Transplantation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in a Swine Model of Geographic Atrophy
Abstract
1. Introduction
2. Results
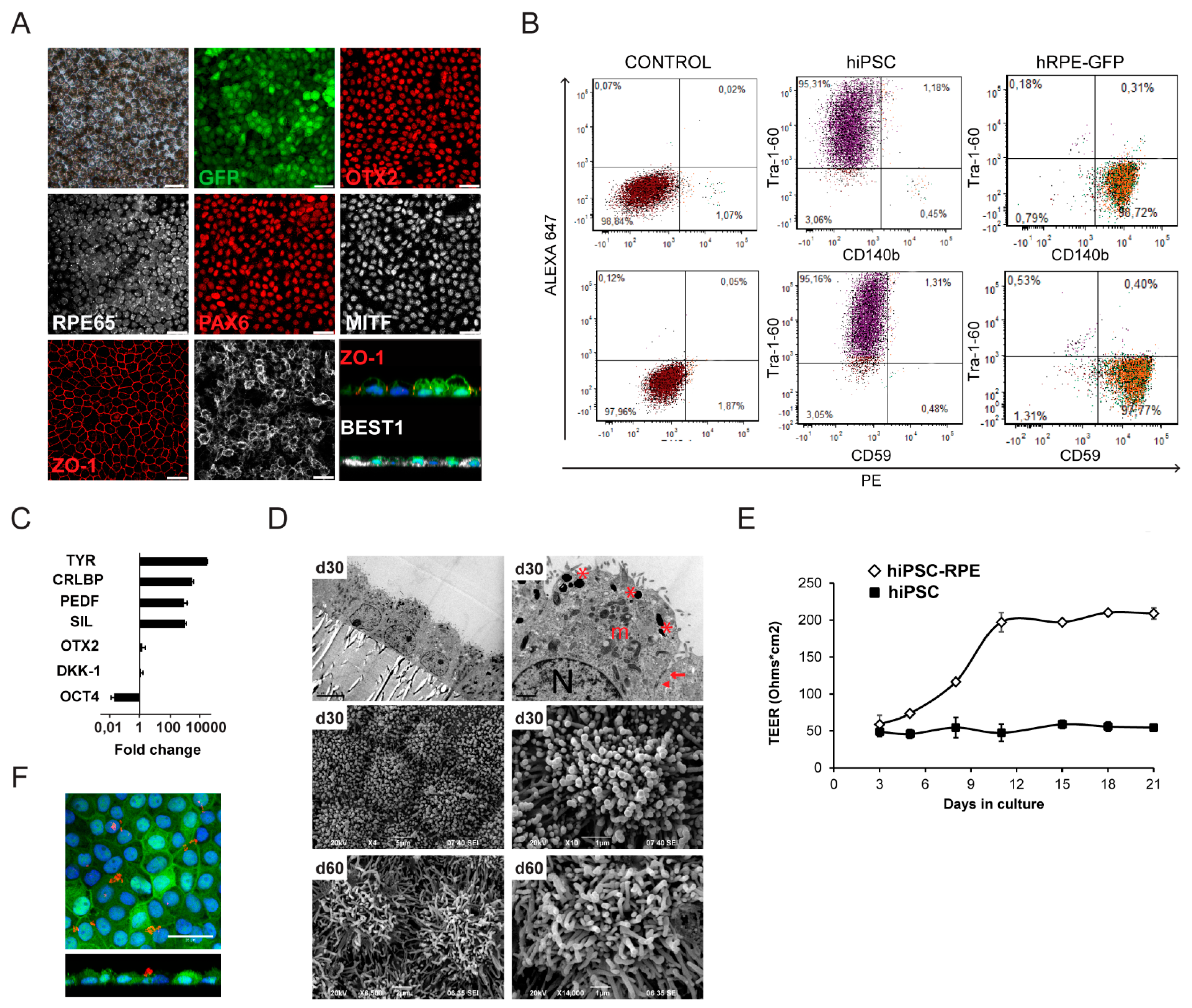
2.1. Generation and Characterization of hiPSC-RPE Cells
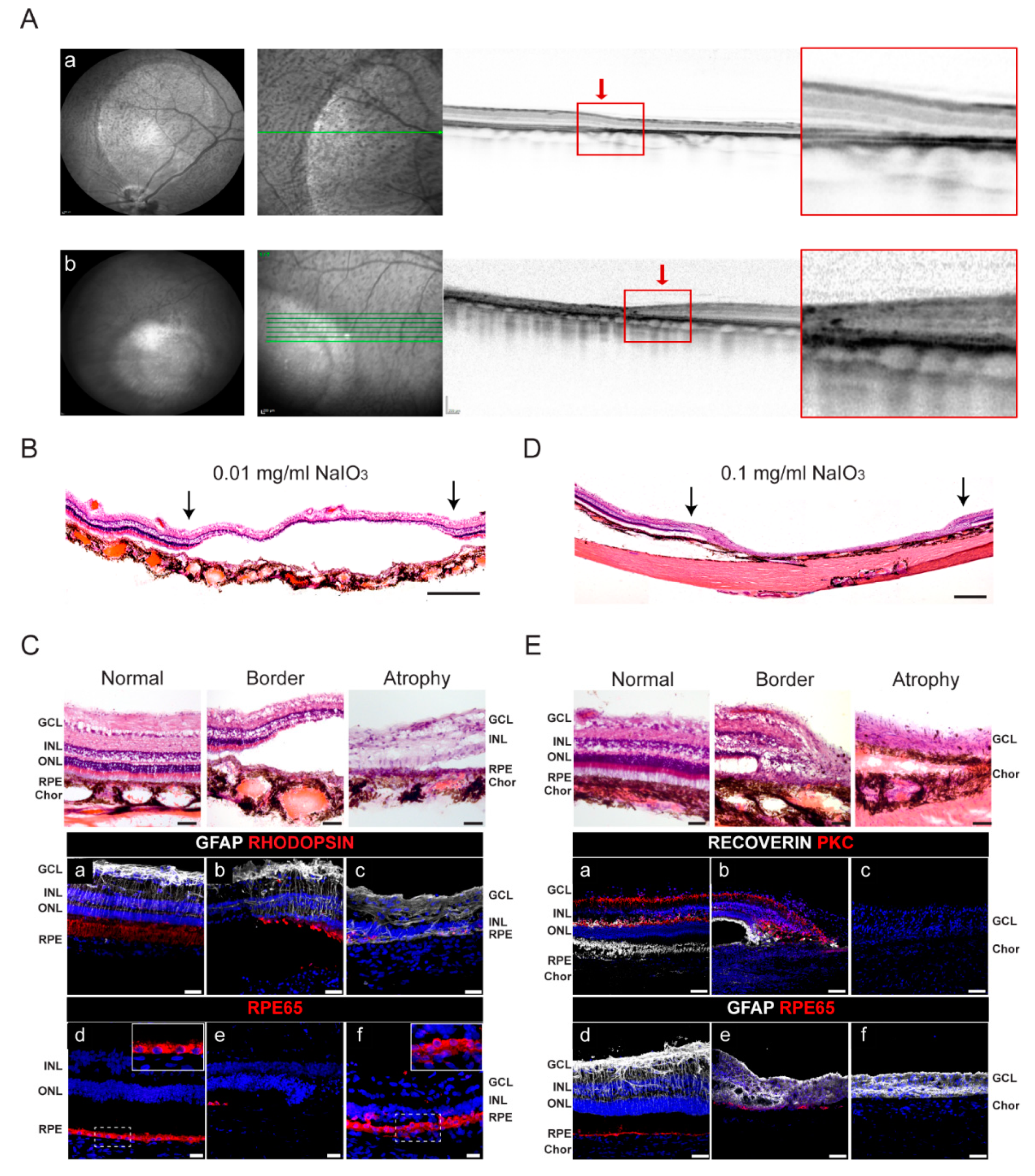
2.2. Generation of a Swine Model with Geographic Atrophy-like Features
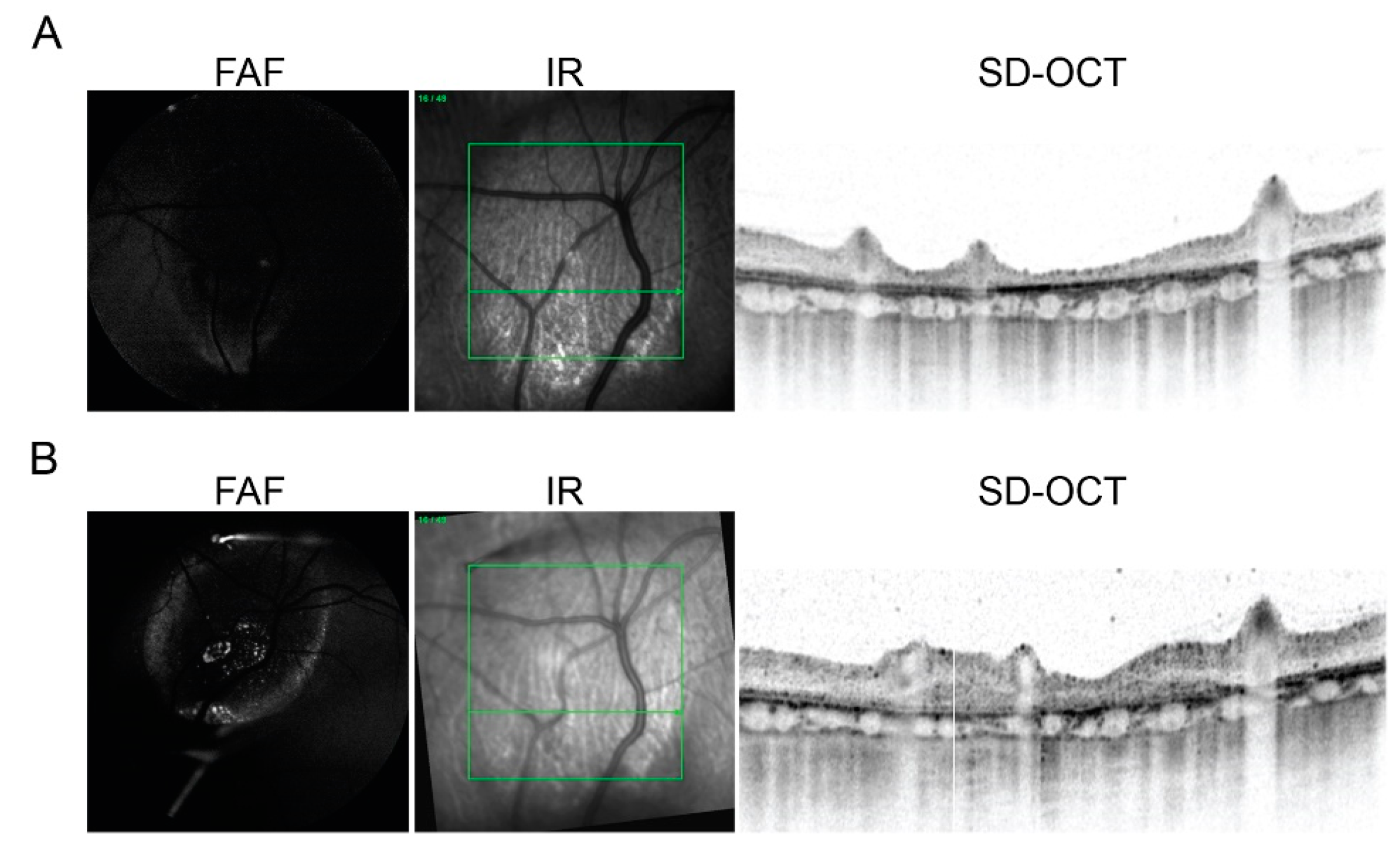
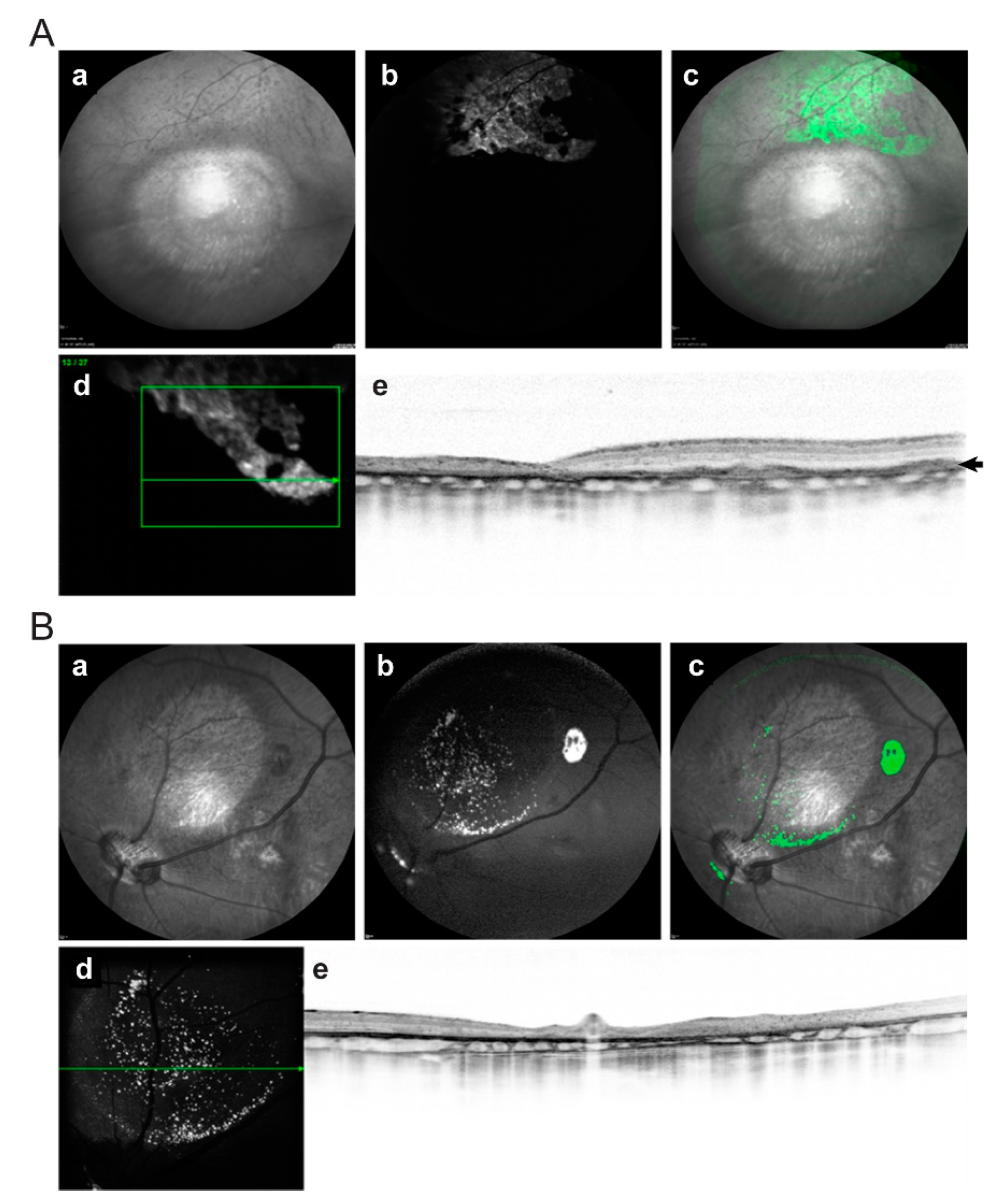
2.3. In Vivo Imaging of Transplanted hiPSC-RPE Cells in the Porcine Eyes
2.4. Post-Mortem Analysis of Retinas Transplanted with hiPSC-RPE Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Generation of hiPSC Expressing GFP and Their Differentiation to RPE Cells
4.3. Immunocytochemistry
4.4. Messenger RNA Expression by Quantitative Reverse-Transcriptase Polymerase Chain Reaction
4.5. Transepithelial Electrical Resistance Measurements
4.6. Electron Microscopy
4.7. Photoreceptor Outer Segment Phagocytosis Assays
4.8. Animals, Preoperative Preparation, and Study Design
4.9. NaIO3 Subretinal Injection
4.10. Subretinal Injection of hiPSC-RPE Single Cell Suspension
4.11. Postoperative Follow-Up
4.12. Histology and Immunohistochemistry
4.13. Image Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colijn, J.M.; Buitendijk, G.H.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular De-generation in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Chirco, K.R.; Sohn, E.; Stone, E.M.; Tucker, B.; Mullins, R.F. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye 2016, 31, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bedell, M.; Zhang, K. Age-related Macular Degeneration: Genetic and Environmental Factors of Disease. Mol. Interv. 2010, 10, 271–281. [Google Scholar] [CrossRef]
- Bracha, P.; Moore, N.A.; Ciulla, T. Induced pluripotent stem cell-based therapy for age-related macular degeneration. Expert Opin. Biol. Ther. 2017, 17, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.M.; Ayton, L.N.; Guymer, R.H.; Lowery, A.J.; Blamey, P.J.; Allen, P.J.; Luu, C.D.; Rosenfeld, J.V. Advances in implantable bionic devices for blindness: A review. ANZ J. Surg. 2016, 86, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.S.; Weiland, J.; Fujii, G.Y.; Greenberg, R.; Williamson, R.; Little, J.; Mech, B.; Cimmarusti, V.; Van Boemel, G.; Dagnelie, G.; et al. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vis. Res. 2003, 43, 2573–2581. [Google Scholar] [CrossRef]
