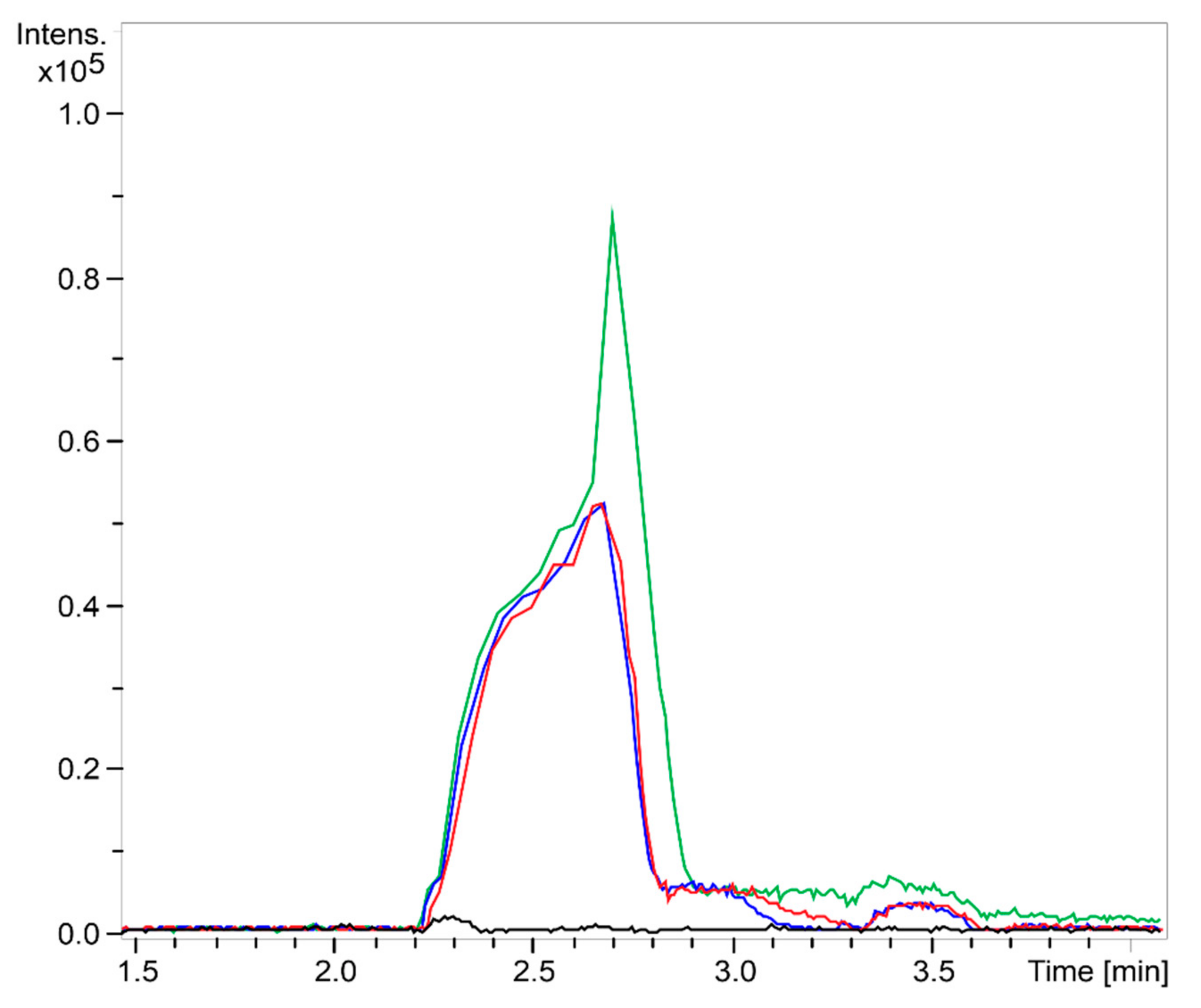
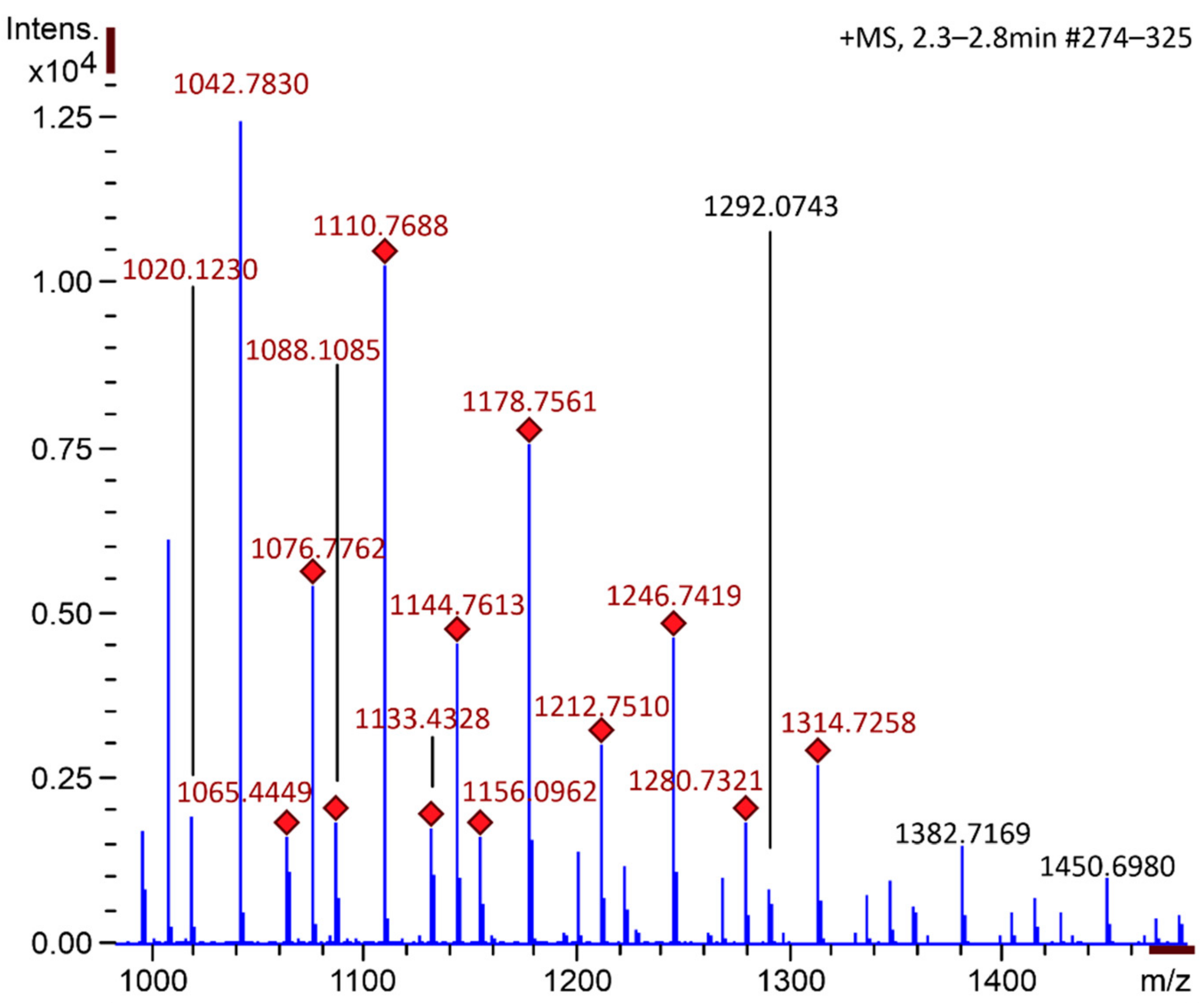
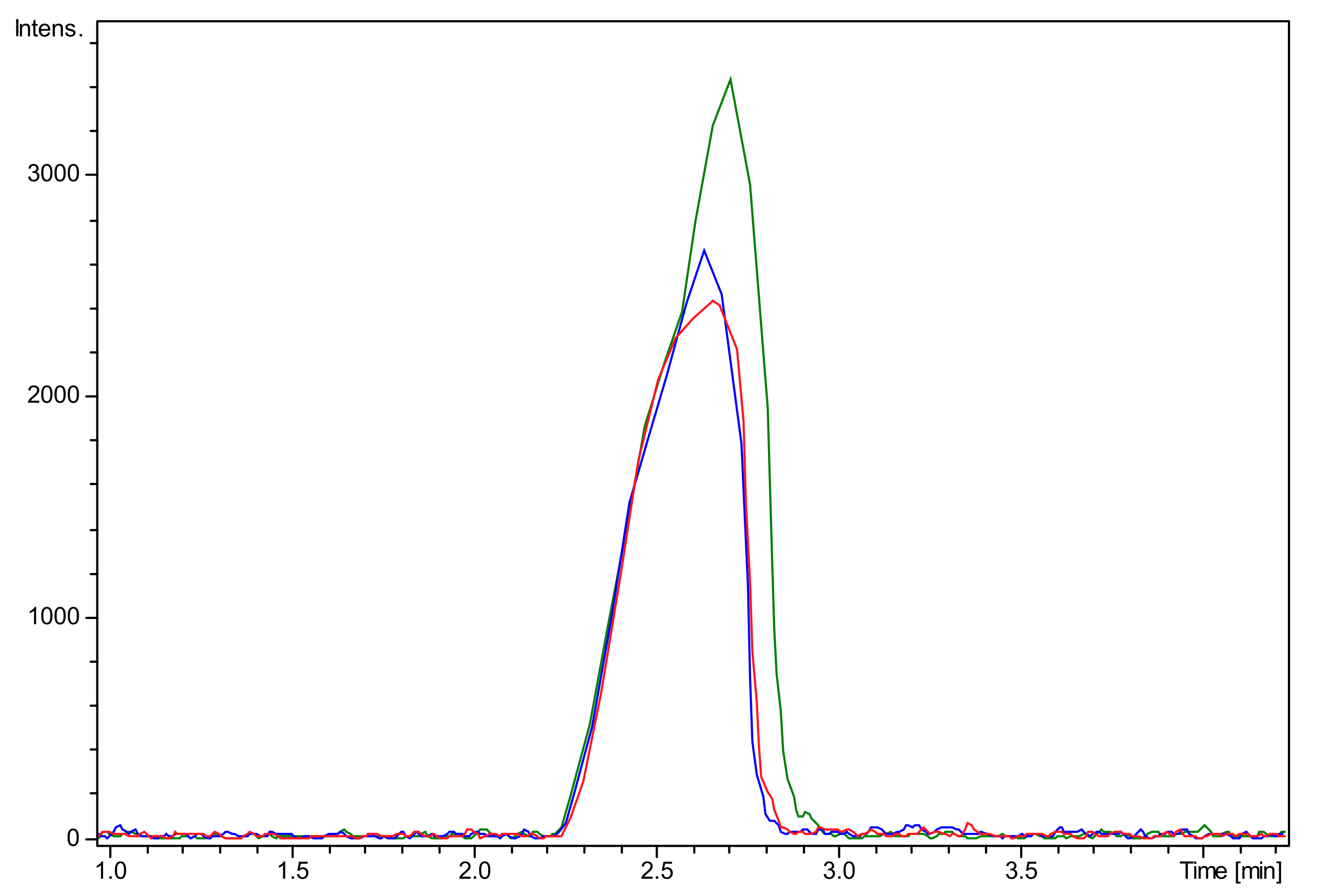
2.1. Chitooligosaccharide Production and Characterization
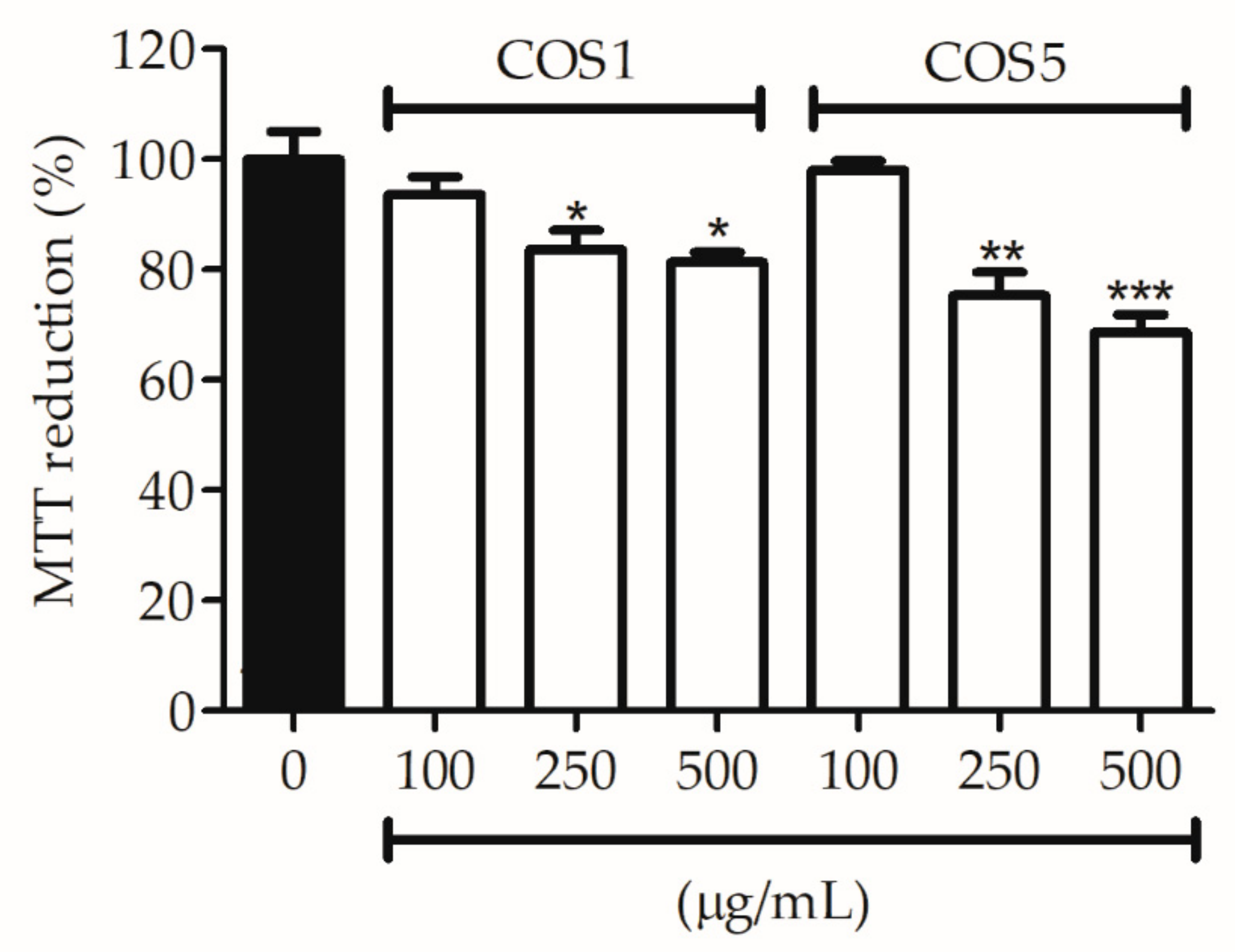
2.2. Cell Viability
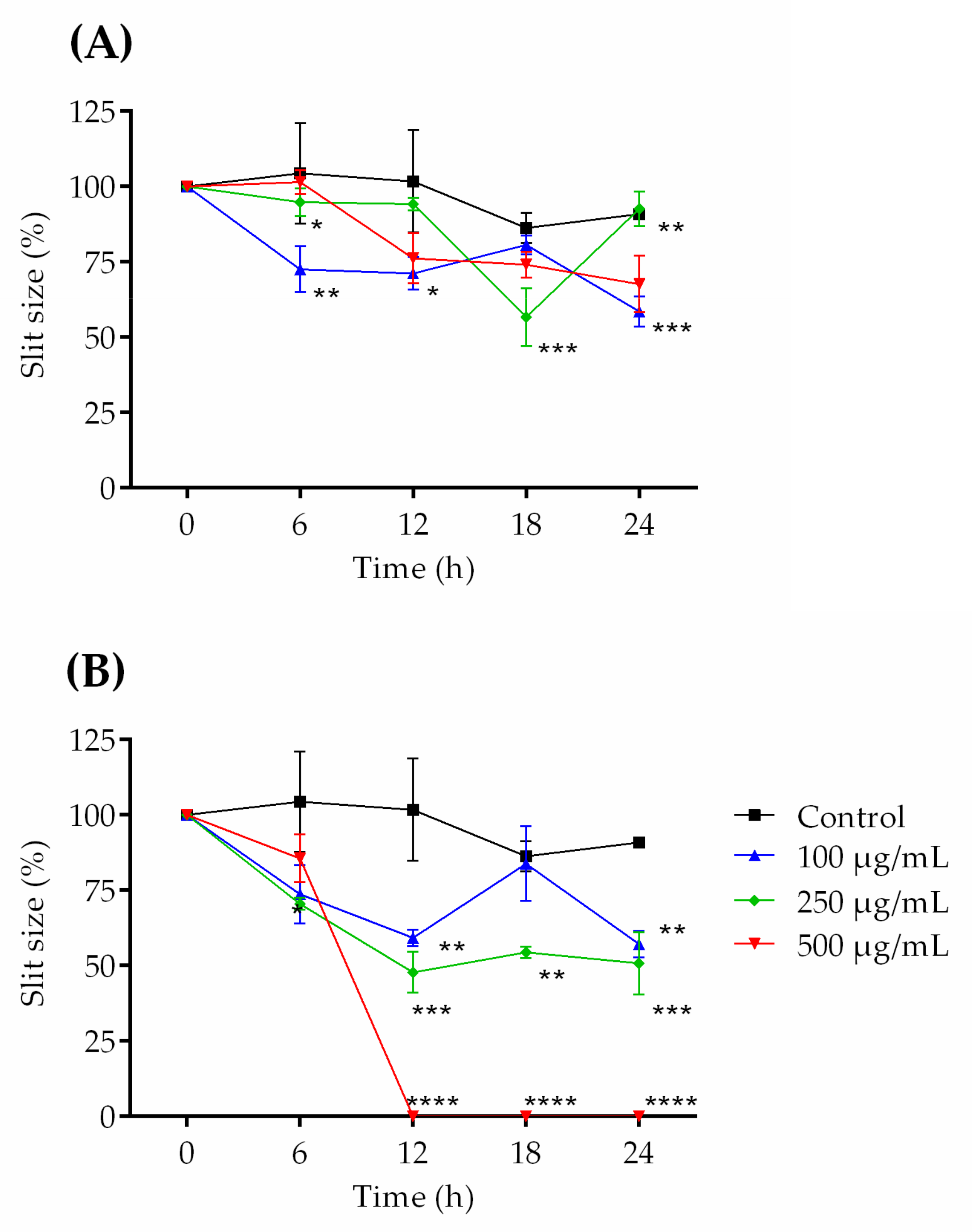
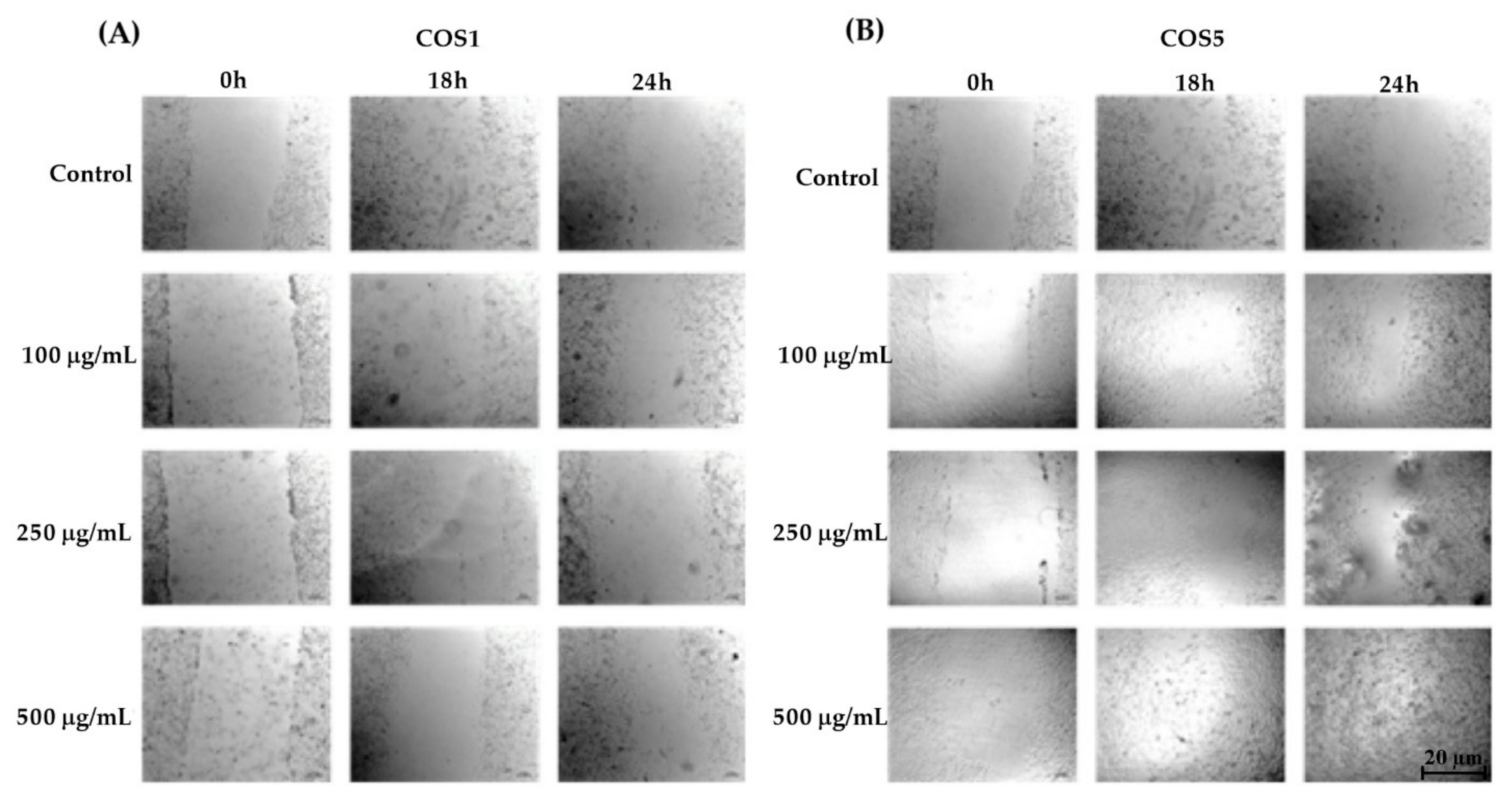
2.3. Healing Test (“Scratch”) In Vitro
2.4. Acute Toxicity Model
2.5. Xylene-Induced Ear Edema Model
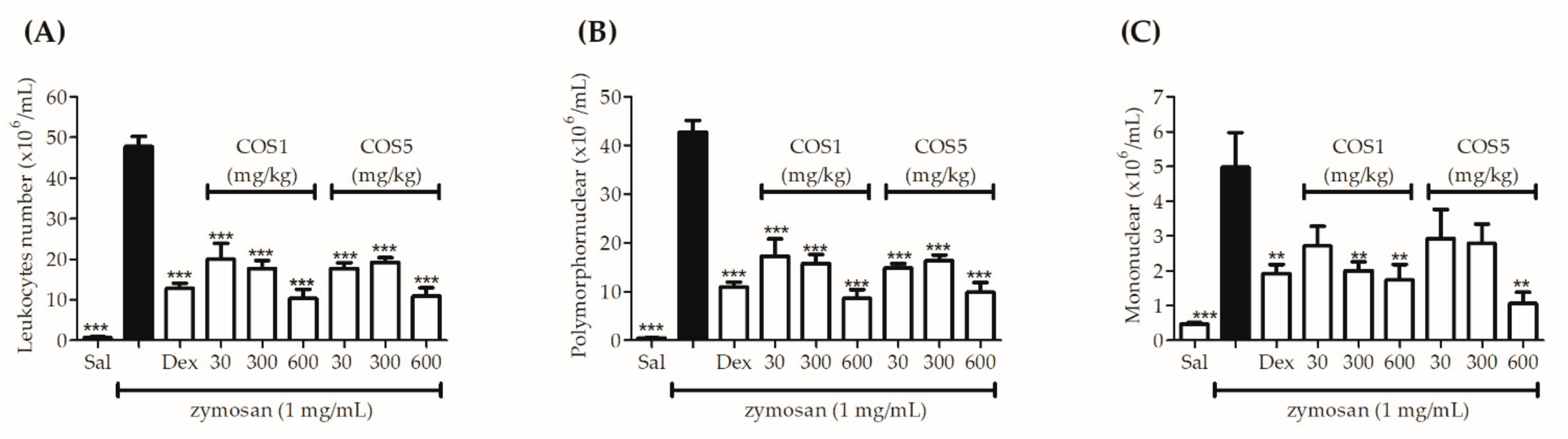
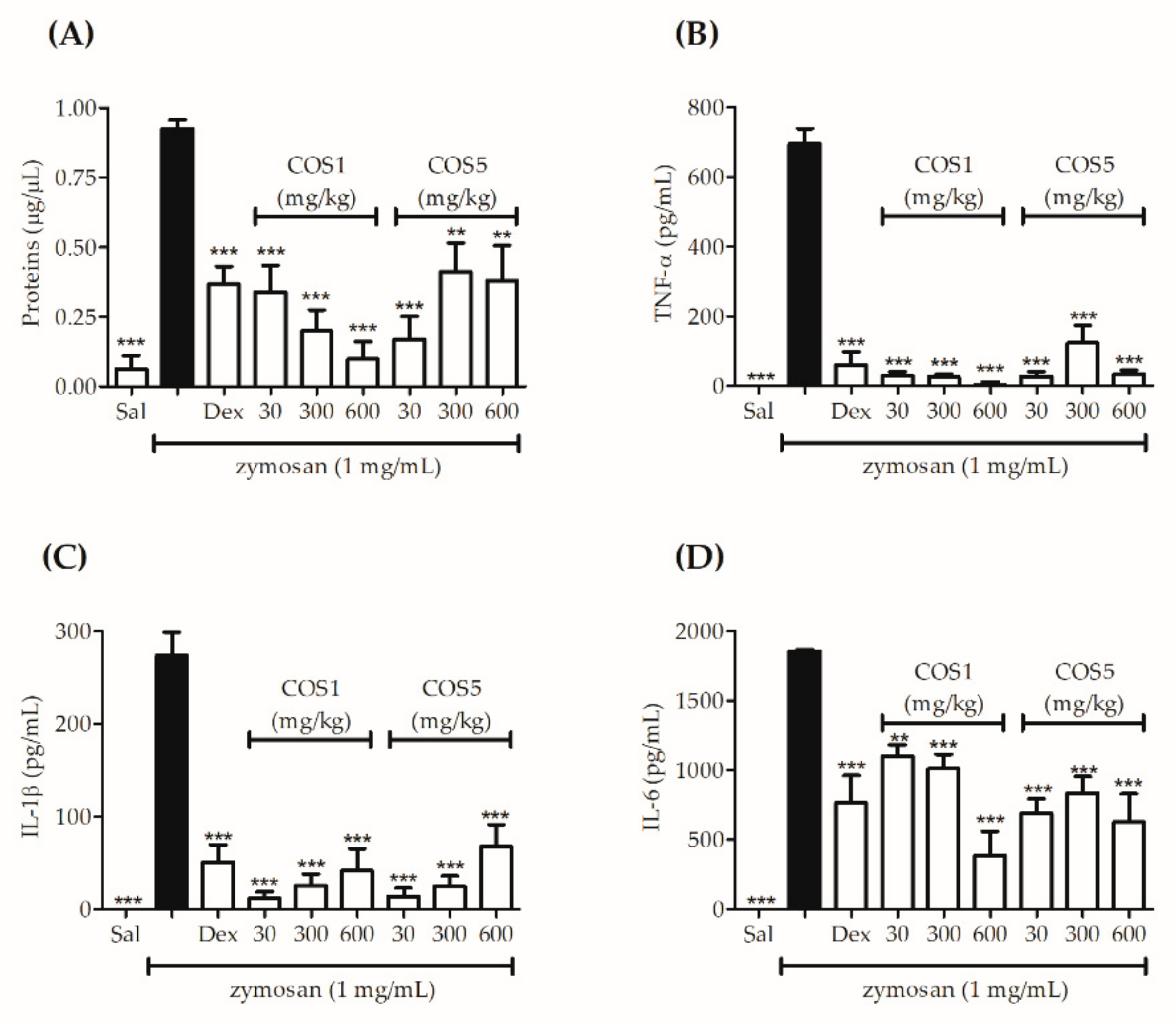
2.6. Zymosan-Induced Air Pouch Model
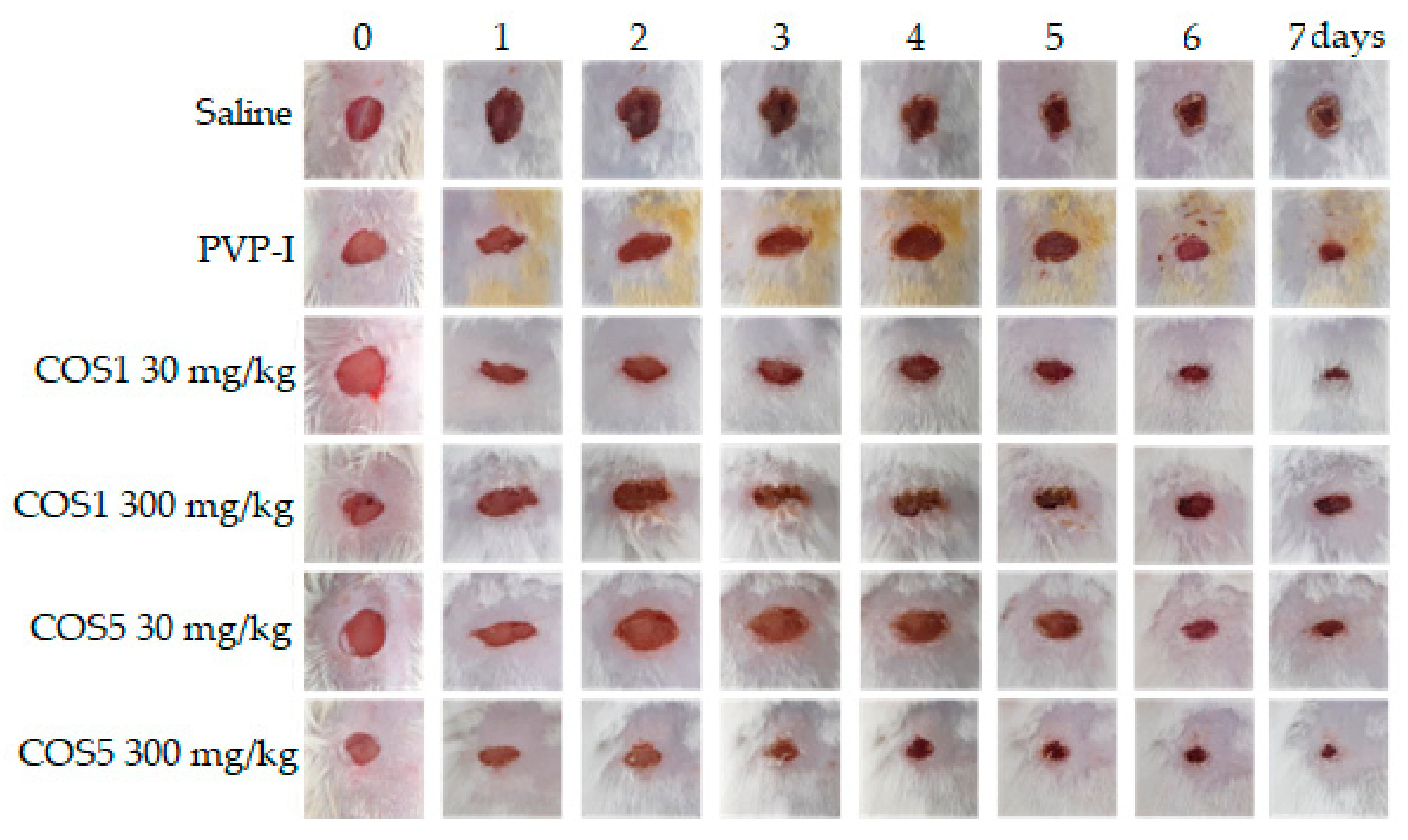
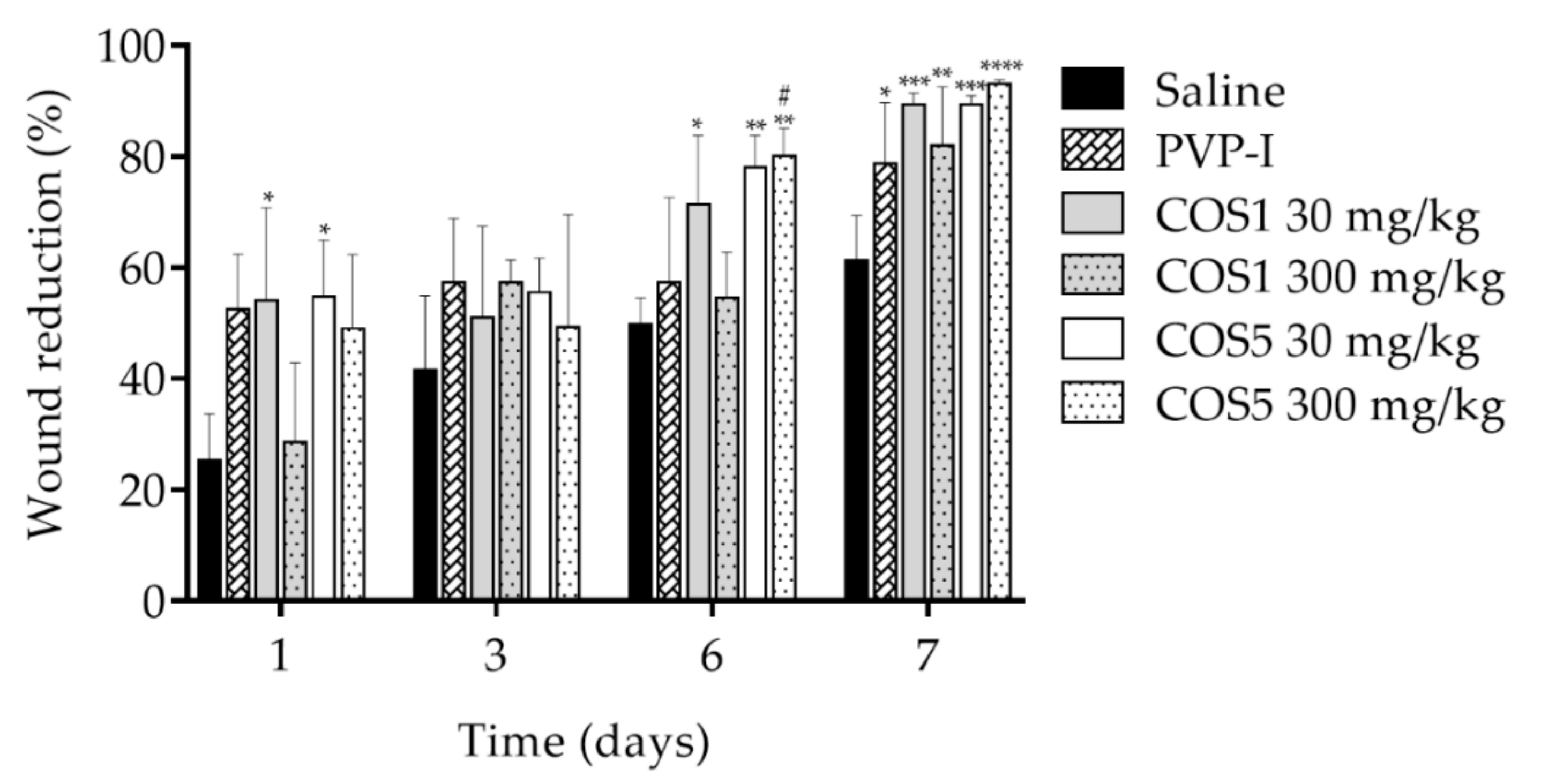
2.7. Healing Test In Vivo
3. Materials and Methods
3.1. Materials
3.2. Chitooligosaccharide Production and Characterization
3.2.1. Chitosanase Production and Chitosan Hydrolysis
3.2.2. Mass Spectrometry
3.3. Tests In Vitro
3.3.1. Cytotoxicity Assay (MTT)
3.3.2. Healing Test (“Scratch”) In Vitro
3.4. Tests In Vivo
3.4.1. Animals
3.4.2. Acute Toxicity Model
3.4.3. Xylene-Induced Ear Edema Model
3.4.4. Zymosan-Induced Air Pouch Model
3.4.5. Wound Induction
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef]
- Usman, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S.; Zia, F. Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications. Int. J. Biol. Macromol. 2016, 86, 630–645. [Google Scholar] [CrossRef]
- Pereira, L.A.; Reis, L.S.; Batista, F.A.; Mendes, A.N.; Osajima, J.A.; Silva-Filho, E.C. Biological properties of chitosan derivatives associated with the ceftazidime drug. Carbohydr. Polym. 2019, 222, 115002. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Chen, W.; Yue, L.; Jiang, Q.; Liu, X.; Xia, W. Synthesis of varisized chitosan-selenium nanocomposites through heating treatment and evaluation of their antioxidant properties. Int. J. Biol. Macromol. 2018, 114, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Chatterji, A.; Dash, B.P.; Nelson, B.R.; Sarkar, T.; Shahimi, S.; Edinur, H.A.; Manan, T.S.B.A.; Jena, P.; Mohanta, Y.K.; et al. Structural characterization and antioxidant potential of chitosan by γ-Irradiation from the carapace of horseshoe crab. Polymers 2020, 12, 2361. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus longirostris shrimp shell waste. Ann. Pharm. Françaises 2016, 74, 27–33. [Google Scholar] [CrossRef]
