Abstract
The pathological elevation of the active thyroid hormone (T3) level results in the manifestation of hyperthyroidism, which is associated with alterations in the differentiation and contractile function of skeletal muscle (SKM). Myosin phosphatase (MP) is a major cellular regulator that hydrolyzes the phosphoserine of phosphorylated myosin II light chain. MP consists of an MYPT1/2 regulatory and a protein phosphatase 1 catalytic subunit. Smoothelin-like protein 1 (SMTNL1) is known to inhibit MP by directly binding to MP as well as by suppressing the expression of MYPT1 at the transcriptional level. Supraphysiological vs. physiological concentration of T3 were applied on C2C12 myoblasts and differentiated myotubes in combination with the overexpression of SMTNL1 to assess the role and regulation of MP under these conditions. In non-differentiated myoblasts, MP included MYPT1 in the holoenzyme complex and its expression and activity was regulated by SMTNL1, affecting the phosphorylation level of MLC20 assessed using semi-quantitative Western blot analysis. SMTNL1 negatively influenced the migration and cytoskeletal remodeling of myoblasts measured by high content screening. In contrast, in myotubes, the expression of MYPT2 but not MYPT1 increased in a T3-dependent and SMTNL1-independent manner. T3 treatment combined with SMTNL1 overexpression impeded the activity of MP. In addition, MP interacted with Na+/K+-ATPase and dephosphorylated its inhibitory phosphorylation sites, identifying this protein as a novel MP substrate. These findings may help us gain a better understanding of myopathy, muscle weakness and the disorder of muscle regeneration in hyperthyroid patients.
1. Introduction
Thyroid hormones (THs) have important regulatory actions in skeletal muscle (SKM) [1,2,3]. The main forms of thyroid hormone produced and secreted by the thyroid gland are tetraiodothyronine (T4) and 3,3′,5-triiodothyronine (T3). Although it has been assumed that THs enter cells by passive diffusion due to their lipophilic properties, THs are primarily transported through the cell membrane by the monocarboxylate transporter 8 (MCT8) in SKM [4]. T4 is considered to be a prohormone and most of the peripheral effects of THs are ascribed to the action of T3. Intracellular monodeiodination of T4 by deiodinase type II (DIO2) produces T3, the active form of thyroid hormone in SKM [5]. The final level of thyroid hormone signaling control is given by the expression of nuclear thyroid hormone receptors (TRs), which positively or negatively regulate the transcription of TH responsive genes [3]. Functional TRs mediate the vast majority of TH actions on the level of gene transcription; however, so-called non-genomic effects using other target molecules have been reported as well [6].
Pathological elevation in serum or tissue TH levels results in the manifestation of hyperthyroidism, which is associated with significant alterations in differentiation, contractile function and the metabolism of SKM [3]. Above all, hyperthyroidism is often accompanied by muscle weakness in the face, throat and respiratory muscle, and wasting of muscles around the shoulders and hips leading to hyperthyroid myopathy [7,8]. Even though several cellular mechanisms have been identified in muscle tissues [9], which can explain the observed effects on the molecular level, the detailed molecular mechanism is not fully characterized yet.
Filamentous contractile motor protein myosin II consists of two 200 kDa heavy chains and two pairs of light chains of 17 and 20 kDa. Regulation of myosin II by phosphorylation is essential in muscle contraction or in the motile and other functions of non-muscle cells, e.g., shape changes, cell division, cell adhesion, cell migration or regulation of ion channels. After the phosphorylation of the 20 kDa myosin light chain (MLC20), myosin II reversibly binds to actin filaments via cross-bridges initiating contractile or motile events [10]. MLC20 phosphorylation depends on the balance between Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) and myosin phosphatase (MP) [11].
Myosin phosphatase (MP) is a heterotrimer enzyme that hydrolyses phosphorylated myosin II light chains, as well as other cytoskeletal and cellular proteins such as adducin, tau, SNAP25 and PRMT5, etc. [12]. MP consists of a type 1 protein phosphatase catalytic subunit δ isoform (PP1cδ), a myosin phosphatase target subunit (MYPT) and a ~20 kDa small subunit of a yet unknown function (M20). The MYPT protein family includes MYPT1 (PPP1R12A) [13], MYPT2 (PPP1R12B) [14], MYPT3 [15], protein phosphatase 1 myosin binding 85 kDa subunit (MBS85) [16] and TGF-β1-inhibited membrane-associated protein (TIMAP) [17], which are responsible for targeting PP1cδ to its substrates [18]. MYPT1, first identified in smooth muscle, is ubiquitously expressed in most eukaryotic cells. MYPT2 was found mainly in cardiac and skeletal muscles [14,19].
The activity of MP holoenzyme is regulated through several mechanisms. The phosphorylation of MYPT by a variety of Ser/Thr kinases is one of the principal regulatory mechanisms leading to the activation or inhibition of MP. The phosphorylation of MYPT1 on Thr696 and/or on Thr853 results in the inhibition of MP activity [20,21]. In addition, the phosphorylation of Thr853 in MYPT1 by RhoA- associated kinase (ROCK) triggers the dissociation of MYPT1 from its substrate, myosin [22]. On the other hand, MP can be regulated via the formation of protein–protein interactions: an example is association with the smoothelin-like protein 1 (SMTNL1). SMTNL1 was identified in gastrointestinal smooth muscle as an early target of protein kinase A and G (PKA/PKG) [23,24]; however, it is also expressed in SKM and steroid hormone sensitive tissues. SMTNL1 inhibits the activity of MP, thereby regulating muscle contraction and cytoskeletal elements in various cells [25,26]. Through the inhibition of MP, SMTNL1 could enhance the phosphorylation of MLC20. Besides that, SMTNL1 is a transcriptional regulator of the progesterone receptor-B (PR-B), thereby regulating the gene expression of numerous metabolic enzymes, cytoskeletal proteins, steroid receptors and cytokines [26]. SMTNL1 is downregulated in hyperthyroid SKM and it selectively inhibits TRα expression, which is a key target of insulin-dependent signaling governed by T3. In addition, SMTNL1 overexpression reduced IRS1 Ser phosphorylation in a hyperthyroid model to restore the normal responsiveness of glucose transport to insulin [27].
