7,8-Dihydroxiflavone Protects Adult Rat Axotomized Retinal Ganglion Cells through MAPK/ERK and PI3K/AKT Activation
Abstract
1. Introduction
2. Results
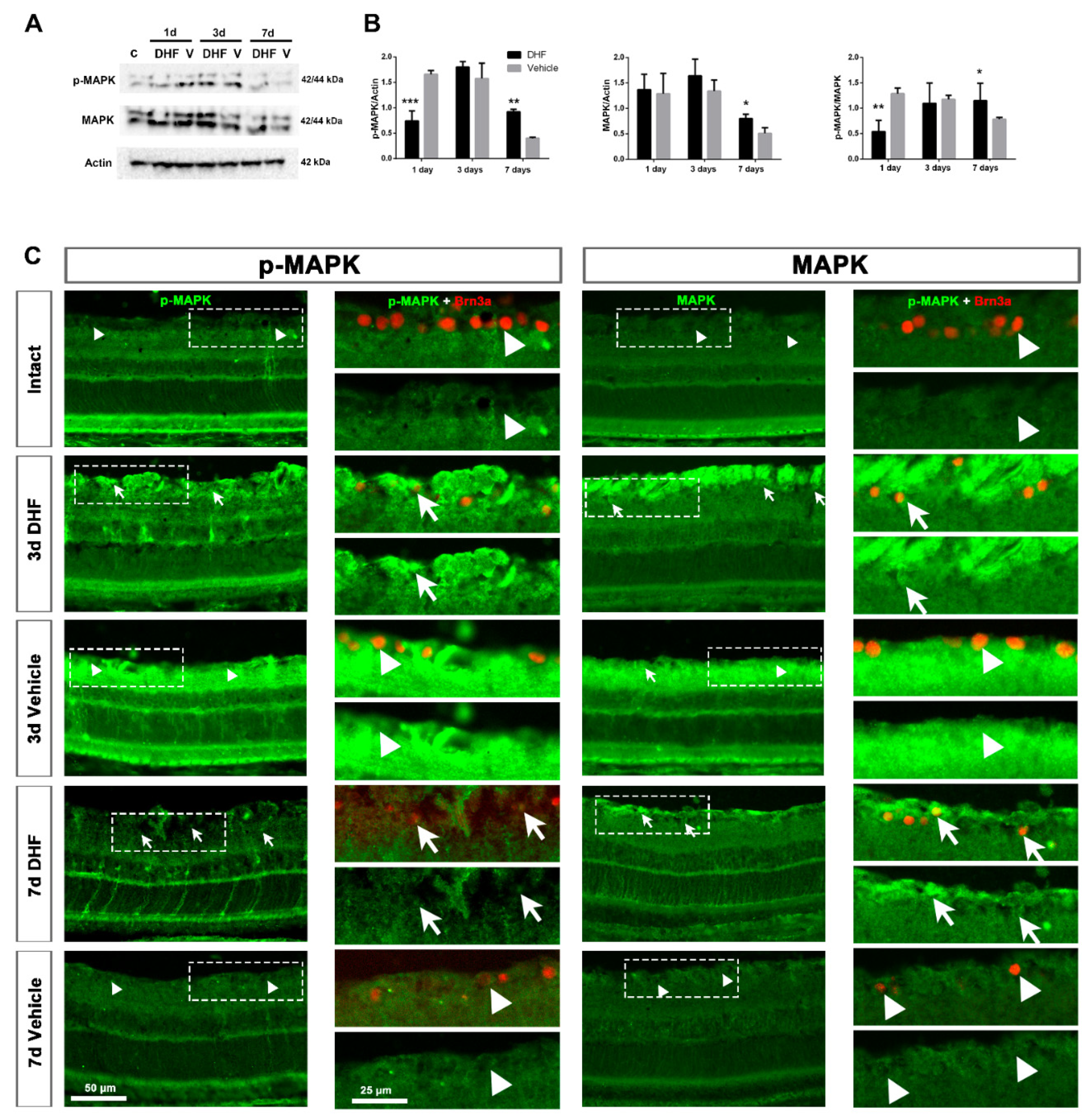
2.1. DHF Treatment Activates MAPK Signaling
2.1.1. MAPK Protein Levels and Expression Pattern
2.1.2. MAPK Activation in Retinal Ganglion Cells
2.1.3. MAPK Activation in Müller Cells
2.2. DHF Treatment Activates PI3K/AKT Signaling Pathway
2.2.1. AKT Protein Levels and Expression Pattern
2.2.2. AKT Activation in Retinal Ganglion Cells
2.2.3. AKT Activation in Müller Cells
3. Discussion
4. Materials and Methods
4.1. Animal Handling
4.2. Intraorbital Optic Nerve Transection
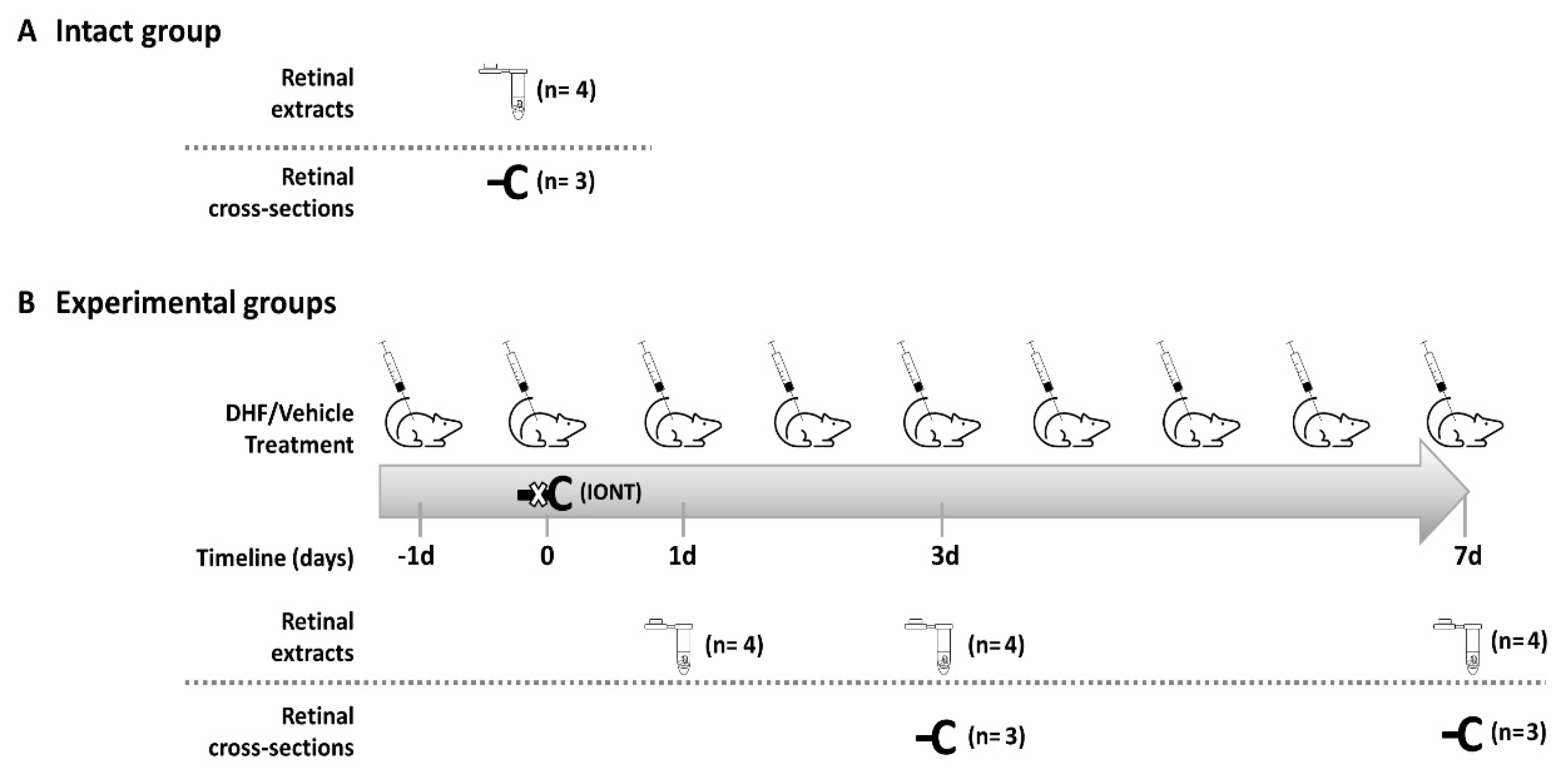
4.3. Experimental Design
4.4. Western Blotting
4.5. Inmunohistofluorescence and Image Acquisition
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| d | Days post-lesion |
| DHF | 7,8-Dihydroxyflavone |
| ERK | Extracellular signal-regulated kinase |
| GCL | Ganglion cell layer |
| HRP | Horseradish peroxidase |
| IONT | Intraorbital nerve transection |
| MAPK | Mitogen-activated protein kinase |
| MC | Müller cells |
| NF-kB | Nuclear factor kappa B |
| NMDA | N-Methyl-D-aspartic acid |
| OHT | Ocular hypertension |
| PI3K | Phosphatidylinositol 3 kinase |
| PLC-Υ | Phospholipase-C |
| PRL | Photoreceptor layer |
| RGC | Retinal ganglion cell |
| RNFL | Retinal nerve fiber layer |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
References
- Vidal-Sanz, M.; Nadal-Nicolas, F.M.; Valiente-Soriano, F.J.; Agudo-Barriuso, M.; Villegas-Perez, M.P. Identifying specific RGC types may shed light on their idiosyncratic responses to neuroprotection. Neural. Regen. Res. 2015, 10, 1228–1230. [Google Scholar] [CrossRef]
- Villegas-Perez, M.P.; Vidal-Sanz, M.; Rasminsky, M.; Bray, G.M.; Aguayo, A.J. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J. Neurobiol. 1993, 24, 23–36. [Google Scholar] [CrossRef]
- Agudo, M.; Perez-Marin, M.C.; Sobrado-Calvo, P.; Lonngren, U.; Salinas-Navarro, M.; Canovas, I.; Nadal-Nicolas, F.M.; Miralles-Imperial, J.; Hallbook, F.; Vidal-Sanz, M. Immediate upregulation of proteins belonging to different branches of the apoptotic cascade in the retina after optic nerve transection and optic nerve crush. Investig. Ophthalmol. Vis. Sci. 2009, 50, 424–431. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Vidal-Sanz, M.; Galindo-Romero, C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Ortin-Martinez, A.; Rovere, G.; Salinas-Navarro, M.; Lucas-Ruiz, F.; Sanchez-Migallon, M.C.; Sobrado-Calvo, P.; et al. Shared and differential retinal responses against optic nerve injury and ocular hypertension. Front. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Aviles-Trigueros, M.; Jimenez-Lopez, M.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolas, F.