- Adnan, S.; Ranjha, N.M.; Hanif, M.; Asghar, S. O-Carboxymethylated chitosan; A promising tool with in-vivo anti-inflammatory and analgesic properties in albino rats. Int. J. Biol. Macromol. 2020, 156, 531–536. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Attjioui, M.; Leitão, A.; Moerschbacher, B.M.; Cavalheiro, É.T.G. Characterization, solubility and biological activity of amphihilic biopolymeric Schiff bases synthesized using chitosans. Carbohydr. Polym. 2019, 220, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.; Kim, S.; Jeon, Y.-J.; Je, J.; Ahn, C.; Moon, S.; Jeon, B.; Park, P. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockuviene, A.; Sereikaite, J. Preparation and characterisation of novel water-soluble β-carotene-chitooligosaccharides complexes. Carbohydr. Polym. 2019, 225, 115226. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Kurozumi, S.; Kiyose, M.; Tsuka, T.; Murahata, Y.; Imagawa, T.; Itoh, N.; Minami, S.; Sato, K.; et al. Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr. Polym. 2015, 115, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef] [Green Version]
- Mollaei, M.; Abbasi, A.; Hassan, Z.M.; Pakravan, N. The intrinsic and extrinsic elements regulating inflammation. Life Sci. 2020, 260, 118258. [Google Scholar] [CrossRef]
- Silva, N.S.; Araújo, N.K.; Daniele-Silva, A.; Oliveira, J.W.F.; Medeiros, J.M.; Araújo, R.M.; Ferreira, L.S.; Rocha, H.A.O.; Silva-Júnior, A.A.; Silva, M.S.; et al. Antimicrobial activity of chitosan oligosaccharides with special attention to antiparasitic potential. Mar. Drugs 2021, 19, 110. [Google Scholar] [CrossRef]
- Katiyar, D.; Singh, B.; Lall, A.M.; Haldar, C. Efficacy of chitooligosaccharides for the management of diabetes in alloxan induced mice: A correlative study with antihyperlipidemic and antioxidative activity. Eur. J. Pharm. Sci. 2011, 44, 534–543. [Google Scholar] [CrossRef]
- Han, F.-S.; Cui, B.-H.; You, X.-F.; Xing, Y.-F.; Sun, X.-W. Anti-proliferation and radiosensitization effects of chitooligosaccharides on human lung cancer line HepG2. Asian Pac. J. Trop. Med. 2015, 8, 757–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assis, C.F.; Costa, L.S.; Melo-Silveira, R.F.; Oliveira, R.M.; Pagnoncelli, M.G.B.; Rocha, H.A.O.; Macedo, G.R.; Santos, E.S. Chitooligosaccharides antagonize the cytotoxic effect of glucosamine. World J. Microbiol. Biotechnol. 2012, 28, 1097–1105. [Google Scholar] [CrossRef]
- Huang, H.-C.; Hong, L.; Chang, P.; Zhang, J.; Lu, S.-Y.; Zheng, B.-W.; Jiang, Z.-F. Chitooligosaccharides attenuate Cu2+-Induced cellular oxidative damage and cell apoptosis involving Nrf2 activation. Neurotox. Res. 2015, 27, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Koo, J.C.; Park, J.K. Antifungal effect of chitosan as Ca2+ channel blocker. Plant Pathol. J. 2016, 32, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Phil, L.; Naveed, M.; Mohammad, I.S.; Bo, L.; Bin, D. Chitooligosaccharide: An evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomed. Pharmacother. 