Given the major role of thyroid hormones in the skeletal muscle system, it is important to study their actions as pathophysiological agents in myopathy. The objective of our work was to investigate the effect of supraphysiological vs. the physiological T3 level on the expression and regulation of MP via the modulation of SMTNL1 in myoblast differentiation and the homeostasis of C2C12 myoblasts and myotubes.
2. Results
2.1. Overexpression of SMTNL1 Promotes C2C12 Mouse Myoblast Differentiation
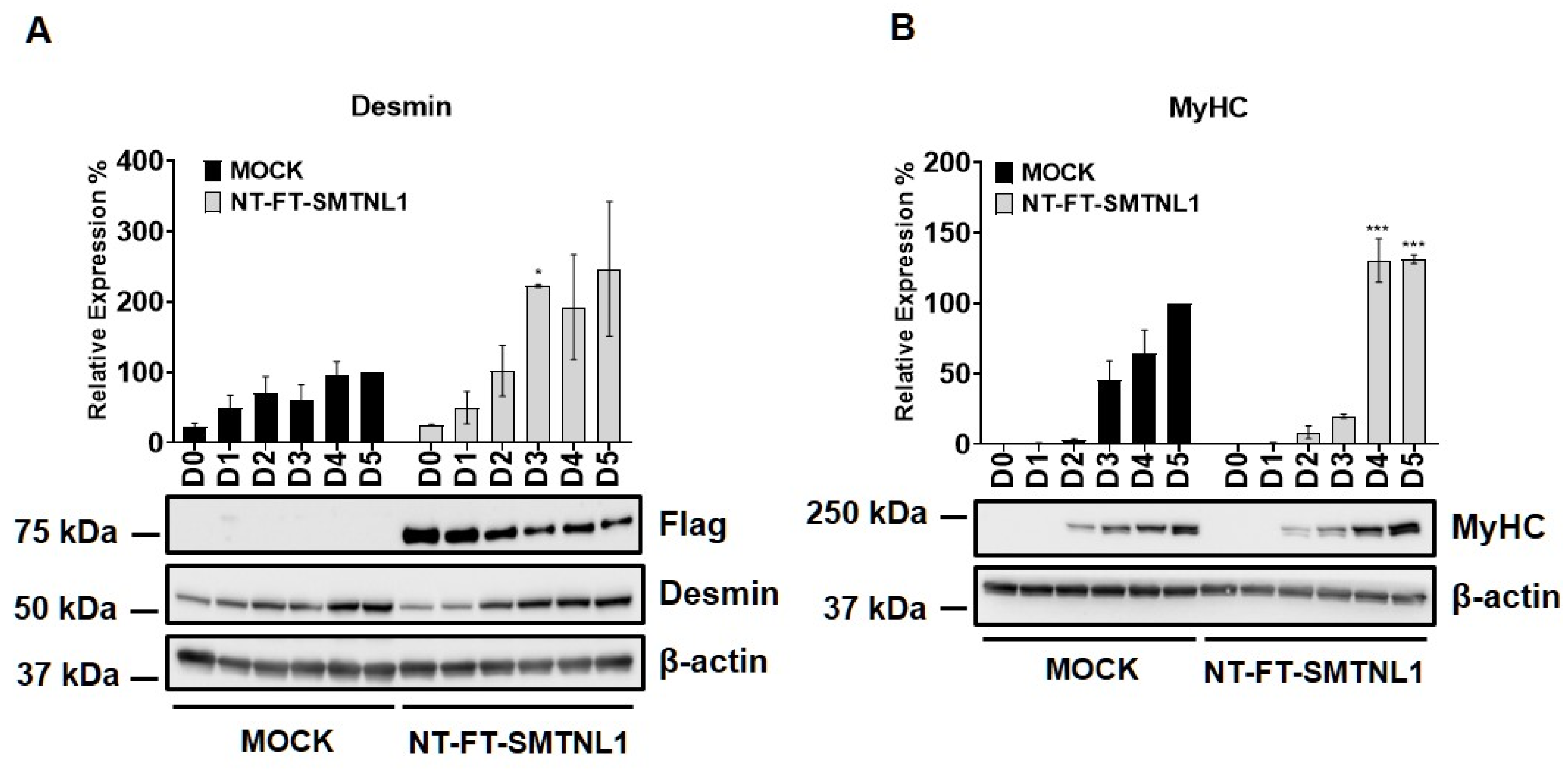
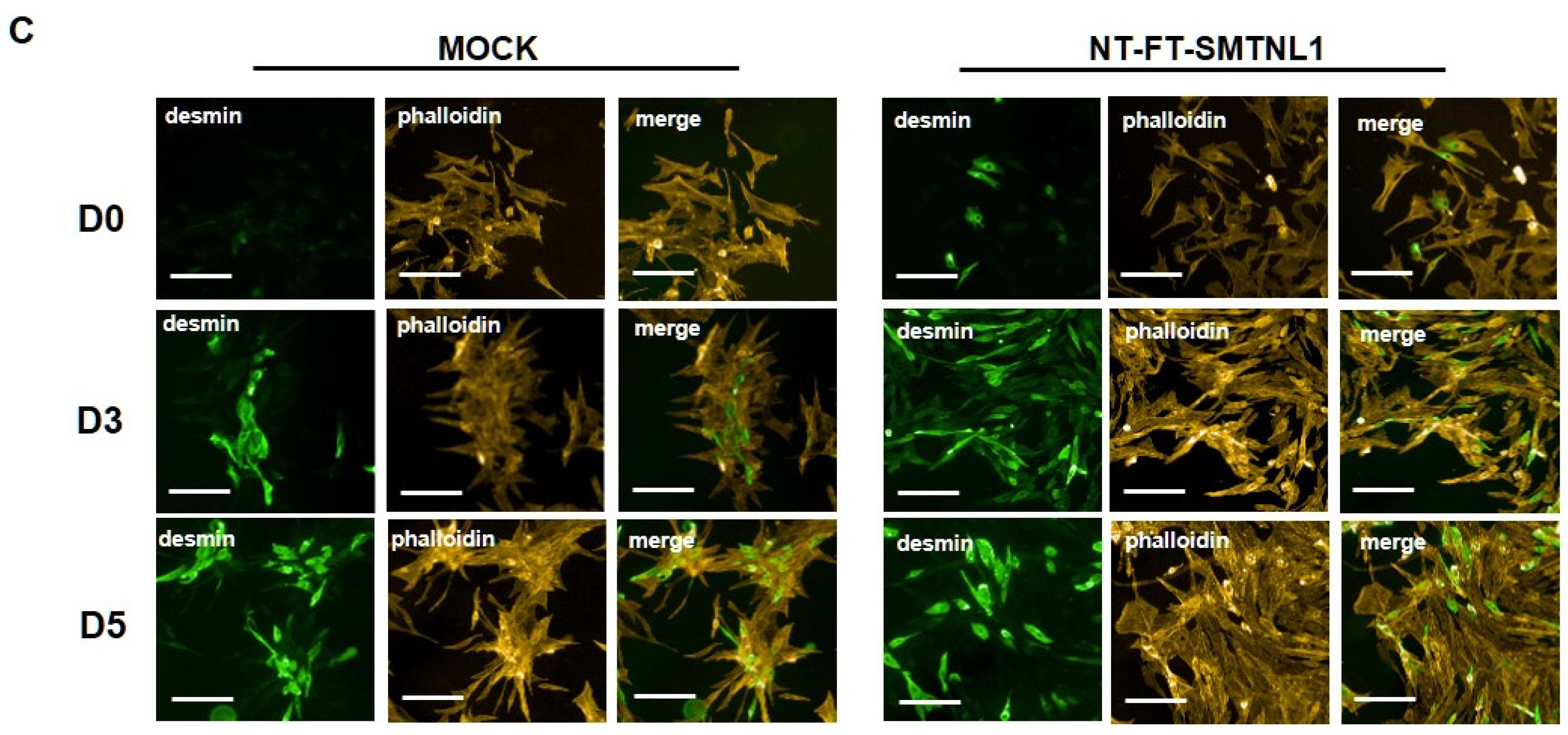
Myogenic differentiation is a highly coordinated process, which is essential for the development and regeneration of skeletal muscle. The mouse myoblast cell line C2C12 is a widely used model to study skeletal muscle myogenesis in vivo [28,29,30]. SMTNL1, a transcriptional cofactor, has been shown to regulate the adaptation of skeletal muscle to sexual development [26] and pregnancy [28] through altering the expression of contractile and metabolic proteins. In order to investigate the possible physiological relevance of SMTNL1 in skeletal muscle differentiation, SMTNL1 was transiently overexpressed in C2C12 cells. Following transfection, myoblasts were differentiated and Western blot analysis (Figure 1A,B) or immunofluorescence staining (Figure 1C) was performed. Desmin, a major intermediate filament protein, is present at low levels at early stages of myogenesis, and its expression increases as normal differentiation proceeds [29]. Our results demonstrate that desmin levels were significantly elevated in the NT-FT-SMTNL1-transfected cells compared to the empty vector-transfected control on Day three (Figure 1A). However, by the end of differentiation, the empty vector-transfected cells showed the same expression level of desmin as the NT-FT-SMTNL1-overexpressed cells. We also examined the expression of a late myogenic marker, myosin heavy chain (MyHC), the expression of which increases sharply during regular differentiation [30]. Here, we show that MyHC expression significantly rose on Day four and Day five due to SMTNL1 overexpression compared to the control (Figure 1B). To further confirm the upregulation of desmin expression caused by SMTNL1 overexpression, we performed immunofluorescence staining on empty vector-transfected or NT-FT-SMTNL1-transfected cells. As shown in Figure 1C, NT-FT-SMTNL1 overexpressing cells showed stronger fluorescent signals even at Day zero of differentiation, which was more pronounced on Day five compared to the control. These results demonstrate that SMTNL1 overexpression leads to the upregulation of early and late myogenic markers, suggesting that SMTNL1 promotes myoblast differentiation. To assess morphological changes throughout the differentiation of empty vector-transfected and NT-FT-SMTNL1-transfected cells, light microscopy images with the same parameter settings were taken and used to calculate the average area, average perimeter and myotube number using ImageJ software (Figure S1A). We observed that SMTNL1 expression significantly decreases the average area and perimeter of myotubes on Day three and Day five of differentiation, respectively. However, SMTNL1 overexpression markedly increased the number of myotubes on Day three and Day five of differentiation. Taken together, these data indicate that SMTNL1 overexpression leads to accelerated myogenesis with myotubes that are significantly smaller in size but bigger in number.
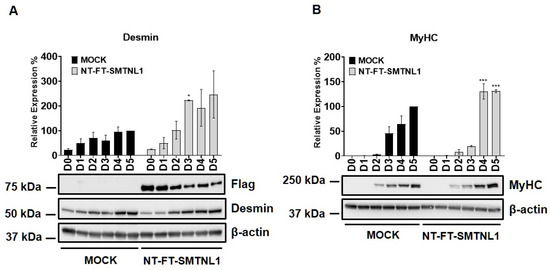
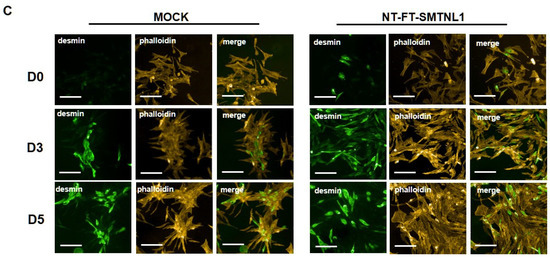
Figure 1.
Effect of SMTNL1 overexpression on desmin and MyHC expression during myogenesis. Lysates from NT-FT-SMTNL1 or empty vector-transfected differentiated C2C12 cells were analyzed using Western blot with antibodies specific for early and late differentiation markers desmin (A) and MyHC (B), respectively. Transfection efficiency was determined using immunoblotting with anti-Flag antibody. Values represent n = 3, mean ± SEM. Differences between group means were determined using Two-way ANOVA followed by Sidak multiple comparisons post hoc test, p < 0.05 (*), p < 0.001 (***). (C) Immunofluorescent staining of cells at Day 0, Day 3 and Day 5 of differentiation after transfection with empty vector or NT-FT-SMTNL1. For staining, anti-desmin antibody (green) and Texas-Red Phalloidin (red) were used. Fluorescent signals were detected using an automated high-content screening instrument. Scale bars: 500 µm.
2.2. Myosin Phosphatase Expression during C2C12 Myogenesis in the Presence of Excess T3
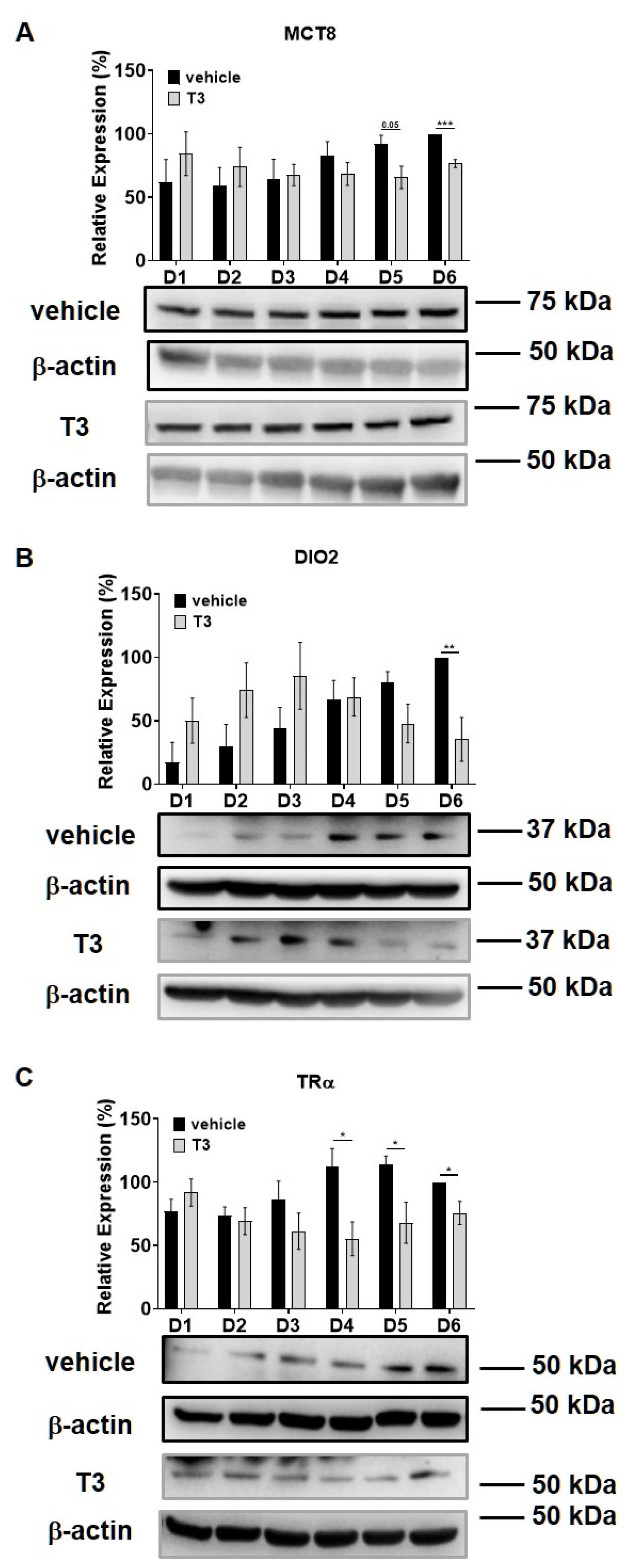
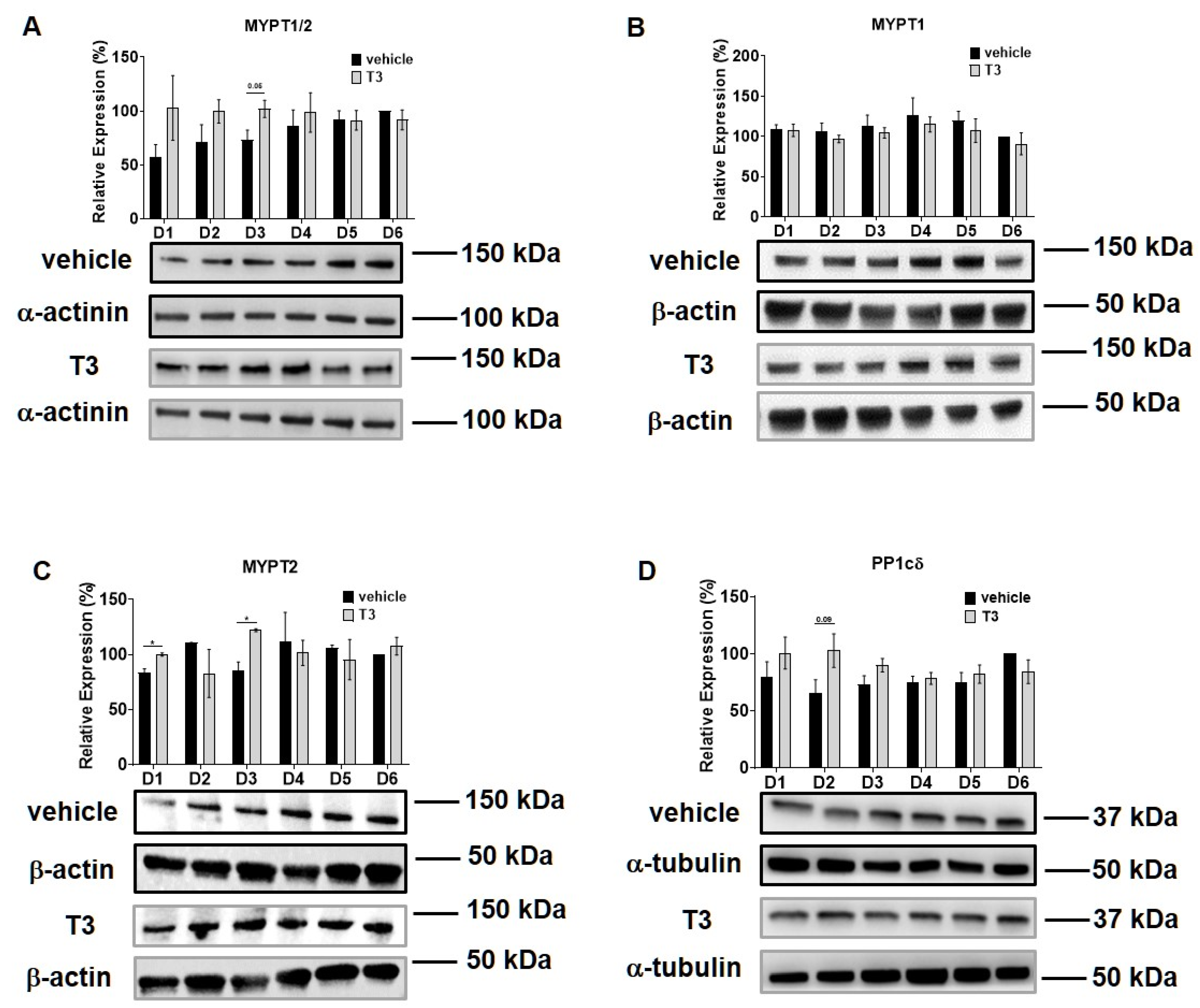
Intracellular T3 hormone availability depends on the presence of TH transporters in the plasma membrane, the activity of iodothyronine deiodinases and the expression of TRs, from which DIO2 and TRα were proved to play crucial roles in myogenesis, too [31,32]. Moreover, Wu et al. described the importance of myosin phosphorylation throughout the course of C2C12 differentiation, which is known to be partially dependent on the activity of MP [33]. Thus, we investigated the effect of excess T3 on these regulatory proteins during myoblast differentiation. MCT8 expression demonstrated a small increase during normal differentiation. In the presence of excess T3, MCT8 expression decreased slightly by Day six, which was 24% smaller compared to the vehicle control (Figure 2A). The expression of DIO2 demonstrated a sharp increase from Day three to Day four and continued to rise until the end of regular differentiation. In response to T3 treatment, DIO2 expression was induced consecutively until Day three and started to decline from Day four. By the end of differentiation, it was significantly diminished by 65% compared to the vehicle control (Figure 2B). In terms of TRα expression, it started to increase from Day four under regular differentiation. Upon T3 treatment, the TRα levels were significantly reduced from Day four until the end of differentiation compared to the vehicle control (Figure 2C). MYPT1/2 and MYPT2 expression was gradually increased during normal differentiation, while it showed an opposite tendency in the presence of excess T3 (Figure 3A,C). Despite the increase in MYPT1 expression on the first days of differentiation, it was reduced by Day six compared to the vehicle control (Figure 3B). Similarly, PP1cδ expression was moderately risen throughout normal differentiation; however, T3 overload caused an increase at first, and a modest decrease in its expression by the end of differentiation (Figure 3D).