- Borooah, S.; Phillips, M.; Bilican, B.; Wright, A.; Wilmut, I.; Chandran, S.; Gamm, D.; Dhillon, B. Using human induced pluripotent stem cells to treat retinal disease. Prog. Retin. Eye Res. 2013, 37, 163–181. [Google Scholar] [CrossRef]
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27. [Google Scholar] [CrossRef]
- Chichagova, V.; Hallam, D.; Collin, J.; Zerti, D.; Dorgau, B.; Felemban, M.; Lako, M.; Steel, D.H. Cellular regeneration strategies for macular degen-eration: Past, present and future. Eye 2018, 32, 946–971. [Google Scholar] [CrossRef]
- Higuchi, A.; Kumar, S.S.; Benelli, G.; Alarfaj, A.A.; Munusamy, M.A.; Umezawa, A.; Murugan, K. Stem Cell Therapies for Reversing Vision Loss. Trends Biotechnol. 2017, 35, 1102–1117. [Google Scholar] [CrossRef]
- Zarbin, M. Cell-Based Therapy for Degenerative Retinal Disease. Trends Mol. Med. 2016, 22, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Reti-nal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pig-ment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Falkner-Radler, C.I.; Krebs, I.; Glittenberg, C.; Považay, B.; Drexler, W.; Graf, A.; Binder, S. Human retinal pigment epithelium (RPE) transplantation: Outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br. J. Ophthalmol. 2011, 95, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Hubschman, J.-P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef]
- Sanchez, I.; Martin, R.; Ussa, F.; Fernandez-Bueno, I. The parameters of the porcine eyeball. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.W.; Allen, D.; Petters, R.M.; Hao, Y.; Peng, Y.-W.; Wong, F. Altered light responses of single rod photoreceptors in transgenic pigs expressing P347L or P347S rhodopsin. Mol. Vis. 2005, 11, 1246–1256. [Google Scholar]
- Petters, R.M.; Alexander, C.A.; Wells, K.D.; Collins, E.B.; Sommer, J.R.; Blanton, M.R.; Rojas, G.; Hao, Y.; Flowers, W.L.; Banin, E.; et al. Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat. Biotechnol. 1997, 15, 965–970. [Google Scholar] [CrossRef]
- Ross, J.W.; De Castro, J.P.F.; Zhao, J.; Samuel, M.; Walters, E.; Rios, C.; Bray-Ward, P.; Jones, B.W.; Marc, R.E.; Wang, W.; et al. Generation of an Inbred Miniature Pig Model of Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2012, 53, 501–507. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wong, F.; Chang, J.H.; Possin, D.E.; Hao, Y.; Petters, R.M.; Milam, A.H. Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 1998, 39, 808–819. [Google Scholar]
