Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets
Abstract
1. Introduction
2. Current Classification of Antimicrobial Molecules in Platelets
3. Initial Findings of Antimicrobial Molecules in Platelets
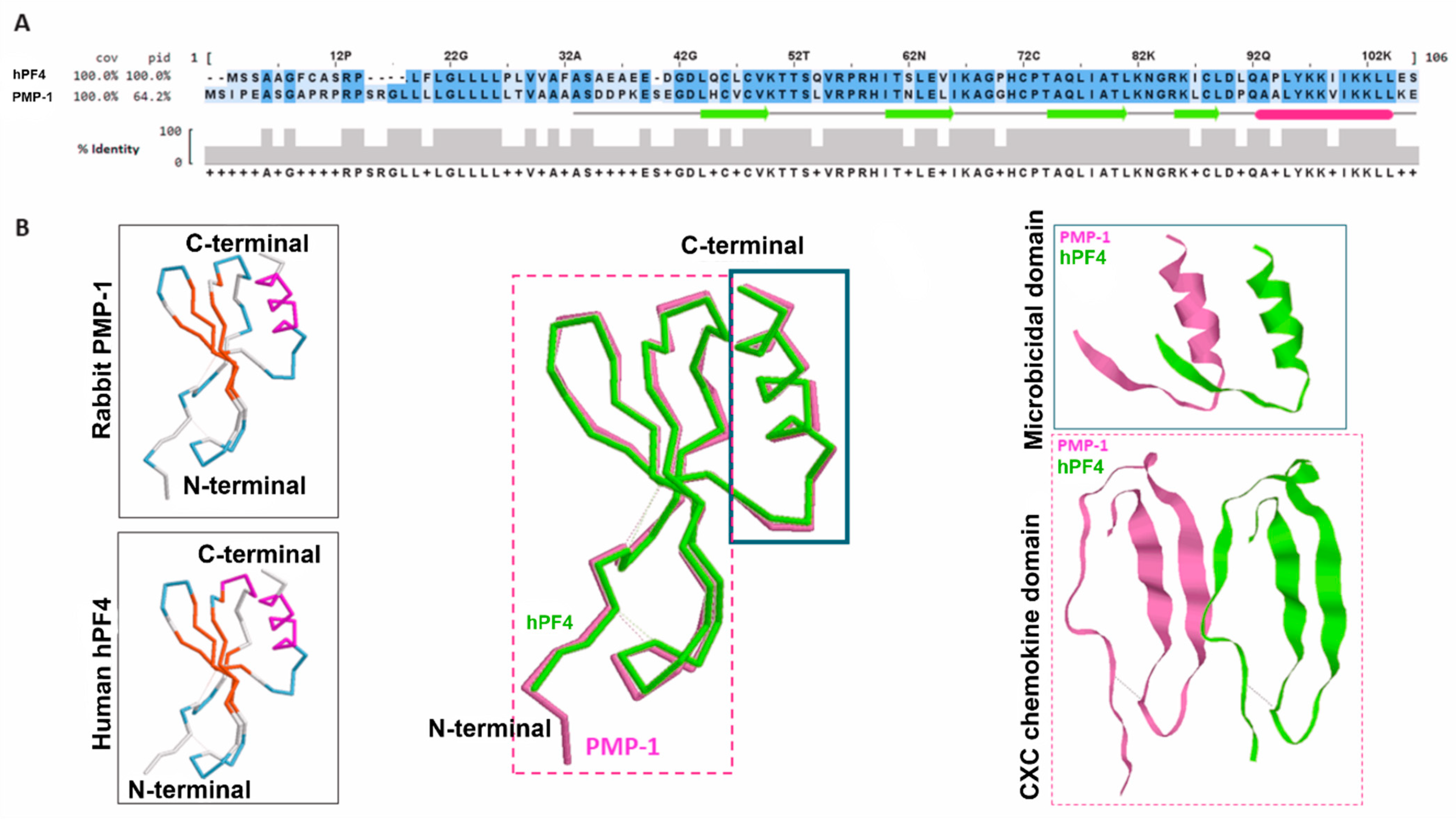
4. Platelet Microbicidal Proteins of Rabbit: PMPs and tPMPs
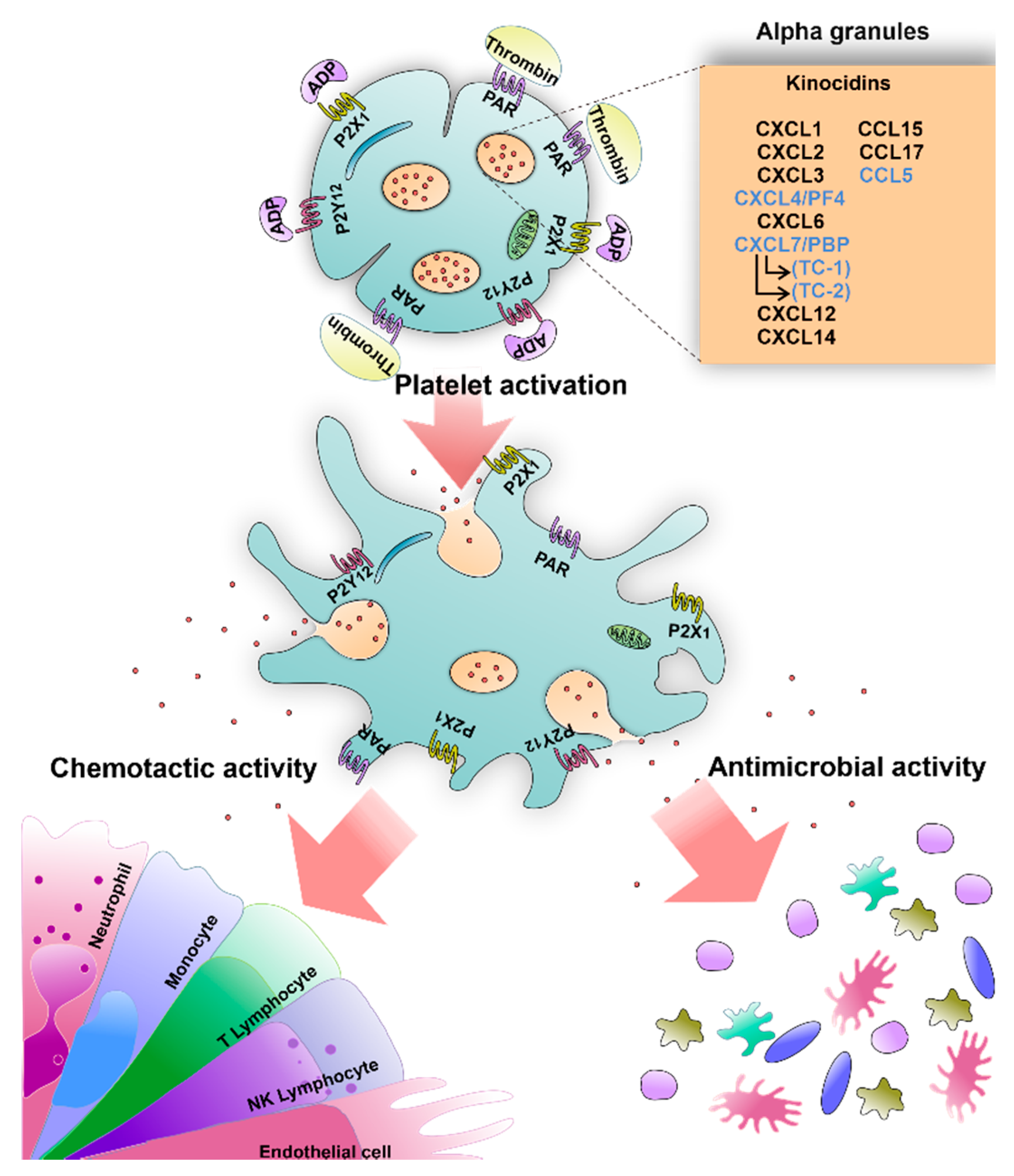
5. Microbicidal Molecules in Human Platelets
5.1. Kinocidins
| Chemokine | Platelet Findings | Target Microorganism |
|---|---|---|
| CXCL1 | Presence of mRNA [66,67] | E. coli, S. aureus, S. typhimurium and C. albicans [35,65] |
| CXCL2 | Proteomic analysis [68] and RNA sequencing [69] | E. coli and S. aureus [65] |
| CXCL3 | Proteomic analysis [68] and RNA sequencing [69] | E. coli and S. aureus [65] |