2018, 102, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Bernaerts, K.V.; Dodi, G.; Shavandi, A. Chitooligosaccharides for wound healing biomaterials engineering. Mater. Sci. Eng. C 2020, 117, 111266. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, W.; Lei, Y.; Gaucher, C.; Pei, S.; Zhang, J.; Xia, X. Edaravone-loaded alginate-based nanocomposite hydrogel accelerated chronic wound healing in diabetic mice. Mar. Drugs 2019, 17, 285. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine collagen peptides from the skin of nile tilapia (Oreochromis niloticus): Characterization and wound healing evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- McEvoy, N.; Avsar, P.; Patton, D.; Curley, G.; Kearney, C.J.; Moore, Z. The economic impact of pressure ulcers among patients in intensive care units. A systematic review. J. Tissue Viability 2021, 30, 168–177. [Google Scholar] [CrossRef]
- Lai, C.-S.; Tu, C.-W.; Kuo, H.-C.; Sun, P.-P.; Tsai, M.-L. Type II collagen from cartilage of Acipenser baerii promotes wound healing in human dermal fibroblasts and in mouse skin. Mar. Drugs 2020, 18, 511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Sun, C.; Hu, W.; Zhao, J.; Li, G.; Zhang, L.; Liu, M.; Liu, Y.; Ding, F.; et al. Chitosan degradation products promote nerve regeneration by stimulating Schwann cell proliferation via miR-27a/FOXO1 axis. Mol. Neurobiol. 2016, 53, 28–39. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; Pei, Y.; Yeo, Y.; Hong, S. Topical application of zwitterionic chitosan suppresses neutrophil-mediated acute skin inflammation. Int. J. Biol. Macromol. 2020, 158, 1184–1193. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.W. Anti-inflammatory glucocorticoid drugs: Reflections after 60 years. Inflammopharmacology 2011, 19, 1–19. [Google Scholar] [CrossRef]
- Dantas, J.M.M.; Araújo, N.K.; Silva, N.S.; Torres-Rêgo, M.; Furtado, A.A.; Assis, C.F.; Araújo, R.M.; Teixeira, J.A.; Ferreira, L.S.; Fernandes-Pedrosa, M.F.; et al. Purification of chitosanases produced by Bacillus toyonensis CCT 7899 and functional oligosaccharides production. Prep. Biochem. Biotechnol. 2021, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harish Prashanth, K.V.; Tharanathan, R.N. Depolymerized products of chitosan as potent inhibitors of tumor-induced angiogenesis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1722, 22–29. [Google Scholar] [CrossRef]