- Shaw, L.C.; Skold, A.; Wong, F.; Petters, R.; Hauswirth, W.W.; Lewin, A. An allele-specific hammerhead ribozyme gene therapy for a porcine model of autosomal dominant retinitis pigmentosa. Mol. Vis. 2001, 7, 6–13. [Google Scholar] [PubMed]
- Monés, J.; Leiva, M.; Peña, T.; Martínez, G.; Biarnés, M.; Garcia, M.; Serrano, A.; Fernandez, E. A Swine Model of Selective Geographic Atrophy of Outer Retinal Layers Mimicking Atrophic AMD: A Phase I Escalating Dose of Subretinal Sodium Iodate. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3974–3983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, L.; Wang, W.; Liu, Y.; De Castro, J.F.; Ezashi, T.; Telugu, B.P.V.; Roberts, R.M.; Kaplan, H.J.; Dean, U.C. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells 2011, 29, 972–980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.Y.; Yin, Z.Q.; Chen, S.J.; Chen, L.F.; Liu, Y. Rescue from light-induced retinal degeneration by human fetal retinal transplantation in minipigs. Curr. Eye Res. 2009, 34, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Warfvinge, K.; Kiilgaard, J.F.; Lavik, E.B.; Scherfig, E.; Langer, R.; Klassen, H.J.; Young, M.J. Retinal progenitor cell xenografts to the pig retina: Morphologic integration and cytochemical differentiation. Arch. Ophthalmol. 2005, 123, 1385–1393. [Google Scholar] [CrossRef]
- Klassen, H.; Warfvinge, K.; Schwartz, P.H.; Kiilgaard, J.F.; Shamie, N.; Jiang, C.; Samuel, M.; Scherfig, E.; Prather, R.S.; Young, M.J. Isolation of Progenitor Cells from GFP-Transgenic Pigs and Transplantation to the Retina of Allorecipients. Cloning Stem Cells 2008, 10, 391–402. [Google Scholar] [CrossRef]
- Klassen, H.; Kiilgaard, J.F.; Zahir, T.; Ziaeian, B.; Kirov, I.; Scherfig, E.; Warfvinge, K.; Young, M.J. Progenitor Cells from the Porcine Neural Retina Express Photoreceptor Markers after Transplantation to the Subretinal Space of Allorecipients. Stem Cells 2007, 25, 1222–1230. [Google Scholar] [CrossRef]
- Ghosh, F.; Engelsberg, K.; English, R.V.; Petters, R.M. Long-term neuroretinal full-thickness transplants in a large animal model of severe retinitis pigmentosa. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 835–846. [Google Scholar] [CrossRef]
