Maternal C-Peptide and Insulin Sensitivity, but Not BMI, Associate with Fatty Acids in the First Trimester of Pregnancy
Abstract
:1. Introduction
2. Results
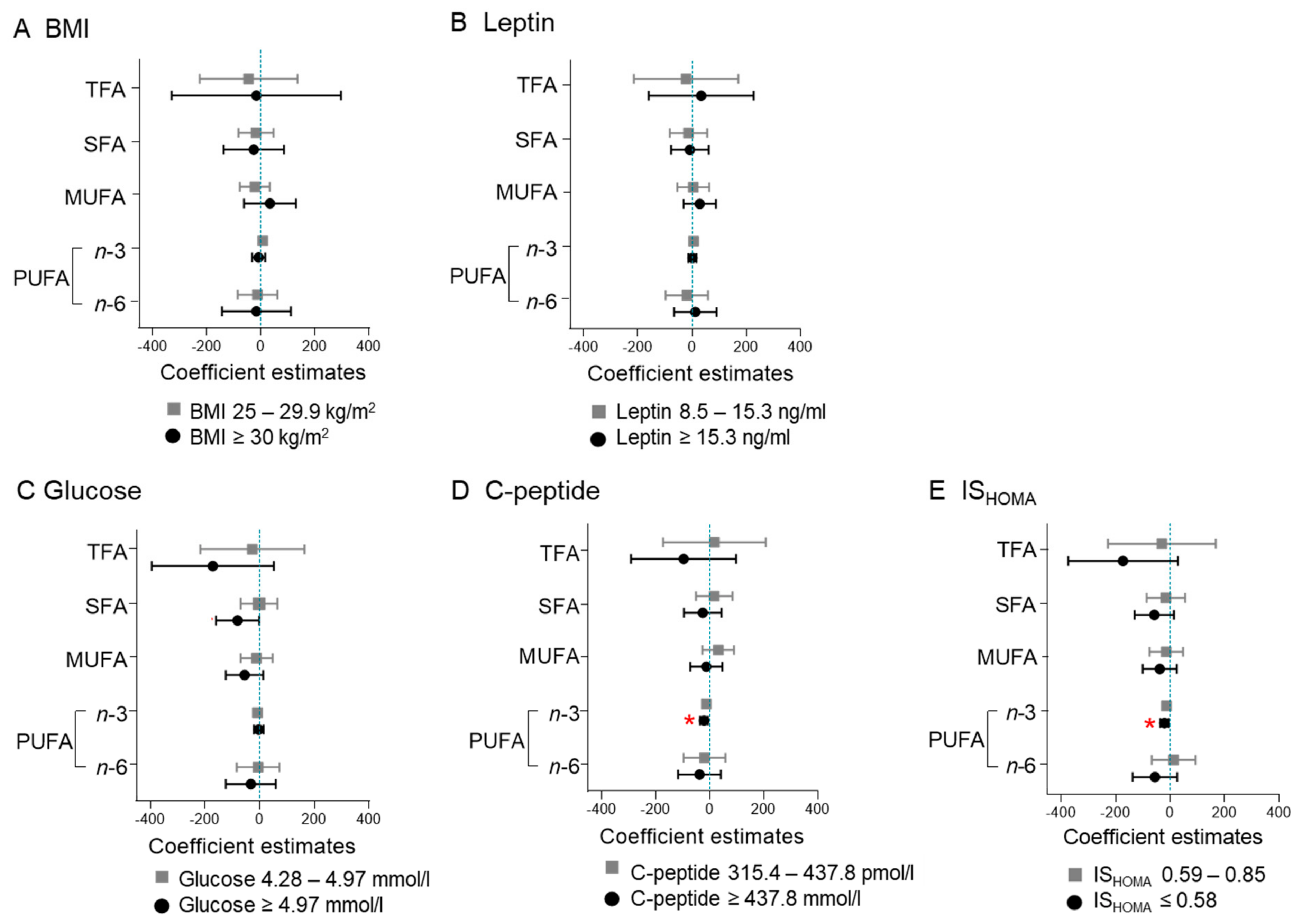
2.1. Maternal BMI and Leptin Do Not Associate with Fatty Acid Concentrations
2.2. High Glucose Associates with Decreased SFA Concentration
2.3. High C-Peptide and Low ISHOMA Associate with Decreased n-3 PUFA Concentration
2.4. High C-Peptide and Low ISHOMA Associate with n-6/n-3 PUFA Ratio
2.5. Indices of Desaturase Activities
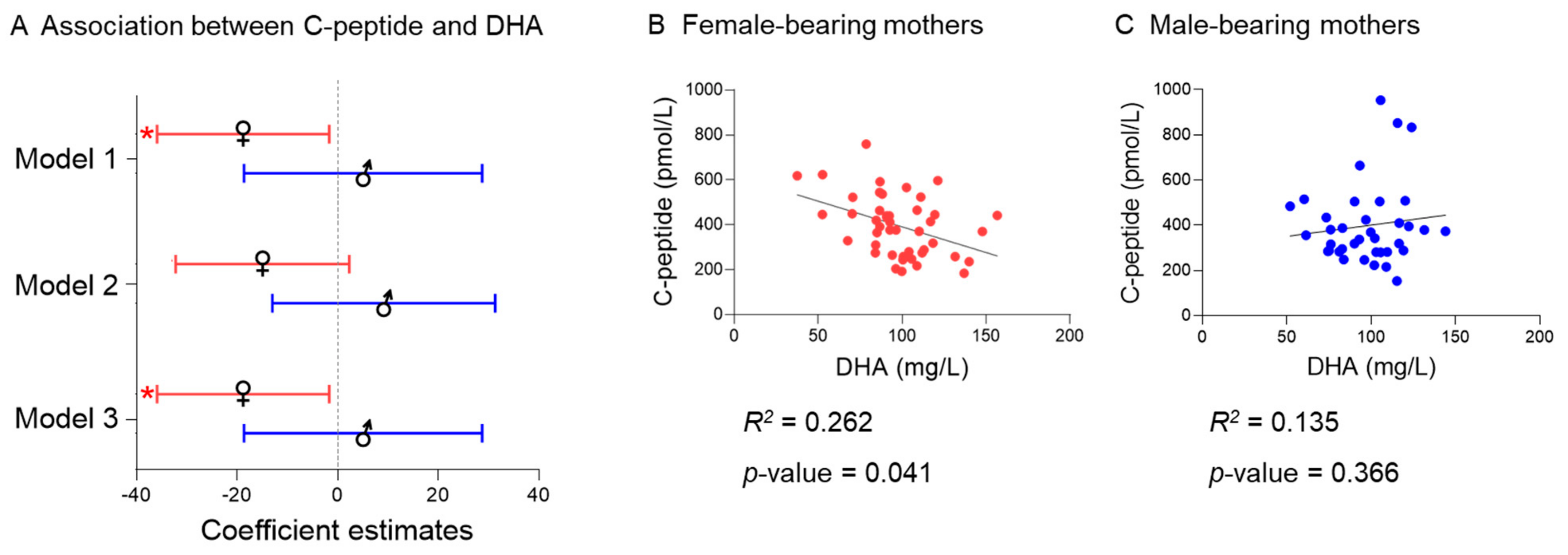
2.6. Fetal Sex
2.7. Sensitivity Analysis Excluding Women with High Fasting Glucose
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Blood Collection and Storage
4.3. Maternal Metabolic Parameters
4.4. Quantification of Serum Fatty Acids
4.5. Fetal Sex Determination
4.6. Exposures and Outcomes
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edlow, A.G. Maternal Obesity and Neurodevelopmental and Psychiatric Disorders in Offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of Obese Mothers Develop Insulin Resistance in Utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Tylavsky, F.A.; Han, J.C.; Kocak, M.; Fowke, J.H.; Davis, R.L.; Lewinn, K.; Bush, N.R.; Zhao, Q. Maternal Metabolic Factors during Pregnancy Predict Early Childhood Growth Trajectories and Obesity Risk: The CANDLE Study. Int. J. Obes. 2019, 43, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Herrera, E. Adipose Tissue Development and Lipid Metabolism in the Human Fetus: The 2020 Perspective Focusing on Maternal Diabetes and Obesity. Prog. Lipid Res. 2021, 81, 101082. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal Obesity and Risk of Gestational Diabetes Mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Metzger, B.E.; Contreras, M.; Sacks, D.A.; Watson, W.; Dooley, S.L.; Foderaro, M.; Niznik, C.; Bjaloncik, J.; Catalano, P.M.; Dierker, L.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2015, 358, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Andrews, C.; Monthé-Drèze, C.; Sacks, D.A.; Ma, R.C.W.; Tam, W.H.; McIntyre, H.D.; Lowe, J.; Catalano, P.; Sen, S. Role of Maternal Glucose Metabolism in the Association between Maternal BMI and Neonatal Size and Adiposity. Int. J. Obes. 2020, 45, 515–524. [Google Scholar] [CrossRef]