- Rafael, O.-H.; Fernándo, Z.-G.L.; Abraham, P.-T.; Alberto, V.-L.P.; Guadalupe, G.-S.; Pablo, P.J. Production of chitosan-oligosaccharides by the chitin-hydrolytic system of Trichoderma harzianum and their antimicrobial and anticancer effects. Carbohydr. Res. 2019, 486, 107836. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-M.; Kim, S.-K. Chitooligosaccharides inhibit activation and expression of matrix metalloproteinase-2 in human dermal fibroblasts. FEBS Lett. 2006, 580, 2661–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.J.; Park, J.K.; Park, Y. Il Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE–antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef]
- Ramaiah, S.K. Preclinical safety assessment: Current gaps, challenges, and approaches in identifying translatable biomarkers of drug-induced liver injury. Clin. Lab. Med. 2011, 31, 161–172. [Google Scholar] [CrossRef]
- Kamran, M.; Khan, M.R.; Khan, H.U.; Abbas, M.; Iqbal, M.; Nazir, A. Phytochemical and cytotoxic evaluation of Medicago monantha: In vivo protective potential in rats. Biomed. Pharmacother. 2018, 102, 1052–1063. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals. Acute Oral Toxicity-Acute Toxic Class Method, Guideline No.423; Adapted 2001; Organisation for Economic Cooperatio and Development: Rome, Italy, 2001; pp. 1–14. [Google Scholar]
- Bitencourt, M.A.O.; Dantas, G.R.; Lira, D.P.; Barbosa-Filho, J.M.; de Miranda, G.E.C.; Santos, B.V.D.O.; Souto, J.T. Aqueous and methanolic extracts of Caulerpa mexicana suppress cell migration and ear edema induced by inflammatory agents. Mar. Drugs 2011, 9, 1332–1345. [Google Scholar] [CrossRef]
- Hu, X.-J.; Jin, H.-Z.; Xu, W.-Z.; Chen, M.; Liu, X.-H.; Zhang, W.-D.; Su, J.; Zhang, C.; Zhang, W.-D. Anti-inflammatory and analgesic activities of Edgeworthia chrysantha and its effective chemical constituents. Biol. Pharm. Bull. 2008, 31, 1761–1765. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.D.; Vasko, M.R. Cellular Mechanisms of Neurogenic Inflammation. J. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Barry, C.M.; Helps, S.C.; Heuvel, C.V.; Vink, R. Characterizing the role of the neuropeptide substance P in experimental subarachnoid hemorrhage. Brain Res. 2011, 1389, 143–151. [Google Scholar] [CrossRef]
- Lee, S.-H.; Senevirathne, M.; Ahn, C.-B.; Kim, S.-K.; Je, J.-Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.A.; Choi, J.S.; Lee, M.C.; Kim, E.; Nam, T.J.; Fujii, H.; Hong, Y.K. Anti-inflammatory activities of methanol extracts from various seaweed species. J. Environ. Biol. 2008, 29, 465–469. [Google Scholar] [PubMed]
- Makni-Maalej, K.; Chiandotto, M.; Hurtado-Nedelec, M.; Bedouhene, S.; Gougerot-Pocidalo, M.A.; Dang, P.M.C.; El-Benna, J. Zymosan induces NADPH oxidase activation in human neutrophils by inducing the phosphorylation of p47phox and the activation of Rac2: Involvement of protein tyrosine kinases, PI3Kinase, PKC, ERK1/2 and p38MAPkinase. Biochem. Pharmacol. 2013, 85, 92–100. [Google Scholar] [CrossRef]