- Klassen, H.; Kiilgaard, J.F.; Warfvinge, K.; Samuel, M.S.; Prather, R.S.; Wong, F.; Petters, R.M.; La Cour, M.; Young, M.J. Photoreceptor Differentiation following Trans-plantation of Allogeneic Retinal Progenitor Cells to the Dystrophic Rhodopsin Pro347Leu Transgenic Pig. Stem Cells Int. 2012, 2012, 939801. [Google Scholar] [CrossRef]
- Brant Fernandes, R.A.; Koss, M.J.; Falabella, P.; Stefanini, F.R.; Maia, M.; Diniz, B.; Ribeiro, R.; Hu, Y.; Hinton, D.; Clegg, D.O.; et al. An Innovative Surgical Technique for Sub-retinal Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigmented Epithelium in Yucatan Mini Pigs: Preliminary Results. Ophthalmic Surg. Lasers Imaging Retina 2016, 47, 342–351. [Google Scholar] [CrossRef]
- Koss, M.J.; Falabella, P.; Stefanini, F.R.; Pfister, M.; Thomas, B.B.; Kashani, A.H.; Brant, R.; Zhu, D.; Clegg, D.O.; Hinton, D.R.; et al. Subretinal implantation of a monolayer of hu-man embryonic stem cell-derived retinal pigment epithelium: A feasibility and safety study in Yucatán minipigs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1553–1565. [Google Scholar] [CrossRef]
- Sohn, E.H.; Jiao, C.; Kaalberg, E.; Cranston, C.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: A pilot study. Sci. Rep. 2015, 5, 11791. [Google Scholar] [CrossRef]
- Bhutto, I.A.; Ogura, S.; Baldeosingh, R.; McLeod, D.S.; Lutty, G.A.; Edwards, M.M. An Acute Injury Model for the Phenotypic Char-acteristics of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD143–AMD151. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.M.; McLeod, D.S.; Bhutto, I.A.; Grebe, R.; Duffy, M.; Lutty, G.A. Subretinal Glial Membranes in Eyes with Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F.; Anand, M.; Almeida, D.; Atabai, K.; Sheppard, D.; Finnemann, S.C. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12005–12010. [Google Scholar] [CrossRef]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic At-rophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Sunness, J.S.; Gonzalez-Baron, J.; Applegate, C.A.; Bressler, N.M.; Tian, Y.; Hawkins, B.; Barron, Y.; Bergman, A. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999, 106, 1768–1779. [Google Scholar] [CrossRef]
- Kyger, M.; Worley, A.; Adamus, G. Autoimmune Responses against Photoreceptor Antigens during Retinal Degeneration and Their Role in Macrophage Recruitment into Retinas of RCS Rats. J. Neuroimmunol. 2013, 254, 91–100. [Google Scholar] [CrossRef]