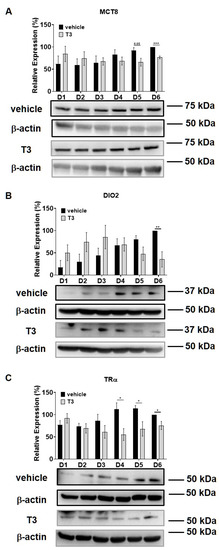
Figure 2.
MCT8, DIO2 and TRα expression throughout the differentiation of C2C12 myoblasts in the presence of T3. (A–C) Myoblasts were differentiated for 6 days receiving either 10 nM T3 or equal amounts of HCl:EtOH solution. Proteins from whole-cell lysates were analyzed using Western blot with anti-MCT8 (A), anti-DIO2 (B) and anti-TRα (C) antibodies. Values represent n = 4–5, mean ± SEM. Data were normalized to the Day 6 vehicle control. Differences between group means were determined using Two-way ANOVA followed by Sidak post hoc test and by multiple unpaired two-tailed t-tests, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
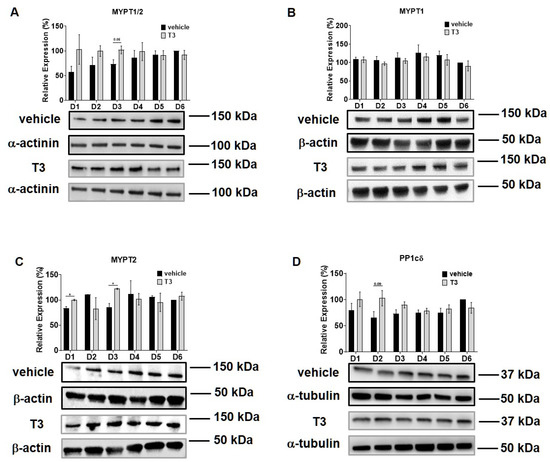
Figure 3.
MYPT1/2 and PP1cδ expression during C2C12 myogenesis in the presence of T3. Myoblasts were differentiated for 6 days in the presence of 10 nM T3 or equal amounts of HCl:EtOH solution. Proteins from whole-cell lysates were analyzed using Western blot with anti-MYPT1−296 (A), anti-MYPT1−38 (B), anti-MYPT2 (C) and anti-PP1cδ (D) antibodies. Values represent n = 4, mean ± SEM. Data were normalized to the Day 6 vehicle control. Differences between group means were determined using Two-way ANOVA followed by Sidak post hoc test and by multiple unpaired two-tailed t-tests, p < 0.05 (*).
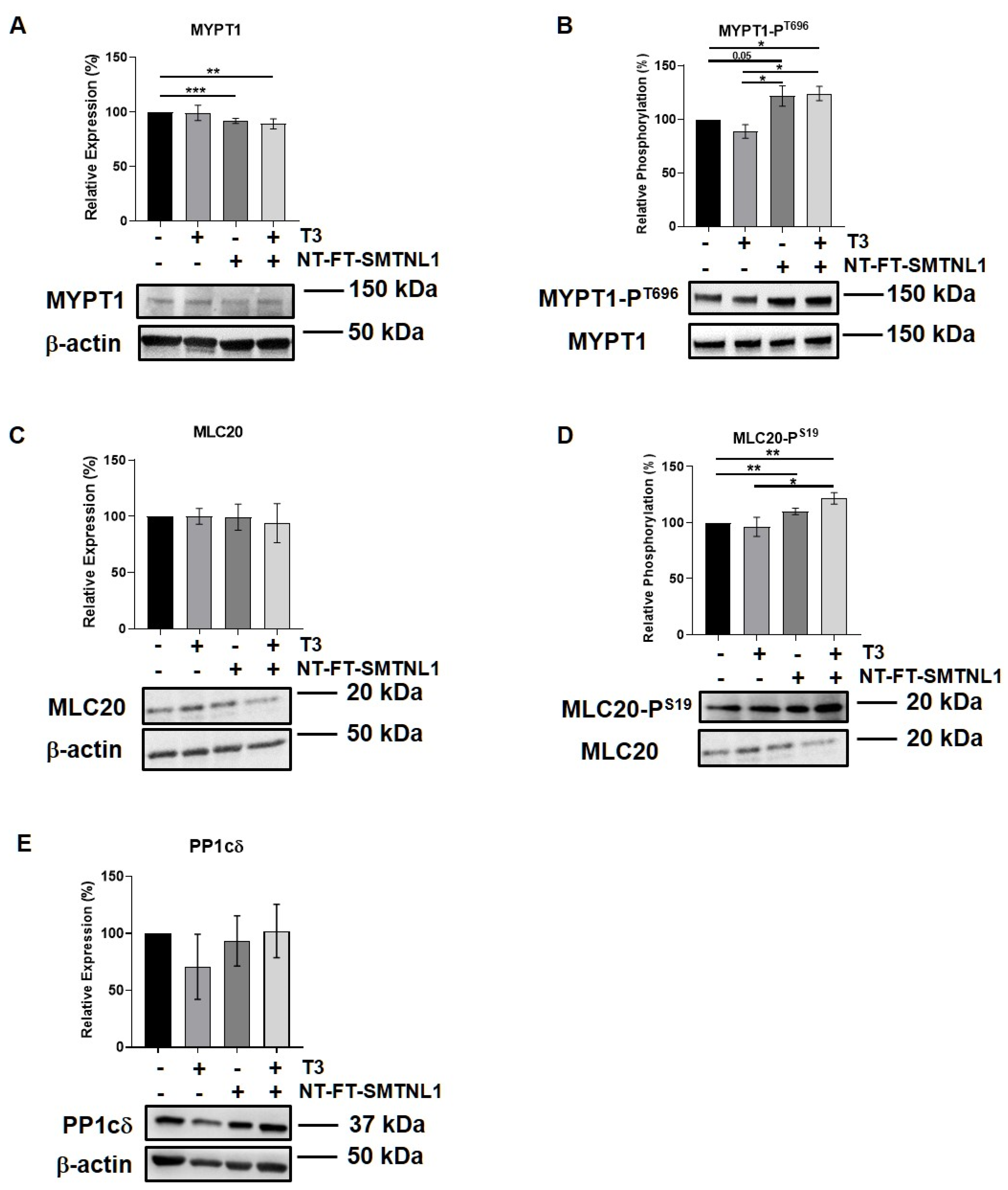
2.3. SMTNL1 Overexpression Induces the Phosphorylation of MYPT1 and MLC20 in Myoblasts
SMTNL1 is known to inhibit the MP holoenzyme by directly binding to MP in the cytoplasm and by suppressing the expression of its regulatory subunit (MYPT1) at the transcriptional level [28]. Since MYPT1 is the main isoform in the MP present in non-differentiated C2C12 cells (Figure 3A,B) we examined the effect of SMTNL1 overexpression on the expression and phosphorylation of MP and its substrate, MLC20, under supraphysiological T3 exposure in myoblasts. Our data demonstrate that SMTNL1 expression was induced by 85% as a result of transfection in myoblasts (Figures S2A and S1C). T3 treatment resulted in a slight decrease in the inhibitory phosphorylation of MYPT1, while the expression of MYPT1 showed no changes (Figure 4A,B). In contrast, SMTNL1 overexpression alone and in combination with T3 significantly reduced the expression of MYPT1, and enhanced the Thr696 phosphorylation of MYPT1 by 22 and 24%, respectively (Figure 4A,B). Remarkably, the SMTNL1-induced elevation in MYPT1 inhibitory phosphorylation was accompanied by an increase in the phosphorylation of MLC20 on Ser19 residue (Figure 4D). T3 treatment caused a small but not significant decrease in MLC20 phosphorylation (Figure 4D). The PP1cδ and MLC20 levels remained unchanged in response to any treatment (Figure 4C,E). Notably, SMTNL1 overexpression did not change the expression of MYPT2, while T3 treatment alone or in combination with SMTNL1 overexpression elevated MYPT2 expression (Figure S3). These results indicate that SMTNL1 has a major impact on MYPT1 in myoblasts.
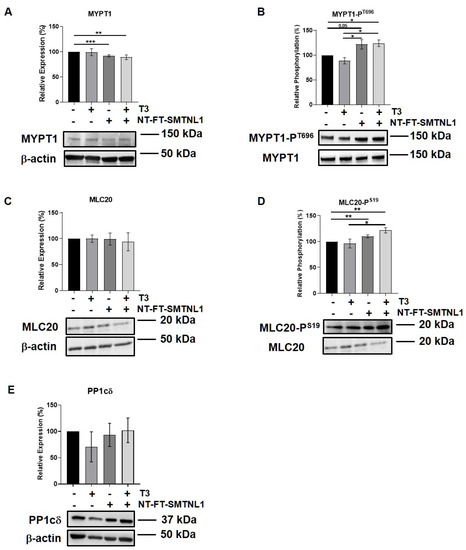
Figure 4.