- Jiang, L.I.; Sternweis, P.C.; Wang, J.E. Zymosan activates protein kinase A via adenylyl cyclase VII to modulate innate immune responses during inflammation. Mol. Immunol. 2013, 54, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, A.T.G.; Cunha, T.M.; Verri, W.A.; Gazzinelli, R.T.; Teixeira, M.M.; Cunha, F.Q.; Ferreira, S.H. Toll-like receptor 2/MyD88 signaling mediates zymosan-induced joint hypernociception in mice: Participation of TNF-α, IL-1β and CXCL1/KC. Eur. J. Pharmacol. 2011, 674, 51–57. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xu, Q.-S.; Du, Y.-G.; Xu, J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr. Polym. 2014, 99, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, Y.; Song, S.; Tong, C.; Shi, X.; Zhao, Y.; Zhang, J.; Hou, M. Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydr. Polym. 2019, 205, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Liu, Y.; Li, K.; Yu, H.; Liu, S.; Yang, Y.; Chen, X.; Li, P. Monomer composition of chitooligosaccharides obtained by different degradation methods and their effects on immunomodulatory activities. Carbohydr. Polym. 2017, 157, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-W.; Li, C.-W.; Wang, Q.; Shi, S.-J.; Hu, M.; Zhang, Q.; Cui, H.-H.; Sun, J.-B.; Zhou, M.; Wu, G.-L.; et al. The cellular and molecular mechanisms underlying silver nanoparticle/chitosan oligosaccharide/poly (vinyl alcohol) nanofiber-mediated wound healing. J. Biomed. Nanotechnol. 2017, 13, 17–34. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Huang, X.; Chen, M.; Wu, H.; Jiao, Y.; Zhou, C. Macrophage polarization mediated by chitooligosaccharide (COS) and associated osteogenic and angiogenic activities. ACS Biomater. Sci. Eng. 2020, 6, 1614–1629. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef]
- Dantas, J.M.M.; Silva, N.S.; Padilha, C.E.A.; Araújo, N.K.; Santos, E.S. Enhancing chitosan hydrolysis aiming chitooligosaccharides production by using immobilized chitosanolytic enzymes. Biocatal. Agric. Biotechnol. 2020, 28, 101759. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Fidelis, G.P.; Costa, M.P.S.S.; Telles, C.B.S.; Dantas-Santos, N.; Elias, S.O.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; Leite, E.L.; et al. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int. J. Mol. Sci. 2012, 13, 409–426. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Balekar, N.; Katkam, N.G.; Nakpheng, T.; Jehtae, K.; Srichana, T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J. Ethnopharmacol. 2012, 141, 817–824. [Google Scholar] [CrossRef]