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axotomy-induced retinal ganglion cell death in adult mice: Quantitative and topographic time course analyses. Exp. Eye Res. 2011, 92, 377–387. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Valiente-Soriano, F.J.; Jimenez-Lopez, M.; Garcia-Ayuso, D.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Rodriguez-Ramirez, K.T.; Vidal-Sanz, M.; Agudo-Barriuso, M. Neuronal death in the contralateral un-injured retina after unilateral axotomy: Role of microglial cells. Int. J. Mol. Sci. 2019, 20, 5733. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Migallon, M.C.; Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Brain derived neurotrophic factor maintains Brn3a expression in axotomized rat retinal ganglion cells. Exp. Eye Res. 2011, 92, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Migallon, M.C.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Nerve fibre layer degeneration and retinal ganglion cell loss long term after optic nerve crush or transection in adult mice. Exp. Eye Res. 2018, 170, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Migallon, M.C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Apoptotic retinal ganglion cell death after optic nerve transection or crush in mice: Delayed RGC Loss with BDNF or a caspase 3 inhibitor. Investig. Ophthalmol. Vis. Sci. 2016, 57, 81–93. [Google Scholar] [CrossRef]
- Lonngren, U.; Napankangas, U.; Lafuente, M.; Mayor, S.; Lindqvist, N.; Vidal-Sanz, M.; Hallbook, F. The growth factor response in ischemic rat retina and superior colliculus after brimonidine pre-treatment. Brain Res. Bull. 2006, 71, 208–218. [Google Scholar] [CrossRef]
- Lindqvist, N.; Peinado-Ramonn, P.; Vidal-Sanz, M.; Hallbook, F. GDNF, Ret, GFRalpha1 and 2 in the adult rat retino-tectal system after optic nerve transection. Exp. Neurol. 2004, 187, 487–499. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Salinas-Navarro, M.; Jimenez-Lopez, M.; Bernal-Garro, J.M.; Villegas-Perez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. BDNF Rescues RGCs but not intrinsically photosensitive RGCs in ocular hypertensive albino rat retinas. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1924–1936. [Google Scholar] [CrossRef]
- Vidal-Sanz, M.; Lafuente, M.; Sobrado-Calvo, P.; Selles-Navarro, I.; Rodriguez, E.; Mayor-Torroglosa, S.; Villegas-Perez, M.P. Death and neuroprotection of retinal ganglion cells after different types of injury. Neurotox. Res. 2000, 2, 215–227. [Google Scholar] [CrossRef]
- Vidal-Sanz, M.; Lafuente, M.P.; Mayor, S.; de Imperial, J.M.; Villegas-Perez, M.P. Retinal ganglion cell death induced by retinal ischemia: Neuroprotective effects of two alpha-2 agonists. Surv. Ophthalmol. 2001, 45, S261–S267. [Google Scholar] [CrossRef]
- Lafuente, M.P.; Villegas-Perez, M.P.; Sobrado-Calvo, P.; Garcia-Aviles, A.; Miralles de Imperial, J.; Vidal-Sanz, M. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2074–2084. [Google Scholar] [PubMed]