SMTNL1 overexpression controls the phosphorylation of myosin phosphatase and its substrate in myoblasts. Myoblasts were transfected with empty vector or NT-FT-SMTNL1 and were treated with 10 nM T3 for 24 h. Whole-cell lysates were analyzed using Western blot with anti-MYPT1 (A), anti-MYPT1-PT696 (B), anti-MLC20 (C), anti-MLC20-PS19 (D) and anti-PP1cδ (E) antibodies. Values represent n = 3, mean ± SEM. Data were normalized to the empty vector-transfected control. Differences between group means were determined using One-way ANOVA and Tukey’s post hoc test, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
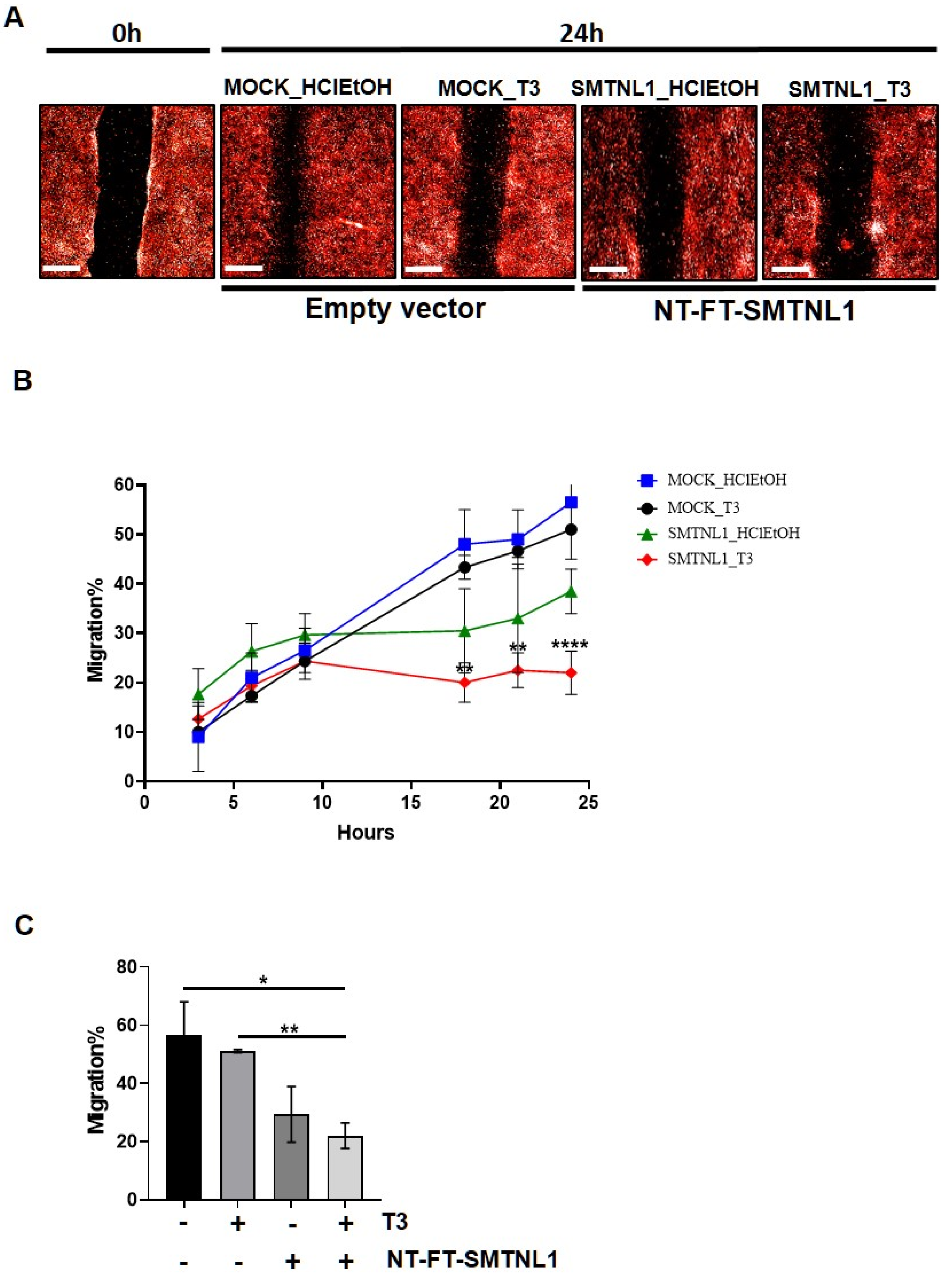
2.4. SMTNL1 Overexpression Inhibits the Migration of Myoblasts
It has previously been shown that the silencing of MP results in the inhibition of migration [34]. Considering the significant impact that SMTNL1 has on the expression and phosphorylation level of MYPT1 and MLC20, we investigated the effect of SMTNL1 overexpression and T3 overload on the migratory capacity of C2C12 myoblasts since this ability is important in skeletal muscle differentiation and regeneration [35]. For this purpose, NT-FT-SMTNL1-transfected C2C12 cells labelled with fluorescent dye were uniformly scratched and cultured in the presence of supraphysiological T3 and changes were monitored for 24 h in real-time. Our results demonstrate that SMTNL1 overexpression alone or in combination with T3 markedly slowed down the closure of the scratch, while T3 treatment had negligible effects (Figure 5A). By monitoring the kinetics of cell migration, we detected a lower migration rate in the NT-FT-SMTNL1-transfected cells compared to the empty vector-transfected and to the T3-treated cells. Remarkably, this effect was more pronounced in response to the combined treatment with T3 and SMTNL1 overexpression (Figure 5B). As shown in Figure 5C, migratory capacity was slightly decreased upon T3 treatment, while SMTNL1 overexpression alone or in combination with T3 caused a 27 and a 34% decrease, respectively, compared to the empty vector-transfected control. Collectively, these data suggest that SMTNL1 negatively influences the migration of myoblasts, possibly through the inhibition of MP.
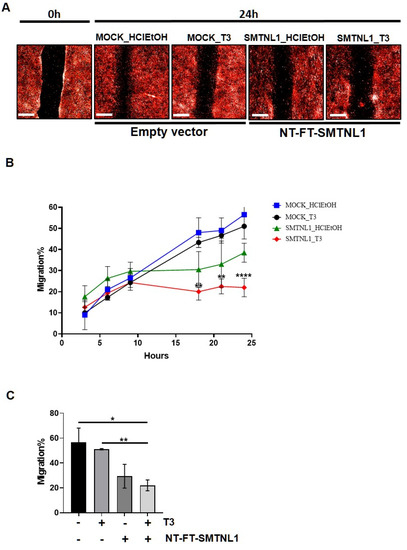
Figure 5.
Scratch assay of T3-treated and SMTNL1 overexpressing myoblasts. Myoblasts were stained and transfected with empty vector or NT-FT-SMTNL1. After 48 h of culturing and a 5-h serum starvation, cells were scratched and were grown for additional 24 h in the presence of 10 nM T3. (A) Photos of scratch areas were taken with a 10× air objective at the indicated times. Scale bars: 500 µm. (B) 24-h kinetic curve of migrating C2C12 myoblasts. (C) Bar charts show covered scratch areas after 24 h. Values represent n = 3, mean ± SEM. Data were normalized to the empty vector-transfected control (MOCK). Differences between group means were determined using Two-way ANOVA (B) or One-way ANOVA (C) followed by Tukey’s post hoc test, p < 0.05 (*), p < 0.01 (**), p < 0.0001 (****).
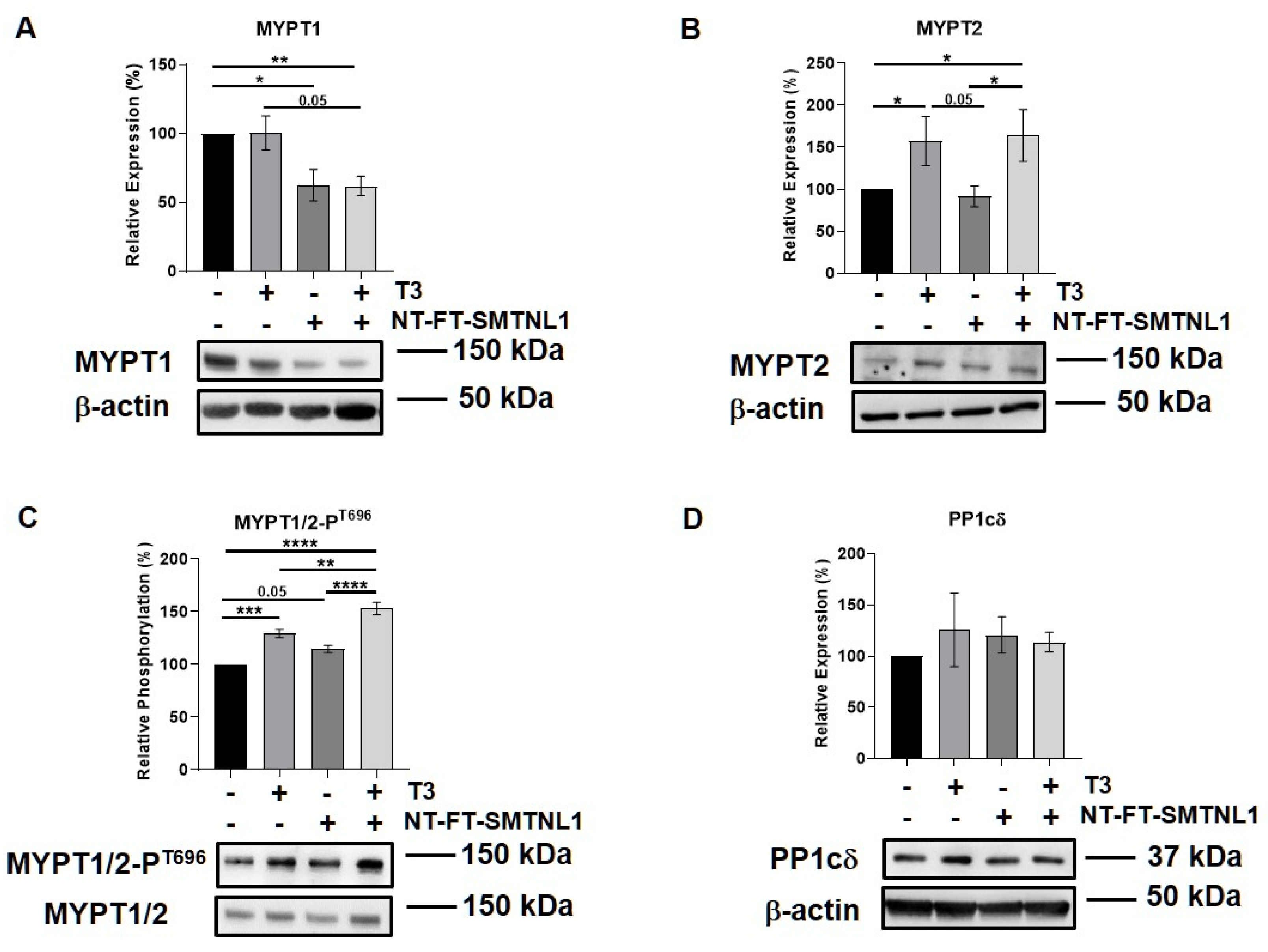
2.5. Differential Regulation of Myosin Phosphatase Target Subunit Isoforms by T3 Treatment and SMTNL1 Overexpression in Myotubes
Previous studies reported that there is a slight shift during myoblast differentiation from MYPT1 to MYPT2 [33]. Therefore, we examined these regulatory subunit isoforms of MP in differentiated C2C12 cells, too. Additionally, Lontay et al. reported that the expression levels of the MYPT2 isoform are unaffected by SMTNL1 deletion in developing muscle [26]. Thus, we investigated whether SMTNL1 overexpression affects MYPT2 expression or not. First, the overexpression of SMTNL1 was successfully confirmed using Western blot analysis showing a 43% increase in total SMTNL1 expression (Figures S2B and S1C). Similarly to the results obtained on C2C12 myoblasts, the expression of MYPT1 did not change in response to excess T3 but it was significantly reduced by 23 and 38%, respectively, owing to SMTNL1 overexpression alone and in combination with T3 in myotubes (Figure 6A). MYPT2 expression was significantly increased by 56 and 64%, respectively, in response to excess T3 and to combined treatment with T3 and SMTNL1 overexpression, while it did not change upon SMTNL1 overexpression (Figure 6B). The phosphorylation of MYPT1/2 on Thr696 residue was significantly elevated upon T3 treatment by 29% and upon combined treatment with T3 and SMTNL1 overexpression by 53%, and it demonstrated a marginally significant increase due to the overexpression of SMTNL1 (Figure 6C). The phosphorylation of Thr853, another inhibitory phosphorylation site of MYPT1, was markedly enhanced under each condition compared to the empty vector-transfected control (Figure S4). Regarding PP1cδ, its levels were slightly increased following T3 treatment and/or SMTNL1 overexpression (Figure 6D). These results suggest that T3 and SMTNL1 are selective to the different MYPT isoforms.