- Tonin, T.D.; Thiesen, L.C.; de Oliveira Nunes, M.L.; Broering, M.F.; Donato, M.P.; Goss, M.J.; Petreanu, M.; Niero, R.; Machado, I.D.; Santin, J.R. Rubus imperialis (Rosaceae) extract and pure compound niga-ichigoside F1: Wound healing and anti-inflammatory effects. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 1235–1244. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Furtado, A.A.; Bitencourt, M.A.O.; Lima, M.C.J.S.; Andrade, R.C.L.C.; Azevedo, E.P.; Soares, T.C.; Tomaz, J.C.; Lopes, N.P.; Silva-Júnior, A.A.; et al. Anti-inflammatory activity of aqueous extract and bioactive compounds identified from the fruits of Hancornia speciosa Gomes (Apocynaceae). BMC Complement. Altern. Med. 2016, 16, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.T.; Guerra, G.C.B.; Marques, J.I.; Torres-Rêgo, M.; Alves, J.S.F.; Vasconcelos, R.C.; Araújo, D.F.S.; Abreu, L.S.; Carvalho, T.G.; Araújo, D.R.C.; et al. Potentialities of cashew nut (Anacardium occidentale) by-product for pharmaceutical applications: Extraction and purification technologies, safety, and anti-inflammatory and anti-arthritis activities. Rev. Bras. Farmacogn. 2020, 30, 652–666. [Google Scholar] [CrossRef]
- Furtado, A.A.; Torres-Rêgo, M.; Lima, M.C.J.S.; Bitencourt, M.A.O.; Estrela, A.B.; Silva, N.S.; Siqueira, E.M.S.; Tomaz, J.C.; Lopes, N.P.; Silva-Júnior, A.A.; et al. Aqueous extract from Ipomoea asarifolia (Convolvulaceae) leaves and its phenolic compounds have anti-inflammatory activity in murine models of edema, peritonitis and air-pouch inflammation. J. Ethnopharmacol. 2016, 192, 225–235. [Google Scholar] [CrossRef]
- Marques, J.I.; Alves, J.S.F.; Torres-Rêgo, M.; Furtado, A.A.; Siqueira, E.M.S.; Galinari, E.; Araújo, D.F.S.; Guerra, G.C.B.; Azevedo, E.P.; Fernandes-Pedrosa, M.F.; et al. Phytochemical analysis by HPLC–HRESI-MS and anti-inflammatory activity of Tabernaemontana catharinensis. Int. J. Mol. Sci. 2018, 19, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, F.O.; Torres-Rêgo, M.; Gomes, J.A.S.; Félix-Silva, J.; Passos, J.G.R.; Santis Ferreira, L.; Silva-Júnior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Mangaba (Hancornia speciosa Gomes) fruit juice decreases acute pulmonary edema induced by Tityus serrulatus venom: Potential application for auxiliary treatment of scorpion stings. Toxicon 2020, 179, 42–52. [Google Scholar] [CrossRef]
- Dantas-Medeiros, R.; Furtado, A.A.; Zanatta, A.C.; Torres-Rêgo, M.; Lourenço, E.M.G.; Alves, J.S.F.; Galinari, É.; Rocha, H.A.O.; Guerra, G.C.B.; Vilegas, W.; et al. Mass spectrometry characterization of Commiphora leptophloeos leaf extract and preclinical evaluation of toxicity and anti-inflammatory potential effect. J. Ethnopharmacol. 2021, 264, 113229. [Google Scholar] [CrossRef] [PubMed]