- Boia, R.; Ruzafa, N.; Aires, I.D.; Pereiro, X.; Ambrosio, A.F.; Vecino, E.; Santiago, A.R. Neuroprotective strategies for retinal ganglion cell degeneration: Current status and challenges ahead. Int. J. Mol. Sci. 2020, 21, 2262. [Google Scholar] [CrossRef]
- Mansour-Robaey, S.; Clarke, D.B.; Wang, Y.C.; Bray, G.M.; Aguayo, A.J. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1994, 91, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sapieha, P.; Kittlerova, P.; Hauswirth, W.W.; Di Polo, A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J. Neurosci. 2002, 22, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Di Polo, A.; Aigner, L.J.; Dunn, R.J.; Bray, G.M.; Aguayo, A.J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3978–3983. [Google Scholar] [CrossRef]
- Peinado-Ramon, P.; Salvador, M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Investig. Ophthalmol. Vis. Sci. 1996, 37, 489–500. [Google Scholar] [PubMed]
- Valiente-Soriano, F.J.; Ortin-Martinez, A.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Gallego-Ortega, A.; Miralles de Imperial-Ollero, J.A.; Jimenez-Lopez, M.; Villegas-Perez, M.P.; Wheeler, L.A.; Vidal-Sanz, M. Topical brimonidine or intravitreal BDNF, CNTF, or bFGF protect cones against phototoxicity. Transl. Vis. Sci. Technol. 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Parrilla-Reverter, G.; Agudo, M.; Sobrado-Calvo, P.; Salinas-Navarro, M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: A quantitative in vivo study. Exp. Eye Res. 2009, 89, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Rovere, G.; Nadal-Nicolas, F.M.; Sobrado-Calvo, P.; Garcia-Bernal, D.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Topical treatment with bromfenac reduces retinal gliosis and inflammation after optic nerve crush. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6098–6106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortin-Martinez, A.; Valiente-Soriano, F.J.; Garcia-Ayuso, D.; Alarcon-Martinez, L.; Jimenez-Lopez, M.; Bernal-Garro, J.M.; Nieto-Lopez, L.; Nadal-Nicolas, F.M.; Villegas-Perez, M.P.; Wheeler, L.A.; et al. A novel in vivo model of focal light emitting diode-induced cone-photoreceptor phototoxicity: Neuroprotection afforded by brimonidine, BDNF, PEDF or bFGF. PLoS ONE 2014, 9, e113798. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Tamai, M.; Mori, N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3319–3326. [Google Scholar]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Baydyuk, M.; Xu, B. BDNF signaling and survival of striatal neurons. Front. Cell Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, A.; Constantine-Paton, M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010, 70, 304–322. [Google Scholar] [CrossRef]