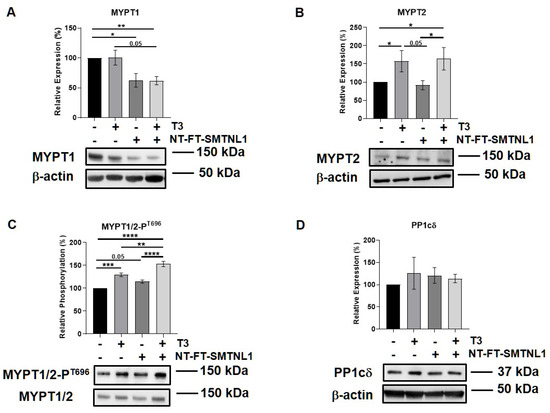
Figure 6.
T3 Treatment and SMTNL1 overexpression influence the expression and phosphorylation of myosin phosphatase target subunits in myotubes. Myoblasts were transfected with empty vector or NT-FT-SMTNL1 and were differentiated with a simultaneous 72-h T3 treatment started from Day 4. Whole-cell lysates were analyzed using Western blot with anti-MYPT1 (A), anti-MYPT2 (B), anti-MYPT1/2-PT696 (C) and anti-PP1cδ (D). Values represent n = 3, mean ± SEM. Data were normalized to the empty vector-transfected control. Differences between group means were determined using One-way ANOVA and Tukey’s post hoc test, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
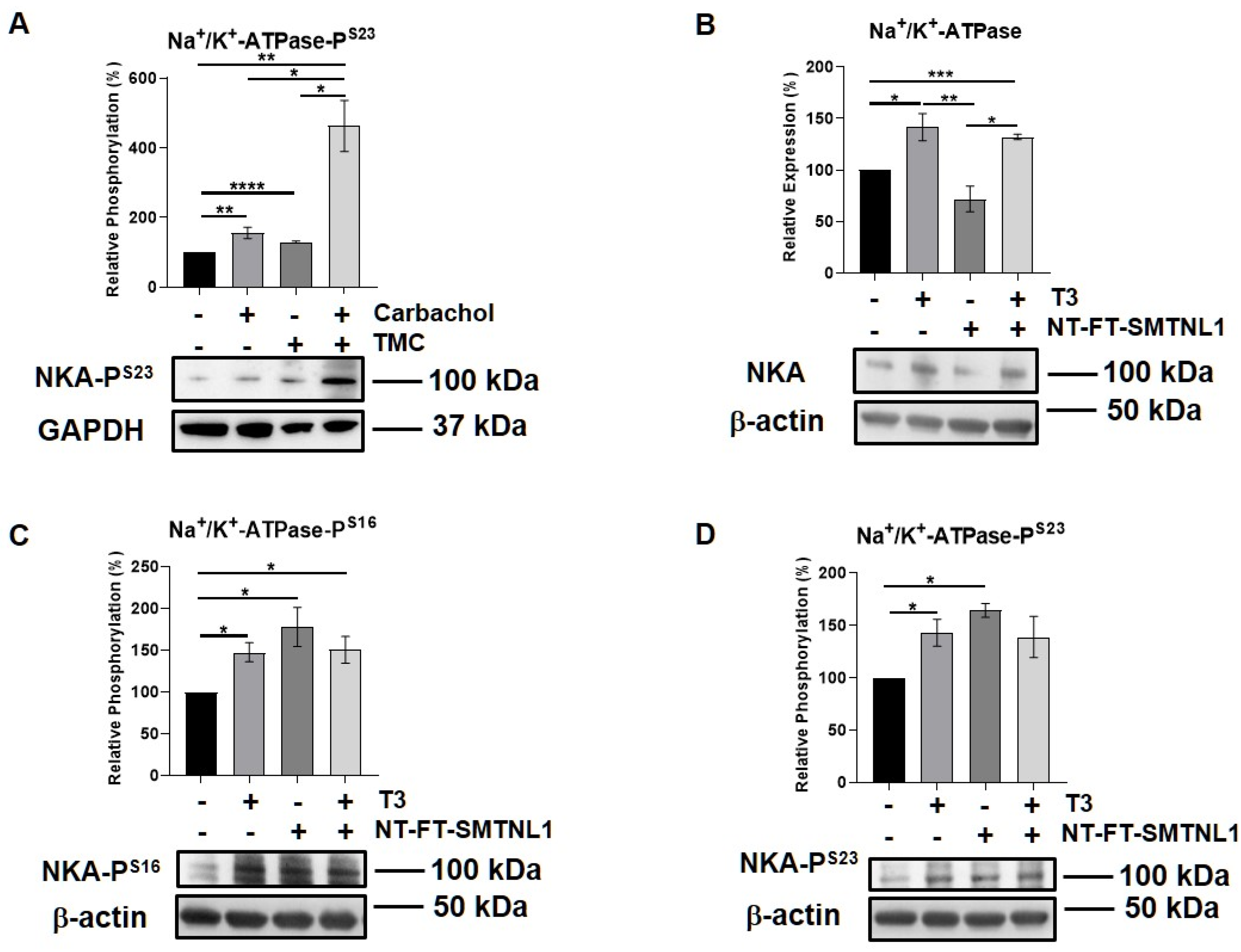
2.6. Effect of T3 Treatment and/or SMTNL1 Overexpression on the Expression and Activity of the Na+/K+-ATPase, an Alternative MP Substrate in Myotubes
Since the expression and importance of the major substrate of MP, MLC20, decreases in SKM, we assumed that MP targets alternative substrate(s) in SKM. Moreover, we have identified Na+/K+-ATPase as a MYPT1 interacting protein using a pull-down assay followed by mass spectrometry analysis from a mouse synaptosome extract [36]. Thus, we examined the expression and activity of Na+/K+-ATPase in our current model system. Na+/K+-ATPase is a widely expressed transmembrane protein, which is essential to maintain whole body homeostasis as well as muscle function [37]. Its expression was found to increase in hyperthyroid muscle [38]. First, we studied the interaction between the Na+/K+-ATPase and MYPT in the C2C12 mouse myoblast cell line. Na+/K+-ATPase and MYPT were reciprocally co-precipitated. These data suggest that the Na+/K+-ATPase is capable of binding to MP, possibly through its regulatory subunit (Figure S5). The G-protein-coupled muscarinic receptor mimetic carbachol, as a positive control [39], and the PP1 selective inhibitor tautomycetin were applied on muscle strips, and the phosphorylation of Na+/K+-ATPase was assessed. Both the carbachol and TMC increased the phosphorylation of Ser23 in the α1 subunit of Na+/K+-ATPase (Figure 7A). We also investigated the effect of T3 treatment and/or SMTNL1 overexpression on Na+/K+-ATPase in C2C12 myotubes. T3 treatment alone and in combination with SMTNL1 overexpression significantly elevated Na+/K+-ATPase expression by 41 and 32%, respectively, while it remained unchanged upon SMTNL1 overexpression (Figure 7B). The phosphorylation of Na+/K+-ATPase on Ser16 and Ser23 residues was significantly increased in response to all the conditions (Figure 7C,D). These results indicate that both T3 and SMTNL1 regulates the activity of Na+/K+-ATPase by increasing its phosphorylation through the inhibition of distinct MYPT isoforms.
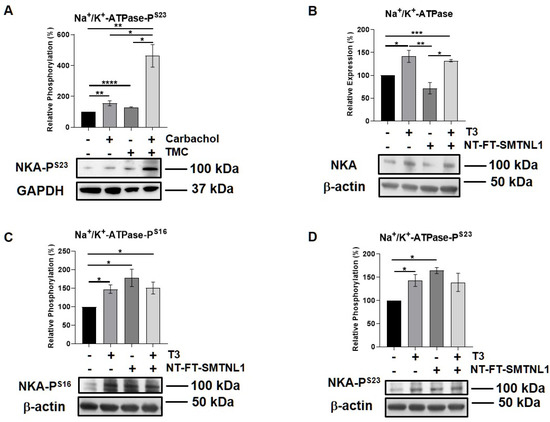
Figure 7.
T3 Treatment and SMTNL1 overexpression influence the expression and phosphorylation of Na+/K+-ATPase in myotubes. (A) Plantaris muscle strips of mice were treated with 10 µM TMC for 30 min followed by 10 µM carbachol for 10 min. Samples were lysed and subjected to Western blot using anti-Na+/K+-ATPase. (B–D) Myoblasts were transfected with empty vector or NT-FT-SMTNL1 and were differentiated in parallel with a 72-h T3 treatment started from Day 4. Proteins from whole-cell lysates were separated by size and were analyzed using Western blot with anti-Na+/K+-ATPase (B), anti-Na+/K+-ATPase-PS16 (C) and anti-Na+/K+-ATPase-PS23 (D). Values represent n = 3, mean ± SEM. Data were normalized to the empty vector-transfected control. Differences between group means were determined using One-way ANOVA and Tukey’s post hoc test, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
3. Discussion
Thyroid hormones are key regulators of the development, regeneration, metabolism and myogenesis of SKM [40]. Myogenic differentiation is a highly coordinated process, which is essential for the development and regeneration of SKM. During differentiation, myoblasts undergo remodeling and fuse to form multinucleated, contractile myotubes in parallel with an increased expression of muscle-specific proteins that coordinate the contractile and metabolic functions of SKM. Moreover, SMTNL1 expression is increased in SKM during development [26]. These data correlate with our findings that SMTNL1 overexpression moderately promoted the differentiation of C2C12 myoblasts to myotubes by increasing the levels of myogenic markers, such as MyHC and desmin (Figure 1). Moreover, SMTNL1 overexpression alters the phenotype of differentiated cells creating smaller myotubes (Figure S1).
In humans, the T3 level is maintained at low at the beginning of the myogenic process but it is increased parallel with the elevation of DIO2 upregulation [41]. It supports our data on C2C12 cell differentiation showing a significant increase in DIO2 expression upon T3 treatment; however, it markedly decreases in response to prolonged T3 treatment, suggesting a negative feedback mechanism (Figure 2B). The effect of MCT8 in myogenesis is debated since MCT8KO mice presented the same motor activity as WT mice [42]. In accord with that, we observed modest changes in MCT8 levels under each experimental setup during differentiation (Figure 2A). TRα was also changed during myoblast differentiation and showed a significant decrease upon prolonged T3 treatment (Figure 2C). TRα is crucial in the proliferation, migration and differentiation of myoblast cells and TRαKO mice showed impaired muscle regeneration [43]. In addition, SMTNL1, a co-regulator of TRα expression, is downregulated by supraphysiological T3 concentration and it hampers TRα expression [27]. Collectively, the C2C12 model proved to provide a sufficient hyperthyroid model for myogenesis with similar effects to that shown in human SKM. The effect of SMTNL1 in combination with the supraphysiological T3 level did not change the cell viability of differentiated C2C12 cells, suggesting that it has an impact not on the proliferation but the differentiation process itself [27].