- Nitz, A.C.; Ely, J.B.; José, A.; Tames, D.R. Estudo morfométrico no processo de cicatrização de feridas cutâneas em ratos, usando: Coronopu didymus e Calendula officinali. Arq. Catarin. Med. 2006, 35, 74–79. [Google Scholar]
- Daniele-Silva, A.; Rodrigues, S.C.S.; Dos Santos, E.C.G.; Queiroz-Neto, M.F.; Rocha, H.A.O.; Silva-Júnior, A.A.; Resende, J.M.; Araújo, R.M.; Fernandes-Pedrosa, M.F. NMR three-dimensional structure of the cationic peptide Stigmurin from Tityus stigmurus scorpion venom: In vitro antioxidant and in vivo antibacterial and healing activity. Peptides 2021, 137, 170478. [Google Scholar] [CrossRef] [PubMed]
| Body Weight Gain | PBS | COS 1 | COS 5 |
|---|---|---|---|
| Initial weight (g) | 29.550 ± 0.610 | 29.500 ± 0.431 ns | 29.310 ± 0.588 ns |
| Final weight (g) | 33.390 ± 1.002 | 33.040 ± 0.716 ns | 32.920 ± 0.643 ns |
| Body weight gain (%) | 11.39 ± 0.944 | 10.63 ± 1.488 ns | 10.92 ± 1.744 ns |
| Relative organ weight (g/10 g of body) | |||
| Lungs | 0.053 ± 0.003 | 0.057 ± 0.003 ns | 0.062 ± 0.003 * |
| Heart | 0.085 ± 0.008 | 0.076 ± 0.006 ns | 0.071 ± 0.004 ns |
| Liver | 0.542 ± 0.020 | 0.500 ± 0.010 ns | 0.508 ± 0.023 ns |
| Spleen | 0.056 ± 0.003 | 0.052 ± 0.008 ns | 0.061 ± 0.007 ns |
| Kidneys | 0.125 ± 0.005 | 0.122 ± 0.005 ns | 0.123 ± 0.009 ns |
| Biochemical Parameters | PBS | COS1 | COS5 |
|---|---|---|---|
| ALT (U/L) | 55.20 ± 1.855 | 62.80 ± 2.245 ns | 60.50 ± 1.360 ns |
| AST (U/L) | 105.20 ± 4.434 | 94.50 ± 2.245 ns | 106.60 ± 4.032 ns |
| Total protein (g/dL) | 5.88 ± 0.558 | 5.92 ± 0.066 ns | 6.32 ± 0.102 ** |
| Albumin (g/dL) | 1.85 ± 0.028 | 1.82 ± 0.020 ns | 1.82 ± 0.047 ns |
| Glucose (mg/dL) | 83.40 ± 5.036 | 84.60 ± 4.173 ns | 83.70 ± 3.675 ns |
| Cholesterol (mg/dL) | 90.00 ± 4.868 | 104.20 ± 6.256 ns | 102.60 ± 4.411 ns |
| Creatinine (mg/dL) | 0.18 ± 0.004 | 0.17 ± 0.013 ns | 0.20 ± 0.023 ns |
| Urea (mg/dL) | 33.00 ± 3.342 | 35.50 ± 0.9574 ns | 32.00 ± 2.214 ns |
| Acid uric (U/L) | 1.32 ± 0.126 | 1.45 ± 0.125 ns | 1.42 ± 0.197 ns |
| Hematological Parameters | PBS | COS1 | COS5 |
|---|---|---|---|
| Erythrocytes (106/mm3) | 8.02 ± 0.300 | 7.58 ± 0.537 ns | 8.85 ± 0.279 ns |
| Hemoglobin (g/dL) | 18.08 ± 0.759 | 16.62 ± 1.175 ns | 18.24 ± 0.201 ns |
| Hematocrit (%) | 45.22 ± 2.364 | 41.24 ± 3.88 ns | 50.04 ± 1.825 ns |
| MCV (fL) | 56.20 ± 1.319 | 54.00 ± 1.673 ns | 55.20 ± 0.374 ns |
| MCH (pg) | 22.54 ± 0.177 | 21.96 ± 0.668 ns | 20.72 ± 0.532 ns |
| MCHC (g/dL) | 40.18 ± 0.774 | 40.78 ± 1.405 ns | 38.40 ± 0.418 ns |
| RDW (%) | 15.98 ± 0.504 | 15.92 ± 0.457 ns | 15.88 ± 0.096 ns |
| Platelets (103/mm3) | 617.6 ± 1.79 | 655.2 ± 2.98 ns | 576.4 ± 1.546 ns |
| MPV (fL) | 7.00 ± 0.151 | 7.30 ± 0.202 ns | 7.58 ± 0.576 ns |