- Cappoli, N.; Tabolacci, E.; Aceto, P.; Dello Russo, C. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 2020, 349, 577406. [Google Scholar] [CrossRef] [PubMed]
- Chitranshi, N.; Gupta, V.; Kumar, S.; Graham, S.L. Exploring the molecular interactions of 7,8-dihydroxyflavone and its derivatives with TrkB and VEGFR2 proteins. Int. J. Mol. Sci. 2015, 16, 21087–21108. [Google Scholar] [CrossRef]
- Liu, X.; Obianyo, O.; Chan, C.B.; Huang, J.; Xue, S.; Yang, J.J.; Zeng, F.; Goodman, M.; Ye, K. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor. J. Biol. Chem. 2014, 289, 27571–27584. [Google Scholar] [CrossRef] [PubMed]
- Cerquone Perpetuini, A.; Mathoux, J.; Kennedy, B.N. The potential of small molecule brain-derived neurotrophic factor: Mimetics to treat inherited retinal degeneration. Neural Regen. Res. 2019, 14, 85–86. [Google Scholar]
- Liu, X.; Qi, Q.; Xiao, G.; Li, J.; Luo, H.R.; Ye, K. O-methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity. Pharmacology 2013, 91, 185–200. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef]
- Emili, M.; Guidi, S.; Uguagliati, B.; Giacomini, A.; Bartesaghi, R.; Stagni, F. Treatment with the flavonoid 7,8-dihydroxyflavone: A promising strategy for a constellation of body and brain disorders. Crit. Rev. Food Sci. Nutr. 2020, 1–38. [Google Scholar] [CrossRef]
- Gupta, V.K.; You, Y.; Li, J.C.; Klistorner, A.; Graham, S.L. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J. Mol. Neurosci. 2013, 49, 96–104. [Google Scholar] [CrossRef]
- Huang, H.M.; Huang, C.C.; Tsai, M.H.; Poon, Y.C.; Chang, Y.C. Systemic 7,8-dihydroxyflavone treatment protects immature retinas against hypoxic-ischemic injury via Muller glia regeneration and MAPK/ERK activation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3124–3135. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Luo, M.; Yue, S.; Han, Y.; Zhang, H.J.; Zhou, Y.H.; Liu, K.; Liu, H.G. 7,8-Dihydroxyflavone protects retinal ganglion cells against chronic intermittent hypoxia-induced oxidative stress damage via activation of the BDNF/TrkB signaling pathway. Sleep Breath 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chan, C.B.; Ye, K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl. Neurodegener. 2016, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Villegas, B.; Di Pierdomenico, J.; Gallego-Ortega, A.; Galindo-Romero, C.; Martinez-de-la-Casa, J.M.; Garcia-Feijoo, J.; Villegas-Perez, M.P.; Vidal-Sanz, M. Systemic treatment with 7,8-Dihydroxiflavone activates TtkB and affords protection of two different retinal ganglion cell populations against axotomy in adult rats. Exp. Eye Res. 2021, 210, 108694. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef]