- Barbour, L.A. Maternal Lipids and Fetal Overgrowth: Making Fat from Fat. Clin. Ther. 2018, 40, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Barbour, L.A. Metabolic Culprits in Obese Pregnancies and Gestational Diabetes Mellitus: Big Babies, Big Twists, Big Picture. Diabetes Care 2019, 42, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Herrera, E.; Ortega-Senovilla, H. Implications of Lipids in Neonatal Body Weight and Fat Mass in Gestational Diabetic Mothers and Non-Diabetic Controls. Curr. Diabetes Rep. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Steenweg-De Graaff, J.C.J.; Tiemeier, H.; Basten, M.G.J.; Rijlaarsdam, J.; Demmelmair, H.; Koletzko, B.; Hofman, A.; Jaddoe, V.W.V.; Verhulst, F.C.; Roza, S.J. Maternal LC-PUFA Status during Pregnancy and Child Problem Behavior: The Generation R Study. Pediatric Res. 2015, 77, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, R.; el Marroun, H.; Voortman, T.; Hillegers, M.; White, T.; Tiemeier, H. Maternal Polyunsaturated Fatty Acids during Pregnancy and Offspring Brain Development in Childhood. Am. J. Clin. Nutr. 2021, 114, 124–133. [Google Scholar] [CrossRef]
- Desoye, G.; Schweditsch, M.O.; Pfeiffer, K.P.; Zechner, R.; Kostner, G.M. Correlation of Hormones with Lipid and Lipoprotein Levels During Normal Pregnancy and Postpartum. Clin. Endocrinol. Metab. 1987, 64, 704–712. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Metabolic Changes in Pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Herrera, E.; Amusquivar, E.; López-Soldado, I.; Ortega, H. Maternal Lipid Metabolism and Placental Lipid Transfer. Horm. Res. 2006, 65, 59–64. [Google Scholar] [CrossRef]