- Romero-Vazquez, S.; Llorens, V.; Soler-Boronat, A.; Figueras-Roca, M.; Adan, A.; Molins, B. Interlink between Inflammation and Oxidative Stress in Age-Related Macular Degeneration: Role of Complement Factor H. Biomedicines 2021, 9, 763. [Google Scholar] [CrossRef]
- Jones, B.W.; Watt, C.B.; Frederick, J.M.; Baehr, W.; Chen, C.-K.; Levine, E.M.; Milam, A.H.; Lavail, M.M.; Marc, R.E. Retinal Remodeling Triggered by Photoreceptor Degenerations. J. Comp. Neurol. 2003, 464, 1–16. [Google Scholar] [CrossRef]
- Sarks, S.H. Drusen Patterns Predisposing to Geographic Atrophy of the Retinal Pigment Epithelium. Aust. J. Ophthalmol. 1982, 10, 91–97. [Google Scholar] [CrossRef]
- Bressler, S.B.; Maguire, M.G.; Bressler, N.M.; Fine, S.L. Relationship of Drusen and Abnormalities of the Retinal Pigment Epithelium to the Prognosis of Neovascular Macular Degeneration. The Macular Photocoagulation Study Group. Arch. Ophthalmol. 1990, 108, 1442–1447. [Google Scholar] [CrossRef]
- Usui, H.; Nishiwaki, A.; Landiev, L.; Kacza, J.; Eichler, W.; Wako, R.; Kato, A.; Takase, N.; Kuwayama, S.; Ohashi, K.; et al. In Vitro Drusen Model - Three-Dimensional Spheroid Culture of Retinal Pigment Epithelial Cells. J. Cell Sci. 2018, 132, jcs215798. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M.Y. Cell-to-Cell Transmission of Pathogenic Proteins in Neurodegenerative Diseases. Nat. Med. 2014, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Toops, K.A.; Tan, L.X.; Lakkaraju, A. Apolipoprotein E Isoforms and AMD. Adv. Exp. Med. Biol. 2016, 854, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Toops, K.A.; Tan, L.X.; Jiang, Z.; Radu, R.A.; Lakkaraju, A. Cholesterol-Mediated Activation of Acid Sphingomyelinase Disrupts Autophagy in the Retinal Pigment Epithelium. Mol. Biol. Cell 2015, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Parish, C.A.; Hashimoto, M.; Nakanishi, K. A2E, a Lipofuscin Fluorophore, in Human Retinal Pigmented Epithelial Cells in Culture. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2988–2995. [Google Scholar]
- Sparrow, J.R.; Cai, B.; Fishkin, N.; Jang, Y.P.; Krane, S.; Vollmer, H.R.; Zhou, J.; Nakanishi, K. A2E, a Fluorophore of RPE Lipofuscin: Can It Cause RPE Degeneration? Adv. Exp. Med. Biol. 2003, 533, 205–211. [Google Scholar]