- Chitranshi, N.; Dheer, Y.; Abbasi, M.; You, Y.; Graham, S.L.; Gupta, V. Glaucoma pathogenesis and neurotrophins: Focus on the molecular and genetic basis for therapeutic prospects. Curr. Neuropharmacol. 2018, 16, 1018–1035. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Liu, X.; Chan, C.B.; Jang, S.W.; Pradoldej, S.; Huang, J.; He, K.; Phun, L.H.; France, S.; Xiao, G.; Jia, Y.; et al. A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J. Med. Chem. 2010, 53, 8274–8286. [Google Scholar] [CrossRef]
- Segal, R.A.; Greenberg, M.E. Intracellular signaling pathways activated by neurotrophic factors. Annu. Rev. Neurosci. 1996, 19, 463–489. [Google Scholar] [CrossRef]
- Galan, A.; Dergham, P.; Escoll, P.; de-la-Hera, A.; D’Onofrio, P.M.; Magharious, M.M.; Koeberle, P.D.; Frade, J.M.; Saragovi, H.U. Neuronal injury external to the retina rapidly activates retinal glia, followed by elevation of markers for cell cycle re-entry and death in retinal ganglion cells. PLoS ONE 2014, 9, e101349. [Google Scholar] [CrossRef] [PubMed]
- González-Riquelme, M.J.; Galindo-Romero, C.; Lucas-Ruiz, F.; Martínez-Carmona, M.; Rodríguez-Ramírez, K.T.; Cabrera-Maqueda, J.M.; Norte-Muñoz, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axonal injuries cast long shadows: Long term glial activation in injured and contralateral retinas after unilateral axotomy. Int. J. Mol. Sci. 2021, 22, 8517. [Google Scholar]
- Harun-Or-Rashid, M.; Diaz-DelCastillo, M.; Galindo-Romero, C.; Hallbook, F. Alpha2-adrenergic-agonist brimonidine stimulates negative feedback and attenuates injury-induced phospho-ERK and dedifferentiation of chicken muller cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5933–5945. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Konjusha, D.; Galindo-Romero, C.; Hallbook, F. Endothelin B Receptors on primary chicken Muller cells and the human MIO-M1 Muller cell line activate ERK signaling via transactivation of epidermal growth factor receptors. PLoS ONE 2016, 11, e0167778. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Salinas-Navarro, M.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Lucas-Ruiz, F.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Villegas-Perez, M.P.; Agudo-Barriuso, M. Tracing the retina to analyze the integrity and phagocytic capacity of the retinal pigment epithelium. Sci. Rep. 2020, 10, 7273. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar]
- Benn, S.C.; Woolf, C.J. Adult neuron survival strategies-slamming on the brakes. Nat. Rev. Neurosci. 2004, 5, 686–700. [Google Scholar] [CrossRef]
- Mao, D.; Sun, X. Reactivation of the PI3K/Akt signaling pathway by the bisperoxovanadium compound bpV(pic) attenuates photoreceptor apoptosis in experimental retinal detachment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5519–5532. [Google Scholar] [CrossRef]
- Chen, J.; Chua, K.W.; Chua, C.C.; Yu, H.; Pei, A.; Chua, B.H.; Hamdy, R.C.; Xu, X.; Liu, C.F. Antioxidant activity of 7,8-Dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci. Lett. 2011, 499, 181–185. [Google Scholar] [CrossRef]