The expression of MYPT isoforms and the enzyme activity of MP have already been assessed by Wu et al. [33] during C2C12 myoblast differentiation; they reported an increase in the expression level in MYPT2 and a decline in the MYPT1 isoform. These findings are supported by our data showing a slight increase in MYPT2 and a non-significant decline of MYPT1 expression (assessed by an anti-MYPT1−38 antibody that recognizes the MYPT1 isoform) [44] with no significant changes in PP1cδ expression (Figure 3). Interestingly, a supraphysiological T3 level elevated MYPT2 and PP1cδ expression but had no effect on MYPT1 expression, suggesting a selective regulation of MYPT isoforms. Surprisingly, the PP1-MYPT1 complex functioned even in differentiated C2C12 cells [33] and MYPT1 expression but not MYPT2 expression, was regulated by SMTNL1 (Figure 6A,B). Since MYPT1 and MYPT2 coding genes are located on chromosome 12q15–q21.2 and 1q32.1, respectively [14], their differential gene expression regulation is feasible. MYPT1 expression showed a significant decrease upon SMTNL1 overexpression, which was independent of T3-exposure in myoblasts and myotubes as well. It is in line with previous findings, showing that SMTNL1 downregulates the gene expression of MYPT1 in SKM [28]. MYPT2 expression is independent of SMTNL1 overexpression, which might be explained by the fact that SMTNL1 and MYPT1 are expressed exclusively in type 2A SKM fibers, while MYPT2 showed a homogenous distribution [28]. In the course of C2C12 differentiation, a shift from type 1 to 2A than to 2X and type 2B fiber marker proteins has been observed [45] and supraphysiological T3 exposure promotes this shift [46], supporting this hypothesis.
Non-muscle myosin (nm-myosin) has a fundamental role in processes that require cellular reshaping and movement, such as cell adhesion, cell migration, morphogenesis, development and cell division through its actin cross-linking and contractile functions in myoblasts [47]. The role of MP is inevitable in these processes [48]. Based on our data and other studies [33], MYPT1 is the major isoform in the MP present in non-differentiated C2C12 cells. Neither MYPT1 expression nor its activity was influenced significantly by T3 but only by the overexpression of SMTNL1 (Figure 4A,B). It has been reported that T3-induced ROCK activation resulted in an increased phosphorylation of MYPT1 in hepatic stellate cells [49]. It was also found to be time-dependent in osteoblast-like cells showing that the phosphorylation of MYPT1 was faded away by 6 h of T3 treatment [50]. It might explain the relatively low decrease in MYPT1 phosphorylation since our treatment lasted for 24 h (Figure 4B). In lung tissues, it was demonstrated that THs are able to modulate MLC20 phosphorylation and a moderate reduction in the level of phospho-MLC20 was detected in hyperthyroid mice compared to their euthyroid counterparts [51]. It supports our data on marginally decreased MLC20 phosphorylation upon T3-treatment, while it was greatly increased in response to the combined effect of T3 and SMTNL1 overexpression (Figure 4D). The T3 and SMTNL1-dependent phosphorylation of MLC20 resembles the migration activity and the ability of cytoskeletal remodeling of C2C12 cells. The combined effect of supraphysiological T3 and SMTNL1 hampered C2C12 migration; however, we need to note that drastic changes happened after 9 h of treatment (Figure 5B). The inhibition of MP by its phosphorylation at Thr696 could result in a slower turnover rate of nm-myosin phosphorylation causing more stable adhesive structures in the cells preventing migration and cytoskeletal remodeling. It is in accordance with a migration assay on HaCaT cells showing that PP1 enzyme inhibition started to cause a significant effect after 9 h of treatment [52]. In addition, SMTNL1 can also inhibit MP activity [26] by altering its phosphorylation that is clearly visible on MLC20 phosphorylation in SMTNL1-overexpressed myoblasts (Figure 4D). Since the MLC20 phosphorylation and MP activity were not fully in accordance with the migration features of the C2C12 myoblast, we assume that T3 has additional, most probably non-genomic effects on the cytoskeletal remodeling. These collectively lead to the arrest of myoblast migration and impede the remodeling of the actomyosin complex in non-differentiated C2C12 cells. We can conclude that MP facilitates myoblast migration that is hampered in a hyperthyroid state regulated by SMTNL1.
By the differentiation of C2C12 cells, a shift from the nm-myosin to the skeletal muscle type of myosin (sk-myosin) is observed, and at Day six the nm-myosin was barely detectable in the myotubes, while the concentration of sk-myosin was increased [33]. This is consistent with the slow rate of dephosphorylation of the sk-myosin phospho-light chain in vivo [53]. The MYPT1-PP1cδ smooth muscle-type holoenzyme shows low affinity to the phosphorylated sk-myosin and the sk-myosin phosphorylation was reduced but not eliminated at Day six of differentiation [33], suggesting that alternative substrates of myosin phosphatase exist in SKM.
One of the potential candidates as a substrate of MP is the Na+/K+-ATPase (NKA). NKA is a transmembrane pump that is essential for cellular ion and water homeostasis, maintaining membrane potentials and Na+-coupled transport of various substances. SKM has one of the largest and most dynamic pools of NKA in the body [37]. Therefore, the regulation of NKA either by the concentrations of its substrates or by protein–protein interactions with FXYD proteins or by covalent modification—predominantly by phosphorylation—is crucial [37]. We have already identified NKA as an MYPT1 interacting protein using a pull-down assay followed by mass spectrometry analysis from a mouse synaptosome extract [36]. The co-immunoprecipitation from C2C12 cell lysate also revealed the interaction between NKA and MYPT (Figure S5). In addition, NKA was proven to be a potential substrate of a PPP1 enzyme, possibly MP, due to the elevated phosphorylation at Ser23 residue upon the selective inhibition of PP1c by tautomycetin (Figure 7A). It is in line with previous studies showing that the inhibition of PP1c/PP2Ac by okadaic acid and calyculin A resulted in a decrease in NKA activity [54]. T3 stimulates the intrinsic activity of NKA in a transcription-independent manner via the stimulation of its cell surface expression through the PI3K/PKB pathway [55]. We have also detected this increase under the supraphysiological T3 exposure of C2C12 myotubes (Figure 7) and it is in accordance with findings showing the increased number of ouabain sites, also known as NKA level, in the SKM of hyperthyroid patients compared to healthy controls [56]. In addition, we proved that NKA gene expression occurs in an SMTNL1-independent manner (Figure 7B). Not only the protein level and translocation but the phosphorylation of the α subunit of NKA by Ser/Thr kinases can regulate the activity of NKA [55]. Although, the effects of THs are controversial due to the tight interplay between different signaling pathways; nevertheless, NKA phosphorylation by novel PKCs at Ser16 and Ser23 possessed mostly negative regulatory effect on NKA activity [57,58,59].
In myotubes, the expression of MYPT2 but not MYPT1 increased in a T3-dependent but SMTNL1-independent manner along with a marginal increase in the expression of PP1 catalytic subunit. Interestingly, T3 and its combined treatment with SMTNL1 overexpression impeded the activity of MP assessed by its regulatory Thr696 phosphorylation (Figure 6C). Parallel with these processes, the phosphorylation of NKA at Ser16 and Ser23 also significantly increased upon T3 treatment and SMTNL1 overexpression as well (Figure 7C,D). We strongly suggest that SMTNL1 took effect on increased NKA phosphorylation and activity via the downregulation of MP. The phosphorylation and inactivation of MP might be evoked by ROCK that was stimulated by T3 in osteoblasts resulting in the phosphorylation of MYPT1 at Thr696 and hampered osteocalcin synthesis [50]. It is important to note that both the overstimulation of NKA expression by T3 or hyper- or downregulation of its activity do not necessarily translate into improved muscle performance, and it could cause muscle weakness and myopathy [37].
In conclusion, we suggest a differential regulation and role of MP in myoblasts and myotubes upon supraphysiological T3 exposure. In non-differentiated myoblasts, MP is in complex with MYPT1 and its expression and activity is regulated by SMTNL1 that modulates the MLC20 phosphorylation, cytoskeletal remodeling and migration. In contrast, in myotubes, where the action of MP is less profound in the regulation of muscle contractility, since sk-myosins are regulated by a separate set of proteins that are bound to the actin filaments, and in the MP holoenzyme, PP1c is predominantly complexed with MYPT2. MYPT2 expression is stimulated by T3, and MP is regulated by the T3-induced ROCK phosphorylation. MP acts as a possible regulator of NKA by dephosphorylating its inhibitory phosphorylation sites. These findings should lead to a better understanding of myopathy and muscle weakness and the disorder of muscle regeneration in hyperthyroid patients.
4. Materials and Methods
4.1. Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
4.2. Antibodies
Western blot analyses were performed using antibodies specific for desmin 1:1500 (Cell Signaling Technology, Danvers, MA, USA, #5332), MyHC 1:1500 (R&D Systems, Minneapolis, MN, USA, MAB4470), Flag 1:2000 (Sigma-Aldrich, St. Louis, MO, USA, F7405), MYPT1−296 1:1000 [44], MYPT1−38 1:1000 [33], MYPT1 1:1000 (BD Transduction Technologies, Becton, NJ, USA, #612164), PP1cδ 1:1000 (Santa Cruz Biotechnology, Dallas, TX, USA, #sc-365678), MYPT1-PT696 1:1000 (Merck Millipore, Burlington, MA, USA, #ABS45), MYPT1-PT853 1:500 (Merck Millipore, #36-003), MLC20 1:1000 (Cell Signaling Technology, #3672), MLC20-PS19 1:500 (Cell Signaling Technology, #3671), MYPT2 1:1000 [14], Na+/K+-ATPase 1:1000 (Cell Signaling Technology, #3010), Na+/K+-ATPase-PS16 1:250 (Cell Signaling Technology, #4020), Na+/K+-ATPase-PS23 1:500 (Cell Signaling Technology, #4006), MCT8 1:1500 (Proteintech, Rosemont, IL, USA, #20676-1-AP), DIO2 1:1500 (NovusBio, Littleton, CO, USA, #NBP1-00178), TRα 1:650 (Abcam, Cambridge, UK, ab53729), SMTNL1 (Santa Cruz Biotechnology, #sc-390369), β-actin 1:20,000 (Santa Cruz Biotechnology, #sc-47778), α-actinin 1:8000 (Sigma-Aldrich, A2543) α-tubulin 1:1000 (Santa Cruz Biotechnology, #sc-53646), anti-mouse 1:5000 (Cell Signaling Technology, #7076), anti-rabbit 1:5000 (Sigma-Aldrich, A0545), anti-goat 1:5000 (Sigma-Aldrich, A5420).
4.3. Cell Culture Maintenance
C2C12 mouse myoblast cells (ECACC 91031101) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1000 mg/L glucose, 2 mM L-glutamine (Lonza; Basel, Switzerland), 10% (v/v) foetal bovine serum (FBS) and phenol red. Cells were grown in a humidified incubator that provided an atmosphere of 5% CO2 at 37 °C.
4.4. Thin Coating of Tissue Culture Plates
Rat tail collagen I (Thermo Fisher Scientific; Waltham, MA, USA) was diluted in 20 mM acetic acid at a final amount of 5 µg/cm2 and was added to each well. After 1 h, the solution was removed, and wells were rinsed twice with 1× phosphate buffered saline (PBS) to remove the acid. Plates (VWR International; West Chester, PA, USA) were used immediately or air dried and stored at 4 °C until use.