- Feeney-Burns, L.; Eldred, G.E. The Fate of the Phagosome: Conversion to “age Pigment” and Impact in Human Retinal Pigment Epithelium. Trans. Ophthalmol. Soc. UK 1983, 103 Pt 4, 416–421. [Google Scholar] [PubMed]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-Grade Stem Cell-Derived Retinal Pigment Epithelium Patch Rescues Retinal Degeneration in Rodents and Pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, L.V.; Tezel, T.H.; Kaplan, H.J. Survival of Allogeneic Porcine Retinal Pigment Epithelial Sheets after Subretinal Transplantation. Investig. Ophthalmol. Vis. Sci. 2004, 45, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Warre-Cornish, K.; Barber, A.C.; Sowden, J.C.; Ali, R.R.; Pearson, R.A. Migration, Integration and Maturation of Photoreceptor Precursors Following Transplantation in the Mouse Retina. Stem Cells Dev. 2014, 23, 941–954. [Google Scholar] [CrossRef]
- Hazim, R.A.; Karumbayaram, S.; Jiang, M.; Dimashkie, A.; Lopes, V.S.; Li, D.; Burgess, B.L.; Vijayaraj, P.; Alva-Ornelas, J.A.; Zack, J.A.; et al. Differentiation of RPE Cells from Integration-Free IPS Cells and Their Cell Biological Characterization. Stem Cell Res. Ther. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Rizzolo, L.J. Barrier Properties of Cultured Retinal Pigment Epithelium. Exp. Eye Res. 2014, 126, 16–26. [Google Scholar] [CrossRef]
- Riera, M.; Fontrodona, L.; Albert, S.; Ramirez, D.M.; Seriola, A.; Salas, A.; Muñoz, Y.; Ramos, D.; Villegas-Perez, M.P.; Zapata, M.A.; et al. Comparative Study of Human Embryonic Stem Cells (HESC) and Human Induced Pluripotent Stem Cells (HiPSC) as a Treatment for Retinal Dystrophies. Mol. Ther. Methods Clin. Dev. 2016, 3, 16010. [Google Scholar] [CrossRef]
- Salas, A.; Duarri, A.; Fontrodona, L.; Ramírez, D.M.; Badia, A.; Isla-Magrané, H.; Ferreira-de-Souza, B.; Zapata, M.Á.; Raya, Á.; Veiga, A.; et al. Cell Therapy with Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium and Retinal Precursor Cells Prevents Visual Function Loss in a Rat Model of Retinal Degeneration. Mol. Ther.-Methods Clin. Dev. 2021, 20, 688–702. [Google Scholar] [CrossRef]
- Maqueda, M.; Mosquera, J.L.; García-Arumí, J.; Veiga, A.; Duarri, A. Repopulation of Decellularized Retinas with HiPSC-Derived Retinal Pigment Epithelial and Ocular Progenitor Cells Shows Cell Engraftment, Organization and Differentiation. Biomaterials 2021, 276, 121049. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Petrus-Reurer, S.; Bartuma, H.; Aronsson, M.; Westman, S.; Lanner, F.; André, H.; Kvanta, A. Integration of Subretinal Suspension Transplants of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in a Large-Eyed Model of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1314–1322. [Google Scholar] [CrossRef]