- Cho, S.J.; Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Fernando, P.; Zhen, A.X.; Hyun, Y.J.; Ahn, M.J.; Kang, H.K.; Hyun, J.W. 7,8-Dihydroxyflavone protects high glucose-damaged neuronal cells against oxidative stress. Biomol. Ther. 2019, 27, 85–91. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Salinas-Navarro, M.; Sobrado-Calvo, P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Microglial dynamics after axotomy-induced retinal ganglion cell death. J. Neuroinflamm. 2017, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, G.Y.; Hyun, J.W.; Hwang, H.J.; Kim, N.D.; Kim, B.W.; Choi, Y.H. 7,8-Dihydroxyflavone exhibits anti-inflammatory properties by downregulating the NF-kappaB and MAPK signaling pathways in lipopolysaccharide-treated RAW264.7 cells. Int. J. Mol. Med. 2012, 29, 1146–1152. [Google Scholar] [PubMed]
- Park, H.Y.; Park, C.; Hwang, H.J.; Kim, B.W.; Kim, G.Y.; Kim, C.M.; Kim, N.D.; Choi, Y.H. 7,8-Dihydroxyflavone attenuates the release of pro-inflammatory mediators and cytokines in lipopolysaccharide-stimulated BV2 microglial cells through the suppression of the NF-kappaB and MAPK signaling pathways. Int. J. Mol. Med. 2014, 33, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sanz, M.; Bray, G.M.; Villegas-Perez, M.P.; Thanos, S.; Aguayo, A.J. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J. Neurosci. 1987, 7, 2894–2909. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galindo-Romero, C.; Vidal-Villegas, B.; Asís-Martínez, J.; Lucas-Ruiz, F.; Gallego-Ortega, A.; Vidal-Sanz, M. 7,8-Dihydroxiflavone Protects Adult Rat Axotomized Retinal Ganglion Cells through MAPK/ERK and PI3K/AKT Activation. Int. J. Mol. Sci. 2021, 22, 10896. https://doi.org/10.3390/ijms221910896
Galindo-Romero C, Vidal-Villegas B, Asís-Martínez J, Lucas-Ruiz F, Gallego-Ortega A, Vidal-Sanz M. 7,8-Dihydroxiflavone Protects Adult Rat Axotomized Retinal Ganglion Cells through MAPK/ERK and PI3K/AKT Activation. International Journal of Molecular Sciences. 2021; 22(19):10896. https://doi.org/10.3390/ijms221910896
Chicago/Turabian StyleGalindo-Romero, Caridad, Beatriz Vidal-Villegas, Javier Asís-Martínez, Fernando Lucas-Ruiz, Alejandro Gallego-Ortega, and Manuel Vidal-Sanz. 2021. "7,8-Dihydroxiflavone Protects Adult Rat Axotomized Retinal Ganglion Cells through MAPK/ERK and PI3K/AKT Activation" International Journal of Molecular Sciences 22, no. 19: 10896. https://doi.org/10.3390/ijms221910896
APA StyleGalindo-Romero, C., Vidal-Villegas, B., Asís-Martínez, J., Lucas-Ruiz, F., Gallego-Ortega, A., & Vidal-Sanz, M. (2021). 7,8-Dihydroxiflavone Protects Adult Rat Axotomized Retinal Ganglion Cells through MAPK/ERK and PI3K/AKT Activation. International Journal of Molecular Sciences, 22(19), 10896. https://doi.org/10.3390/ijms221910896