4.5. Transient Transfection and Differentiation
GeneJuice transfection reagent was added dropwise to serum-free DMEM and incubated at room temperature (RT) for 5 min. According to the manufacturer’s intructions, 2 µg of pM13-NT-FT-SMTNL1 per well was added to the serum-free DMEM/transfection reagent mixture and was incubated at RT for 15 min. Meanwhile, cells were washed with 1× PBS and were detached with 0.25% (w/v) trypsin/EDTA solution. After the 15-min incubation ended, the entire volume of the transfection mixture was added dropwise to cells in complete growth medium. Finally, cells were seeded in 6-well tissue culture plates and incubated at 37 °C overnight (O/N). Transfection with empty vector (MOCK) acted as the control in all experiments. The day after transfection, cells were washed with 1× PBS and complete growth medium was replaced by phenol red-free DMEM supplemented with 1000 mg/L glucose, 2 mM L-glutamine and 2% (v/v) horse serum (HS). From then on, differentiation medium was changed daily for 5 days.
4.6. Immunofluorescence Staining
Transfected myoblasts were seeded into collagen-coated OptiPlate-96 Black plates (PerkinElmer; Waltham, MA, USA) and were differentiated for 5 days. On days 0, 3 and 5 of differentiation, cells were fixed with 4% (w/v) paraformaldehyde for 15 min at RT. Then, cells were washed 3 times with 1× PBS and were permeabilized with 0.2% (w/v) Triton-X-100 for 4 min at RT. After blocking with 1% HS for 1 h at 4 °C, immunofluorescence staining was carried out using anti-desmin (1:100) antibody (green), anti-Flag (1:100) antibody (green) and F-actin-binding peptide phalloidin (1:500) (red). Fluorescent signals were detected using an automated high-content imaging reader (PerkinElmer; Waltham, MA, USA).
4.7. 3,3′,5-Triiodo-L-thyronine (T3) Hormone Treatment
According to the manufacturer’s instructions, 3,3′,5-Triiodo-L-thyronine sodium salt was dissolved in 1 M HCl:EtOH with a ratio of 1:4. In Figure 2 and Figure 3, cells received 10 nM of T3 from Day 1 to Day 6 of differentiation. In Figure 4, 48 h after transfection, myoblasts were treated with 10 nM T3 for 24 h. In Figure 6 and Figure 7, myoblasts were transfected the day before the beginning of differentiation. From the beginning of Day 4 to the end of Day 6 of differentiation, differentiation medium was supplemented with 10 nM of T3. In all cases, equal amounts of HCl:EtOH (1:4) were added to the non-treated control called ‘vehicle’ in Figure 2 and Figure 3.
4.8. Cell Lysis
Myotubes were washed with 1× PBS and were harvested in ice-cold RIPA buffer complemented with protease and phosphatase inhibitors (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton-X 100, 1 mM PMSF, 10× protease inhibitor cocktail, 50× phosphatase inhibitor cocktail, 1 µM microcystin-LR). Cell suspensions were sonicated for 30 s with a 10% pulse using a Branson Sonifier 250 sonicator (Thermo Fisher Scientific; Waltham, MA, USA) and were centrifuged at 16,000× g, 4 °C for 10 min. Supernatants were transferred to fresh tubes and total protein concentration was measured with a Bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific; Waltham, MA, USA).
4.9. Western Blot Analysis
Whole-cell lysates were boiled in 4× sodium dodecyl sulphate (SDS) sample buffer (Bio-Rad Laboratories; Hercules, CA, USA) at 100 °C for 5 min. A total of 30 µg of protein was loaded onto 4–20% precast Criterion gels (Bio-Rad Laboratories; Hercules, CA, USA) and was separated by size at a constant voltage of 200 V. Next, proteins were transferred onto nitrocellulose membranes using a Criterion blotter (Bio-Rad Laboratories; Hercules, CA, USA) at 100 V for 75 min. Membranes were blocked in 5% (w/v) bovine serum albumin (BSA) dissolved in 1× Tris buffered saline (TBS) containing 0.1% (v/v) Tween 20 at RT for 1.5 h. After that, membranes were incubated with primary antibodies, diluted in 5% BSA/TBST, at 4 °C O/N. On the next day, membranes were incubated with HRP-conjugated secondary antibodies at RT for 1.5 h and immunoreactions were visualized by enhanced chemiluminescence (ECL) using a mixture of WesternBright ECL or WesternBright Sirius and WesternBright Peroxide reagents (Advansta Inc.; San Jose, CA, USA) with a ratio of 1:1 in a ChemiDoc Touch Imaging System (Bio-Rad Laboratories; Hercules, CA, USA). In case of phospho-blots, membranes were stripped as described in Supplementary Methods and were reprobed with non-phospho antibodies.
4.10. Scratch Assay and High Content Screening (HCS) Analysis
Myoblasts were suspended in serum-free medium containing 5 µL/mL of DiI (Thermo Fischer Scientific; Waltham, MA, USA), a highly lipophilic carbocyanine dye, and were incubated for 15 min at 37 °C. After washing 3 times with warm complete medium, myoblasts were transfected with an empty vector or NT-FT-SMTNL1 and were seeded into collagen-coated OptiPlate-96 Black plates (PerkinElmer; Waltham, MA, USA). After 48 h, cells were serum-starved for 5 h and were scratched with a 10-microliter pipette tip. Myoblasts were washed with 1× PBS and were cultured in the presence of 10 nM T3 for an additional 24 h under real-time screening with an HCS equipment using a 10× air objective. Data were analyzed and evaluated using the Harmony software (PerkinElmer; Waltham, MA, USA).
4.11. Carbachol and Tautomycetin Treatment
Mice were housed as described before [28]. All procedures were approved by the University of Debrecen Faculty of Medicine Ethical Committee and are consistent with the NIH ‘Guide for the Care and Use of Laboratory Animals’. Plantaris muscle strips were removed and maintained as described before [24] in an organ bath filled with Krebs solution (118 mM NaCl; 4.75 mM KCl; 1.2 mM MgSO4; 1.2 mM KH2PO4; 2.5 mM CaCl2; 25 mM NaHCO2; 11.5 mM glucose) warmed at 37 °C and gassed with 95% O2 and 5% CO2. The effects of PP1 inhibitor on muscle tissues were investigated by a 30-min preincubation with 10 μM tautomycetin (TMC) followed by a 10-min incubation with 10 μM carbachol. Tissues were lysed and subjected to Western blot analysis.
4.12. Statistical Analysis
Immunoblots were analyzed using ImageJ software. Bar charts were created and statistical analyses were performed using GraphPad Prism 8 software. Phosphorylated proteins were normalized to non-phosphorylated protein expression, while non-phosphorylated protein expression was normalized to the loading control. All data are presented as mean ± SEM, where n is the number of independently performed experiments. Differences were considered to be statistically significant at p < 0.05.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms221910293/s1.
Author Contributions
B.L., conceptualization; E.M. and B.L., data curation; E.M. and I.K., visualization; E.M., D.H., I.T. and I.K., methodology; E.M., Figure preparation and data analysis; B.L. and F.E., funding acquisition; E.M. and B.L., writing—original draft preparation and editing; F.E., writing—review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Research, Development and Innovation Office FK125043 (to B.L.) and K129104 (to F.E.), the University of Debrecen, Faculty of Medicine Research Fund (1G3DBKJ0BFTK 247) for B.L. and from the EU co-financed by the European Regional Development Fund under project EFOP-3.6.2-16-2017-00006 and from the EFOP-3.6.3-VEKOP-16-2017-00009 project co-financed by EU and the European Social Fund. B. L. MECENATURA from the University of Debrecen.
Acknowledgments
Thank for Karen Uray for providing the animals for the study and for Tibor Docsa and for Ádám Ungvári for his technical help with the mouse experiment. We also thank for Andrea Docsa and Ágota Kelemenné Szántó for their technical assistance.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Vale, C.; Neves, J.S.; von Hafe, M.; Borges-Canha, M.; Leite-Moreira, A. The Role of Thyroid Hormones in Heart Failure. Cardiovasc. Drugs Ther. 2019, 33, 179–188. [Google Scholar] [CrossRef]
- Goncalves, A.; Resende, E.S.; Fernandes, M.L.; da Costa, A.M. Effect of thyroid hormones on cardiovascular and muscle systems and on exercise tolerance: A brief review. Arq. Bras. Cardiol. 2006, 87, e45–e47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of thyroid hormone in skeletal muscle physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef]
- Friesema, E.C.; Jansen, J.; Visser, T.J. Thyroid hormone transporters. Biochem. Soc. Trans. 2005, 33, 228–232. [Google Scholar] [CrossRef]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Samanci, N.; Balci, N.; Balci, M.K. Musculoskeletal manifestations in patients with thyroid disease. Clin. Endocrinol. 2003, 59, 162–167. [Google Scholar] [CrossRef]
- Olson, B.R.; Klein, I.; Benner, R.; Burdett, R.; Trzepacz, P.; Levey, G.S. Hyperthyroid myopathy and the response to treatment. Thyroid 1991, 1, 137–141. [Google Scholar] [CrossRef]
- Short, K.R.; Nygren, J.; Barazzoni, R.; Levine, J.; Nair, K.S. T3 increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and -3. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E761–E769. [Google Scholar] [CrossRef]
- Chacko, S.; Conti, M.A.; Adelstein, R.S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc. Natl. Acad. Sci. USA 1977, 74, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, M.; Hartshorne, D.J. The role of myosin phosphorylation in the contraction-relaxation cycle of smooth muscle. Experientia 1985, 41, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Erdodi, F.; Lontay, B. Myosin phosphatase: Unexpected functions of a long-known enzyme. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 2–15. [Google Scholar] [CrossRef]
- Shimizu, H.; Ito, M.; Miyahara, M.; Ichikawa, K.; Okubo, S.; Konishi, T.; Naka, M.; Tanaka, T.; Hirano, K.; Hartshorne, D.J.; et al. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J. Biol. Chem. 1994, 269, 30407–30411. [Google Scholar] [CrossRef]
- Fujioka, M.; Takahashi, N.; Odai, H.; Araki, S.; Ichikawa, K.; Feng, J.; Nakamura, M.; Kaibuchi, K.; Hartshorne, D.J.; Nakano, T.; et al. A new isoform of human myosin phosphatase targeting/regulatory subunit (MYPT2): cDNA cloning, tissue expression, and chromosomal mapping. Genomics 1998, 49, 59–68. [Google Scholar] [CrossRef]
- Skinner, J.A.; Saltiel, A.R. Cloning and identification of MYPT3: A prenylatable myosin targetting subunit of protein phosphatase 1. Biochem. J. 2001, 356 Pt 1, 257–267. [Google Scholar] [CrossRef]
- Tan, I.; Ng, C.H.; Lim, L.; Leung, T. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J. Biol. Chem. 2001, 276, 21209–21216. [Google Scholar] [CrossRef]