- Plaza Reyes, A.; Petrus-Reurer, S.; Antonsson, L.; Stenfelt, S.; Bartuma, H.; Panula, S.; Mader, T.; Douagi, I.; André, H.; Hovatta, O.; et al. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Stem Cell Rep. 2015, 6, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Seiler, M.J.; Zhu, D.; Falabella, P.; Hinton, D.R.; Clegg, D.O.; Humayun, M.S.; Thomas, B.B. Assessment of Safety and Functional Efficacy of Stem Cell-Based Therapeutic Approaches Using Retinal Degenerative Animal Models. Stem Cells Int. 2017, 2017, 9428176. [Google Scholar] [CrossRef]
- Nakatsuji, N.; Nakajima, F.; Tokunaga, K. HLA-Haplotype Banking and IPS Cells. Nat. Biotechnol. 2008, 26, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Montserrat, N.; Aasen, T.; Gonzalez, F.; Rodríguez-Pizà, I.; Vassena, R.; Raya, A.; Boué, S.; Barrero, M.J.; Corbella, B.A.; et al. Generation of Induced Pluripotent Stem Cells from Human Cord Blood Using OCT4 and SOX2. Cell Stem Cell 2009, 5, 353–357. [Google Scholar] [CrossRef]
- Badia, A.; Salas, A.; Duarri, A.; Ferreira-de-Souza, B.; Zapata, M.Á.; Fontrodona, L.; García-Arumí, J. Transcriptomics Analysis of Ccl2/Cx3cr1/Crb1rd8 Deficient Mice Provides New Insights into the Pathophysiology of Progressive Retinal Degeneration. Exp. Eye Res. 2021, 203, 108424. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F.; Kim, Y.; Brodie, S.E.; Huang, X.; Sheppard, D.; Finnemann, S.C. Loss of Synchronized Retinal Phagocytosis and Age-Related Blindness in Mice Lacking Alphavbeta5 Integrin. J. Exp. Med. 2004, 200, 1539–1545. [Google Scholar] [CrossRef] [PubMed]



| Animal | NaIO3 dose | In vivo | hiPSC-RPE Cells Injected | Follow-up | Evidence of hiPSC-RPE in vivo | Post-Mortem Analysis | ||
|---|---|---|---|---|---|---|---|---|
| (mg/mL) | Months | FAF | SD-OCT | Affected Layers in the Atrophy | GFP+ Cells (Area and Layers) | |||
| 1 | 0.01 | Selective atrophy of ONL | 3.3 × 105 | 6 | Yes | Yes | ONL, RPE | Healthy retina, atrophy (RPE) |
| 2 | 0.01 | Selective atrophy of ONL | 3.3 × 105 | 0.5 * | ND | ND | ONL, RPE | Healthy retina, atrophy and border (RPE) |
| 3 | 0.01 | Selective atrophy of ONL | 3.3 × 105 | 3 | No | No | ONL, RPE | No cells |