- Scifres, C.M.; Catov, J.M.; Simhan, H.N. The Impact of Maternal Obesity and Gestational Weight Gain on Early and Mid-Pregnancy Lipid Profiles. Obesity 2014, 22, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Penfield-Cyr, A.; Monthe-Dreze, C.; Smid, M.C.; Sen, S. Maternal BMI, Mid-Pregnancy Fatty Acid Concentrations, and Perinatal Outcomes. Clin. Ther. 2018, 40, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Bandres-Meriz, J.; Dieberger, A.M.; Hoch, D.; Pöchlauer, C.; Bachbauer, M.; Glasner, A.; Niedrist, T.; vann Poppel, M.N.; Desoye, G. Maternal Obesity Affects the Glucose-Insulin Axis during the First Trimester of Human Pregnancy. Front. Endocrinol. 2020, 11, 566673. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Qaraghouli, M.; Fang, Y.M.V. Effect of Fetal Sex on Maternal and Obstetric Outcomes. Front. Pediatrics 2017, 5, 144. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.A.; Desoye, G.; Simmons, D.; Devlieger, R.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; Kautzky-Willer, A.; et al. Temporal Relationships between Maternal Metabolic Parameters with Neonatal Adiposity in Women with Obesity Differ by Neonatal Sex: Secondary Analysis of the DALI Study. Pediatric Obes. 2020, 15, e12628. [Google Scholar] [CrossRef] [Green Version]
- Retnakaran, R.; Kramer, C.K.; Ye, C.; Kew, S.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B. Fetal Sex and Maternal Risk of Gestational Diabetes Mellitus: The Impact of Having a Boy. Diabetes Care 2015, 38, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaglioni, S.; Verduci, E.; Salvioni, M.; Bruzzese, M.G.; Radaelli, G.; Zetterström, R.; Riva, E.; Agostoni, C. Plasma Long-Chain Fatty Acids and the Degree of Obesity in Italian Children. Acta Paediatr. Int. J. Paediatr. 2006, 95, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.; Munro, I.; Phang, M.; Garg, M. Plasma N-3 Polyunsaturated Fatty Acids Are Negatively Associated with Obesity. Br. J. Nutr. 2009, 102, 1370–1374. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, E.; Rafraf, M.; Farzadi, L.; Asghari-Jafarabadi, M.; Sabour, S. Effects of Omega-3 Fatty Acids Supplementation on Serum Adiponectin Levels and Some Metabolic Risk Factors in Women with Polycystic Ovary Syndrome. Asia Pac. J. Clin. Nutr. 2012, 21, 511–518. [Google Scholar] [CrossRef]