- Cao, W.S.; Mattagajasingh, S.N.; Xu, H.X.; Kim, K.H.; Fierlbeck, W.; Deng, J.; Lowenstein, C.J.; Ballermann, B.J. TIMAP, a novel CAAX box protein regulated by TGF-beta 1 and expressed in endothelial cells. Am. J. Physiol.-Cell Physiol. 2002, 283, C327–C337. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, F.; Hartshorne, D.J. Myosin phosphatase target subunit: Many roles in cell function. Biochem. Biophys. Res. Commun. 2008, 369, 149–156. [Google Scholar] [CrossRef]
- Arimura, T.; Suematsu, N.; Zhou, Y.B.; Nishimura, J.; Satoh, S.; Takeshita, A.; Kanaide, H.; Kimura, A. Identification, characterization, and functional analysis of heart-specific myosin light chain phosphatase small subunit. J. Biol. Chem. 2001, 276, 6073–6082. [Google Scholar] [CrossRef]
- Ichikawa, K.; Ito, M.; Hartshorne, D.J. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J. Biol. Chem. 1996, 271, 4733–4740. [Google Scholar] [CrossRef]
- Muranyi, A.; Derkach, D.; Erdodi, F.; Kiss, A.; Ito, M.; Hartshorne, D.J. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: Inhibitory effects and occurrence in A7r5 cells. FEBS Lett. 2005, 579, 6611–6615. [Google Scholar] [CrossRef]
- Velasco, G.; Armstrong, C.; Morrice, N.; Frame, S.; Cohen, P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002, 527, 101–104. [Google Scholar] [CrossRef]
- Borman, M.A.; MacDonald, J.A.; Haystead, T.A. Modulation of smooth muscle contractility by CHASM, a novel member of the smoothelin family of proteins. FEBS Lett. 2004, 573, 207–213. [Google Scholar] [CrossRef]
- Wooldridge, A.A.; Fortner, C.N.; Lontay, B.; Akimoto, T.; Neppl, R.L.; Facemire, C.; Datto, M.B.; Kwon, A.; McCook, E.; Li, P.; et al. Deletion of the protein kinase A/protein kinase G target SMTNL1 promotes an exercise-adapted phenotype in vascular smooth muscle. J. Biol. Chem. 2008, 283, 11850–11859. [Google Scholar] [CrossRef] [PubMed]
- Hartshorne, D.J.; Ito, M.; Erdodi, F. Role of protein phosphatase type 1 in contractile functions: Myosin phosphatase. J. Biol. Chem. 2004, 279, 37211–37214. [Google Scholar] [CrossRef] [PubMed]
- Lontay, B.; Bodoor, K.; Weitzel, D.H.; Loiselle, D.; Fortner, C.; Lengyel, S.; Zheng, D.; Devente, J.; Hickner, R.; Haystead, T.A. Smoothelin-like 1 protein regulates myosin phosphatase-targeting subunit 1 expression during sexual development and pregnancy. J. Biol. Chem. 2010, 285, 29357–29366. [Google Scholar] [CrossRef] [PubMed]
- Major, E.; Győry, F.; Horváth, D.; Keller, I.; Tamás, I.; Uray, K.; Fülöp, P.; Lontay, B. Smoothelin-like protein 1 regulates development and metabolic transformation of skeletal muscle in hyperthyroidism. Front. Endocrinol 2021, in press. [Google Scholar]
- Lontay, B.; Bodoor, K.; Sipos, A.; Weitzel, D.H.; Loiselle, D.; Safi, R.; Zheng, D.; Devente, J.; Hickner, R.C.; McDonnell, D.P.; et al. Pregnancy and Smoothelin-like Protein 1 (SMTNL1) Deletion Promote the Switching of Skeletal Muscle to a Glycolytic Phenotype in Human and Mice. J. Biol. Chem. 2015, 290, 17985–17998. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Choudhary, S.K.; Milner, D.J.; Munir, M.I.; Kuisk, I.R.; Capetanaki, Y. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J. Cell Biol. 1994, 124, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Chen, H.; Guo, C.; Yuan, Z.; Zhou, X.; Zhang, Y.; Zhang, X.; Mo, D.; Chen, Y. Palmdelphin promotes myoblast differentiation and muscle regeneration. Sci. Rep. 2017, 7, 41608. [Google Scholar] [CrossRef]
- Dentice, M.; Marsili, A.; Ambrosio, R.; Guardiola, O.; Sibilio, A.; Paik, J.H.; Minchiotti, G.; DePinho, R.A.; Fenzi, G.; Larsen, P.R.; et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J. Clin. Investig. 2010, 120, 4021–4030. [Google Scholar] [CrossRef]
- Milanesi, A.; Lee, J.W.; Kim, N.H.; Liu, Y.Y.; Yang, A.; Sedrakyan, S.; Kahng, A.; Cervantes, V.; Tripuraneni, N.; Cheng, S.Y.; et al. Thyroid Hormone Receptor alpha Plays an Essential Role in Male Skeletal Muscle Myoblast Proliferation, Differentiation, and Response to Injury. Endocrinology 2016, 157, 4–15. [Google Scholar] [CrossRef]
- Wu, Y.; Erdodi, F.; Muranyi, A.; Nullmeyer, K.D.; Lynch, R.M.; Hartshorne, D.J. Myosin phosphatase and myosin phosphorylation in differentiating C2C12 cells. J. Muscle Res. Cell Motil. 2003, 24, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Lontay, B.; Kiss, A.; Gergely, P.; Hartshorne, D.J.; Erdodi, F. Okadaic acid induces phosphorylation and translocation of myosin phosphatase target subunit 1 influencing myosin phosphorylation, stress fiber assembly and cell migration in HepG2 cells. Cell Signal. 2005, 17, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Lehka, L.; Redowicz, M.J. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef]
- Lontay, B.; Pal, B.; Serfozo, Z.; Koszeghy, A.; Szucs, G.; Rusznak, Z.; Erdodi, F. Protein phosphatase-1M and Rho-kinase affect exocytosis from cortical synaptosomes and influence neurotransmission at a glutamatergic giant synapse of the rat auditory system. J. Neurochem. 2012, 123, 84–99. [Google Scholar] [CrossRef]
- Pirkmajer, S.; Chibalin, A.V. Na,K-ATPase regulation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E1–E31. [Google Scholar] [CrossRef]
- Riis, A.L.; Jorgensen, J.O.; Moller, N.; Weeke, J.; Clausen, T. Hyperthyroidism and cation pumps in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E1265–E1269. [Google Scholar] [CrossRef]
- Soltoff, S.P.; Asara, J.M.; Hedden, L. Regulation and identification of Na,K-ATPase alpha1 subunit phosphorylation in rat parotid acinar cells. J. Biol. Chem. 2010, 285, 36330–36338. [Google Scholar] [CrossRef]
- Ambrosio, R.; De Stefano, M.A.; Di Girolamo, D.; Salvatore, D. Thyroid hormone signaling and deiodinase actions in muscle stem/progenitor cells. Mol. Cell Endocrinol. 2017, 459, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Dentice, M.; Ambrosio, R.; Damiano, V.; Sibilio, A.; Luongo, C.; Guardiola, O.; Yennek, S.; Zordan, P.; Minchiotti, G.; Colao, A.; et al. Intracellular inactivation of thyroid hormone is a survival mechanism for muscle stem cell proliferation and lineage progression. Cell Metab. 2014, 20, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Di Cosmo, C.; Liao, X.H.; Ye, H.; Ferrara, A.M.; Weiss, R.E.; Refetoff, S.; Dumitrescu, A.M. Mct8-deficient mice have increased energy expenditure and reduced fat mass that is abrogated by normalization of serum T3 levels. Endocrinology 2013, 154, 4885–4895. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, A.; Lee, J.W.; Yang, A.; Liu, Y.Y.; Sedrakyan, S.; Cheng, S.Y.; Perin, L.; Brent, G.A. Thyroid Hormone Receptor Alpha is Essential to Maintain the Satellite Cell Niche During Skeletal Muscle Injury and Sarcopenia of Aging. Thyroid 2017, 27, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Lontay, B.; Serfozo, Z.; Gergely, P.; Ito, M.; Hartshorne, D.J.; Erdodi, F. Localization of myosin phosphatase target subunit 1 in rat brain and in primary cultures of neuronal cells. J. Comp. Neurol. 2004, 478, 72–87. [Google Scholar] [CrossRef]
- Brown, D.M.; Parr, T.; Brameld, J.M. Myosin heavy chain mRNA isoforms are expressed in two distinct cohorts during C2C12 myogenesis. J. Muscle Res. Cell Motil. 2012, 32, 383–390. [Google Scholar] [CrossRef]
- Izumo, S.; Nadal-Ginard, B.; Mahdavi, V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science 1986, 231, 597–600. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Horwitz, A.R. Myosin light chain mono- and di-phosphorylation differentially regulate adhesion and polarity in migrating cells. Biochem. Biophys. Res. Commun. 2010, 402, 537–542. [Google Scholar] [CrossRef]
- Tullio, A.N.; Accili, D.; Ferrans, V.J.; Yu, Z.X.; Takeda, K.; Grinberg, A.; Westphal, H.; Preston, Y.A.; Adelstein, R.S. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc. Natl. Acad. Sci. USA 1997, 94, 12407–12412. [Google Scholar] [CrossRef]
- Zvibel, I.; Atias, D.; Phillips, A.; Halpern, Z.; Oren, R. Thyroid hormones induce activation of rat hepatic stellate cells through increased expression of p75 neurotrophin receptor and direct activation of Rho. Lab. Investig. 2010, 90, 674–684. [Google Scholar] [CrossRef]
- Kondo, A.; Tokuda, H.; Kato, K.; Matsushima-Nishiwaki, R.; Kuroyanagi, G.; Mizutani, J.; Kozawa, O.; Otsuka, T. Rho-kinase negatively regulates thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Biochimie 2013, 95, 719–724. [Google Scholar] [CrossRef]
- Pajouhi, N.; Owji, M.; Naghibalhossaini, F.; Omrani, G.H.; Varedi, M. Modulation by thyroid hormone of myosin light chain phosphorylation and aquaporin 5 protein expression in intact lung. J. Physiol. Biochem. 2015, 71, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.; Sipos, A.; Major, E.; Konya, Z.; Batori, R.; Dedinszki, D.; Szoll Si, A.; Tamas, I.; Ivan, J.; Kiss, A.; et al. Myosin phosphatase accelerates cutaneous wound healing by regulating migration and differentiation of epidermal keratinocytes via Akt signaling pathway in human and murine skin. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3268–3280. [Google Scholar] [CrossRef]
- Stull, J.T.; Kamm, K.E.; Taylor, D.A. Calcium control of smooth muscle contractility. Am. J. Med. Sci. 1988, 296, 241–245. [Google Scholar] [CrossRef]
- Ragolia, L.; Cherpalis, B.; Srinivasan, M.; Begum, N. Role of serine/threonine protein phosphatases in insulin regulation of Na+/K+-ATPase activity in cultured rat skeletal muscle cells. J. Biol. Chem. 1997, 272, 23653–23658. [Google Scholar] [CrossRef][Green Version]
- Lei, J.; Mariash, C.N.; Ingbar, D.H. 3,3’,5-Triiodo-L-thyronine up-regulation of Na,K-ATPase activity and cell surface expression in alveolar epithelial cells is Src kinase- and phosphoinositide 3-kinase-dependent. J. Biol. Chem. 2004, 279, 47589–47600. [Google Scholar] [CrossRef]
- Kjeldsen, K.; Norgaard, A.; Gotzsche, C.O.; Thomassen, A.; Clausen, T. Effect of thyroid function on number of Na-K pumps in human skeletal muscle. Lancet 1984, 2, 8–10. [Google Scholar] [CrossRef]
- Logvinenko, N.S.; Dulubova, I.; Fedosova, N.; Larsson, S.H.; Nairn, A.C.; Esmann, M.; Greengard, P.; Aperia, A. Phosphorylation by protein kinase C of serine-23 of the alpha-1 subunit of rat Na+,K(+)-ATPase affects its conformational equilibrium. Proc. Natl. Acad. Sci. USA 1996, 93, 9132–9137. [Google Scholar] [CrossRef] [PubMed]
- Feraille, E.; Beguin, P.; Carranza, M.L.; Gonin, S.; Rousselot, M.; Martin, P.Y.; Favre, H.; Geering, K. Is phosphorylation of the alpha1 subunit at Ser-16 involved in the control of Na,K-ATPase activity by phorbol ester-activated protein kinase C? Mol. Biol. Cell 2000, 11, 39–50. [Google Scholar] [CrossRef]
- Vasilets, L.A.; Postina, R.; Kirichenko, S.N. Mutations of Ser-23 of the alpha1 subunit of the rat Na+/K+-ATPase to negatively charged amino acid residues mimic the functional effect of PKC-mediated phosphorylation. FEBS Lett. 1999, 455, 8–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).