| 4 | 0.01 | Selective atrophy of ONL | 3.3 × 105 | 3 | No | No | ONL, RPE | No cells |
| 5 | 0.01 | Selective atrophy of ONL | 2.5 × 105 | 3 | Yes | Yes | ONL, RPE | Healthy retina and vitreous |
| 6 | 0.01 | Selective atrophy of ONL | 2.5 × 105 | 3 | Yes | Yes | ONL, RPE | Healthy retina (RPE) |
| 7 | 0.01 | Selective atrophy of ONL | 2.5 × 105 | 3 | Yes | Yes | ONL, RPE | Atrophy (INL, RPE), border and vitreous |
| 8 | 0.1 | Marked atrophy and thinning of all retinal layers | 3.3 × 105 | 3 | No | No | INL, ONL, RPE | No cells |
| 9 | 0.1 | Marked atrophy and thinning of all retinal layers | 3.3 × 105 | 3 | Yes | Yes | INL, ONL, RPE | Healthy retina and border (RPE) |
| 10 | 0.1 | Retinal detachment | ND | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarri, A.; Rodríguez-Bocanegra, E.; Martínez-Navarrete, G.; Biarnés, M.; García, M.; Ferraro, L.L.; Kuebler, B.; Aran, B.; Izquierdo, E.; Aguilera-Xiol, E.; et al. Transplantation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in a Swine Model of Geographic Atrophy. Int. J. Mol. Sci. 2021, 22, 10497. https://doi.org/10.3390/ijms221910497
Duarri A, Rodríguez-Bocanegra E, Martínez-Navarrete G, Biarnés M, García M, Ferraro LL, Kuebler B, Aran B, Izquierdo E, Aguilera-Xiol E, et al. Transplantation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in a Swine Model of Geographic Atrophy. International Journal of Molecular Sciences. 2021; 22(19):10497. https://doi.org/10.3390/ijms221910497
Chicago/Turabian StyleDuarri, Anna, Eduardo Rodríguez-Bocanegra, Gema Martínez-Navarrete, Marc Biarnés, Miriam García, Lucía Lee Ferraro, Bernd Kuebler, Begoña Aran, Elisabeth Izquierdo, Eli Aguilera-Xiol, and et al. 2021. "Transplantation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in a Swine Model of Geographic Atrophy" International Journal of Molecular Sciences 22, no. 19: 10497. https://doi.org/10.3390/ijms221910497
APA StyleDuarri, A., Rodríguez-Bocanegra, E., Martínez-Navarrete, G., Biarnés, M., García, M., Ferraro, L. L., Kuebler, B., Aran, B., Izquierdo, E., Aguilera-Xiol, E., Casaroli-Marano, R. P., Trias, E., Fernandez, E., Raya, Á., Veiga, A., & Monés, J. (2021). Transplantation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in a Swine Model of Geographic Atrophy. International Journal of Molecular Sciences, 22(19), 10497. https://doi.org/10.3390/ijms221910497