- Liu, H.Q.; Qiu, Y.; Mu, Y.; Zhang, X.J.; Liu, L.; Hou, X.H.; Zhang, L.; Xu, X.N.; Ji, A.L.; Cao, R.; et al. A High Ratio of Dietary n-3/n-6 Polyunsaturated Fatty Acids Improves Obesity-Linked Inflammation and Insulin Resistance through Suppressing Activation of TLR4 in SD Rats. Nutr. Res. 2013, 33, 849–858. [Google Scholar] [CrossRef]
- Alvarado, F.; Tsai, P.; Miinium, J.; Catalano, P.; O’Tierney-Ginn, P. High Maternal DHA Levels in Hawaiian Women Decrease Insulin Resistance. Diabetes 2018, 67, 1404. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (N-3) Fatty Acids Alleviate Adipose Tissue Inflammation and Insulin Resistance: Mechanistic Insights. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Sedlmeier, E.M.; Brunner, S.; Much, D.; Pagel, P.; Ulbrich, S.E.; Meyer, H.H.D.; Amann-Gassner, U.; Hauner, H.; Bader, B.L. Human Placental Transcriptome Shows Sexually Dimorphic Gene Expression and Responsiveness to Maternal Dietary N-3 Long-Chain Polyunsaturated Fatty Acid Intervention during Pregnancy. BMC Genom. 2014, 15, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, J.A.; Fong, J.; Bernert, J.T.; Browner, W.S. Relation of Smoking and Alcohol Consumption to Serum Fatty Acids. Am. J. Epidemiol. 1996, 144, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Sowell, K.; Holt, R.; Uriu-Adams, J.; Chambers, C.; Coles, C.; Kable, J.; Yevtushok, L.; Zymak-Zakutnya, N.; Wertelecki, W.; Keen, C. Alcohol Consumption and Smoking During Pregnancy Alters Maternal Plasma Fatty Acid Composition: Association with Fetal Alcohol Spectrum Disorders (P11-028-19). Curr. Dev. Nutr. 2019, 3, 989. [Google Scholar] [CrossRef] [Green Version]
- Salahuddin, M.; Pérez, A.; Ranjit, N.; Hoelscher, D.M.; Kelder, S.H. The Effect of Prenatal Maternal Cigarette Smoking on Children’s BMI z-Score with SGA as a Mediator. Int. J. Obes. 2018, 42, 1008–1018. [Google Scholar] [CrossRef]