| Leukocytes (103/mm3) | 6.26 ± 0.728 | 7.22 ± 1.304 ns | 5.90 ± 0.862 ns |
| Lymphocytes (103/mm3) | 5.17 ± 0.773 | 6.16 ± 1.127 ns | 4.94 ± 0.681 ns |
| Monocytes (103/mm3) | 0.52 ± 0.058 | 0.66 ± 0.074 ns | 0.42 ± 0.048 ns |
| Granulocytes (103/mm3) | 0.38 ± 0.073 | 0.40 ± 0.083 ns | 0.44 ± 0.092 ns |
| Groups | Dose (mg/kg) | Difference (mg) | Inhibition (%) |
|---|---|---|---|
| Saline Dexamethasone | ---- 2 | 28.380 ± 4.547 5.360 ± 0.980 *** | ---- 81.11 |
| COS1 | 30 | 11.12 ± 1.192 *** | 60.81 |
| COS1 | 300 | 3.100 ± 2.691 *** | 89.07 |
| COS1 | 600 | 6.360 ± 1.589 *** | 77.58 |
| COS5 | 30 | 5.360 ± 2.654 *** | 81.11 |
| COS5 | 300 | 2.380 ± 2.216 *** | 91.61 |
| COS5 | 600 | 8.440 ± 2.758 *** | 70.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade, R.C.L.C.; de Araújo, N.K.; Torres-Rêgo, M.; Furtado, A.A.; Daniele-Silva, A.; de Souza Paiva, W.; de Medeiros Dantas, J.M.; da Silva, N.S.; da Silva-Júnior, A.A.; Ururahy, M.A.G.; et al. Production and Characterization of Chitooligosaccharides: Evaluation of Acute Toxicity, Healing, and Anti-Inflammatory Actions. Int. J. Mol. Sci. 2021, 22, 10631. https://doi.org/10.3390/ijms221910631
de Andrade RCLC, de Araújo NK, Torres-Rêgo M, Furtado AA, Daniele-Silva A, de Souza Paiva W, de Medeiros Dantas JM, da Silva NS, da Silva-Júnior AA, Ururahy MAG, et al. Production and Characterization of Chitooligosaccharides: Evaluation of Acute Toxicity, Healing, and Anti-Inflammatory Actions. International Journal of Molecular Sciences. 2021; 22(19):10631. https://doi.org/10.3390/ijms221910631
Chicago/Turabian Stylede Andrade, Rafael Caetano Lisbôa Castro, Nathália Kelly de Araújo, Manoela Torres-Rêgo, Allanny Alves Furtado, Alessandra Daniele-Silva, Weslley de Souza Paiva, Julia Maria de Medeiros Dantas, Nayara Sousa da Silva, Arnóbio Antônio da Silva-Júnior, Marcela Abbott Galvão Ururahy, and et al. 2021. "Production and Characterization of Chitooligosaccharides: Evaluation of Acute Toxicity, Healing, and Anti-Inflammatory Actions" International Journal of Molecular Sciences 22, no. 19: 10631. https://doi.org/10.3390/ijms221910631
APA Stylede Andrade, R. C. L. C., de Araújo, N. K., Torres-Rêgo, M., Furtado, A. A., Daniele-Silva, A., de Souza Paiva, W., de Medeiros Dantas, J. M., da Silva, N. S., da Silva-Júnior, A. A., Ururahy, M. A. G., de Assis, C. F., De Santis Ferreira, L., Rocha, H. A. O., & de Freitas Fernandes-Pedrosa, M. (2021). Production and Characterization of Chitooligosaccharides: Evaluation of Acute Toxicity, Healing, and Anti-Inflammatory Actions. International Journal of Molecular Sciences, 22(19), 10631. https://doi.org/10.3390/ijms221910631