- Albers, L.; Sobotzki, C.; Kuß, O.; Ajslev, T.; Batista, R.F.; Bettiol, H.; Brabin, B.; Buka, S.L.; Cardoso, V.C.; Clifton, V.L.; et al. Maternal Smoking during Pregnancy and Offspring Overweight: Is There a Dose–Response Relationship? An Individual Patient Data Meta-Analysis. Int. J. Obes. 2018, 42, 1249–1264. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Bernert, J.T.; Caraballo, R.S.; Holiday, D.B.; Wang, J. Optimal Serum Cotinine Levels for Distinguishing Cigarette Smokers and Nonsmokers within Different Racial/Ethnic Groups in the United States between 1999 and 2004. Am. J. Epidemiol. 2009, 169, 236–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radaelli, T.; Farrell, K.A.; Huston-Presley, L.; Amini, S.B.; Kirwan, J.P.; McIntyre, H.D.; Catalano, P.M. Estimates of Insulin Sensitivity Using Glucose and C-Peptide from the Hyperglycemia and Adverse Pregnancy Outcome Glucose Tolerance Test. Diabetes Care 2010, 33, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Amusquivar, E.; Schiffner, S.; Herrera, E. Evaluation of Two Methods for Plasma Fatty Acid Analysis by GC. Eur. J. Lipid Sci. Technol. 2011, 113, 711–716. [Google Scholar] [CrossRef]
- Hoch, D.; Novakovic, B.; Tokic, S.; Saffery, R.; Majali-Martinez, A. Sex Matters: XIST and DDX3Y Gene Expression as a Tool to Determine Fetal Sex in Human First Trimester Placenta. Placenta 2020, 97, 68–70. [Google Scholar] [CrossRef]
- Beccarelli, L.M.; Scherr, R.E.; Newman, J.W.; Borkowska, A.G.; Gray, I.J.; Linnell, J.D.; Keen, C.L.; Young, H.M. Associations Among Fatty Acids, Desaturase and Elongase, and Insulin Resistance in Children. J. Am. Coll. Nutr. 2018, 37, 44–50. [Google Scholar] [CrossRef]
- Warensjö, E.; Rosell, M.; Hellenius, M.L.; Vessby, B.; De Faire, U.; Risérus, U. Associations between Estimated Fatty Acid Desaturase Activities in Serum Lipids and Adipose Tissue in Humans: Links to Obesity and Insulin Resistance. Lipids Health Dis. 2009, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.A.; Ochoa, C.R.; Mitsche, M.A.; Hammer, R.E.; Horton, J.D. Deletion of ELOVL6 Blocks the Synthesis of Oleic Acid but Does Not Prevent the Development of Fatty Liver or Insulin Resistance. J. Lipid Res. 2015, 55, 2597–2605. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. BMI Classification. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 September 2021).

| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | CI | p | β | CI | p | β | CI | p | ||
| C-peptidea | Total fatty acids | −14.9 | −201.4–171.7 | 0.875 | −7.5 | −202.1–187.1 | 0.939 | −43.3 | −243.0–156.5 | 0.669 |
| SFA | 0.6 | −66.0–67.3 | 0.985 | 6.5 | −62.9–75.9 | 0.853 | −0.7 | −72.3–70.8 | 0.984 | |
| MUFA | 19.0 | −38.3–76.3 | 0.512 | 21.2 | −38.5–81.0 | 0.483 | 8.5 | −52.7–69.8 | 0.783 | |
| PUFA n-3 | −17.0 | −31.2–−2.8 | 0.019 | −17.1 | −32.0–−2.3 | 0.024 | −21.5 | −36.5–−6.4 | 0.006 | |
| PUFA n-6 | −17.5 | −93.25–−58.3 | 0.648 | −18.1 | −97.1–61.0 | 0.652 | −29.7 | −110.8–−51.4 | 0.470 | |
| Ratio n-6/n-3 | 0.69 | 0.12–1.25 | 0.018 | 0.69 | 0.10–1.28 | 0.022 | 0.78 | 0.17–1.38 | 0.012 | |
| ISHOMAa | Total fatty acids | 35.0 | −131.2–201.3 | 0.677 | 30.9 | −142.1–203.8 | 0.677 | 69.6 | −111.4–250.5 | 0.448 |
| SFA | 10.5 | −48.9–69.9 | 0.727 | 6.5 | −55.2–68.1 | 0.836 | 14.4 | −50.4–79.2 | 0.661 | |
| MUFA | −8.5 | −59.7–42.6 | 0.742 | −9.1 | −62.3–44.1 | 0.735 | 3.3 | −52.3– 58.8 | 0.908 | |
| PUFA n-3 | 15.1 | 2.5–27.8 | 0.020 | 15.2 | 2.0–28.3 | 0.025 | 20.1 | 6.4–33.7 | 0.004 | |
| PUFA n-6 | 18.0 | −49.6–85.5 | 0.599 | 18.4 | −51.9–88.6 | 0.605 | 31.8 | −41.6–105.3 | 0.392 | |
| Ratio n-6/n-3 | −0.59 | −1.09–−0.09 | 0.022 | −0–0.59 | −1.12–−0.07 | 0.027 | −0.69 | −1.24–−1.14 | 0.014 | |
| n (%) | Mean ± SD | Median (IQR) | |
|---|---|---|---|
| Age (years) | 122 | 31.4 (± 7.2) | |
| Gestational age (days) | 123 | 51.0 (± 15.4) | |
| 4–6 weeks | 58 (47.0%) | 35.0 (35.0–42.0) | |
| 7–9 weeks | 48 (39.0%) | 56.0 (51.0–61.0) | |
| 10–12 weeks | 17 (13.8%) | 81.0 (78.0–82.0) | |
| BMI (kg/m2) | 123 | 22.6 (20.6–25.7) | |
| Under-/normal-weight (<25 kg/m2) | 85 (69.2%) | 21.1 (19.8–22.8) | |
| Overweight (25.0–29.9 kg/m2) | 30 (24.4%) | 26.9 (25.9–27.4) | |
| Obese (≥30.0 kg/m2) | 8 (6.5%) | 32.6 (31.9–39.5) | |
| Metabolic parameters | |||
| Leptin (ng/mL) | 123 | 11.8 (7.1–17.4) | |
| 1st tertile (<8.5) | 41 | 5.4 (3.5–7.1) | |
| 2nd tertile (8.5–15.3) | 41 | 12.0 (10.2–13.9) | |
| 3rd tertile (≥15.3) | 41 | 19.6 (17.4–28.6) | |
| Glucose (mmol/L) | 118 | 4.76 (± 0.85) | |
| 1st tertile (<4.28) | 39 | 4.01 (3.65–4.14) | |
| 2nd tertile (4.28–4.97) | 40 | 4.65 (4.49–4.82) | |
| 3rd tertile (≥4.97) | 39 | 5.52 (5.16–5.98) | |
| C-peptide (pmol/L) | 123 | 371.0 (281.5–484.9) | |
| 1st tertile (<315.4) | 41 | 258.3 (218.5–281.7) | |
| 2nd tertile (315.4–437.8) | 41 | 371.0 (346.6–402.8) | |
| 3rd tertile (≥437.8) | 41 | 537.0 (476.4–621.3) | |
| ISHOMA | 118 | 0.74 (0.53–1.00) | |
| 1st tertile (≤0.59) | 40 | 0.46 (0.36–0.53) | |
| 2nd tertile (0.58–0.85) | 39 | 0.73 (0.68–0.79) | |
| 3rd tertile (>0.85) | 39 | 1.13 (1.00–1.41) | |
| Fatty acids (mg/L) | |||
| Total fatty acids | 123 | 2724.2 (± 415.4) | |
| SFA | 123 | 918.9 (± 148.2) | |
| C 14:0 | 123 | 22.7 (17.3–32.8) | |
| C 16:0 | 123 | 618.3 (539.4–694.0) | |
| C 18:0 | 123 | 273.6 (± 45.5) | |
| MUFA | 123 | 610.5 (± 132.8) | |
| C 16:1 | 123 | 54.8 (39.7–67.8) | |
| C 18:1 (n-9) | 123 | 546.1 (± 114.2) | |
| C 22:1 (n-9) | 123 | 6.5 (± 2.8) | |
| n-3 PUFA | 123 | 158.0 (± 28.5) | |
| C 18:3 (n-3) | 123 | 10.7 (8.7–14.8) | |
| C 20:3 (n-3) | 123 | 4.9 (3.6–7.3) | |
| C 20:5 (n-3) | 123 | 17.2 (± 7.3) | |
| C 22:5 (n-3) | 123 | 26.6 (± 9.6) | |
| C 22:6 (n-3) | 123 | 97.5 (± 24.1) | |
| n-6 PUFA | 123 | 1036.9 (± 161.8) | |
| C 18:2 (n-6) | 123 | 751.4 (± 131.9) | |
| C 18:3 (n-6) | 123 | 9.7 (7.8–12.9) | |
| C 20:2 (n-6) | 123 | 6.2 (5.0–7.3) | |
| C 20:3 (n-6) | 123 | 45.4 (± 14.0) | |
| C 20:4 (n-6) | 123 | 216.4 (± 49.7) | |
| C 22:4 (n-6) | 123 | 7.4 (± 2.4) | |
| C 22:5 (n-6) | 123 | 5.8 (± 4.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandres-Meriz, J.; Majali-Martinez, A.; Hoch, D.; Morante, M.; Glasner, A.; van Poppel, M.N.M.; Desoye, G.; Herrera, E. Maternal C-Peptide and Insulin Sensitivity, but Not BMI, Associate with Fatty Acids in the First Trimester of Pregnancy. Int. J. Mol. Sci. 2021, 22, 10422. https://doi.org/10.3390/ijms221910422
Bandres-Meriz J, Majali-Martinez A, Hoch D, Morante M, Glasner A, van Poppel MNM, Desoye G, Herrera E. Maternal C-Peptide and Insulin Sensitivity, but Not BMI, Associate with Fatty Acids in the First Trimester of Pregnancy. International Journal of Molecular Sciences. 2021; 22(19):10422. https://doi.org/10.3390/ijms221910422
Chicago/Turabian StyleBandres-Meriz, Julia, Alejandro Majali-Martinez, Denise Hoch, Milagros Morante, Andreas Glasner, Mireille N. M. van Poppel, Gernot Desoye, and Emilio Herrera. 2021. "Maternal C-Peptide and Insulin Sensitivity, but Not BMI, Associate with Fatty Acids in the First Trimester of Pregnancy" International Journal of Molecular Sciences 22, no. 19: 10422. https://doi.org/10.3390/ijms221910422
APA StyleBandres-Meriz, J., Majali-Martinez, A., Hoch, D., Morante, M., Glasner, A., van Poppel, M. N. M., Desoye, G., & Herrera, E. (2021). Maternal C-Peptide and Insulin Sensitivity, but Not BMI, Associate with Fatty Acids in the First Trimester of Pregnancy. International Journal of Molecular Sciences, 22(19), 10422. https://doi.org/10.3390/ijms221910422