| CXCL4 | Isolation and characterization [70] and release by stimulation [71] | E. coli, S. aureus, S. typhimurium and C. albicans [35,62] |
| CXCL6 | Proteomic analysis [68] | N. gonorrhoeae, E. faecalis, P. aeruginosa, S. pyogenes, S. dysgalactiae subsp, S. aureus, E. coli, and B. subtilis [72,73] |
| CXCL7 | Purification of the secretion product and sequence analysis [33] | E. coli, S. aureus and C. neoformans [33] |
| CXCL7 (fragment TC-1) | Purification from granule-α and sequence analysis [32] | E. coli, B. subtilis, C. neoformans and S. aureus [32,74] |
| CXCL7 (fragment TC-2) | Purification from granule-α and sequence analysis [32] | E. coli, S. aureus and B. subtilis [32] |
| CXCL12 | Protein expression and release by stimulation [75,76] | E. coli and S. aureus [65] |
| CXCL14 | Surface expression and release by stimulation [77] | E. coli, S. aureus, E.coli and C. albicans [65,78] |
| CCL5 | Expression of mRNA [66] and release by stimulation [79] | E. coli, S. aureus and S. typhimurium, [33,35] |
| CCL15 | Release by stimulation [80] | E. coli, S. aureus [33] |
| CCL17 | Release by stimulation (in vitro) and during coagulation [80,81] | E. coli, S. aureus [33] |
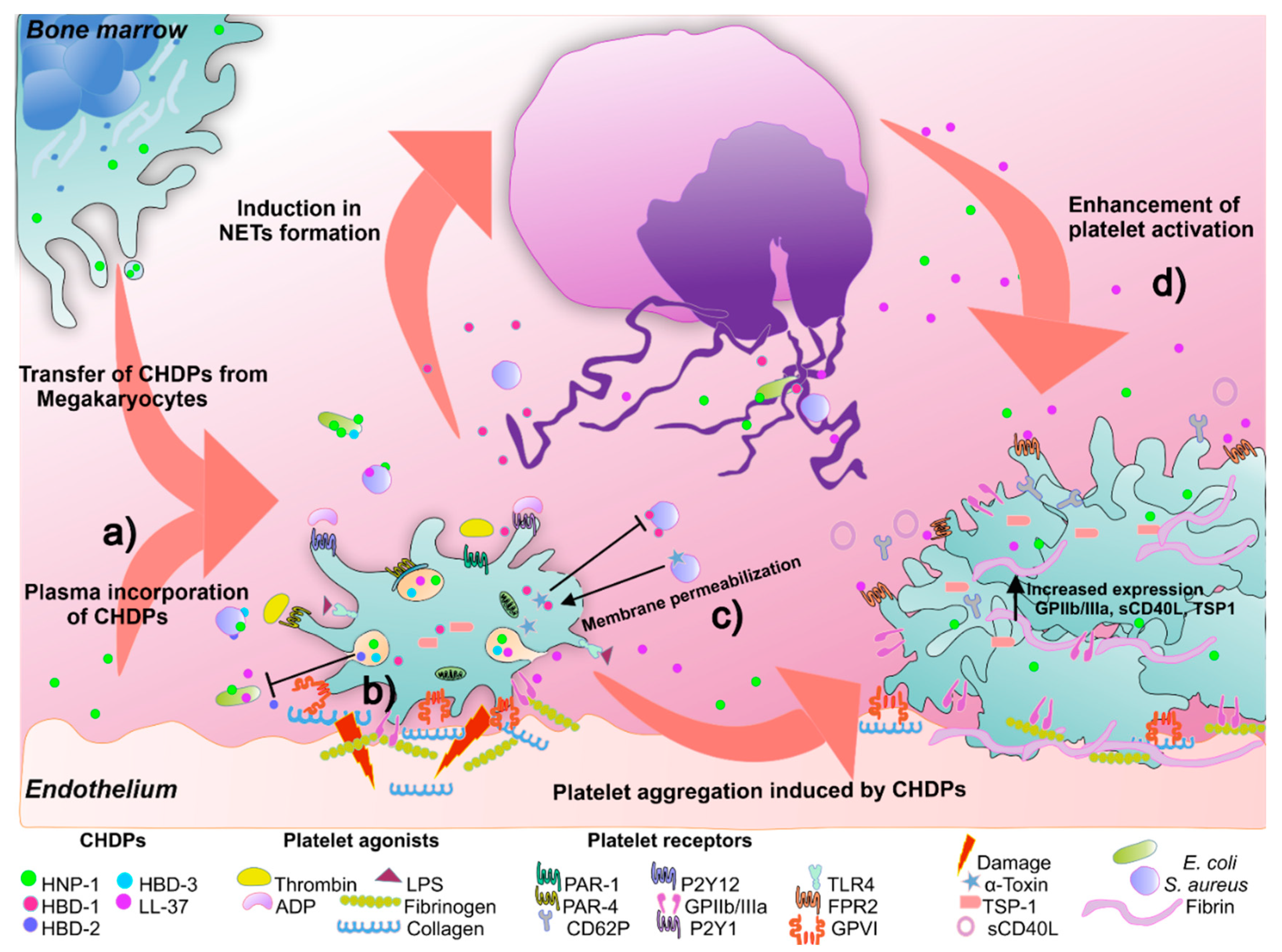
5.2. Cationic Host Defense Peptides (CHDPs) in Platelets
5.3. RNAse 7
6. Platelet Antibacterial Molecules Participation against Other Infectious Agents
6.1. Antiviral Activity
6.2. Antiparasitic Activity
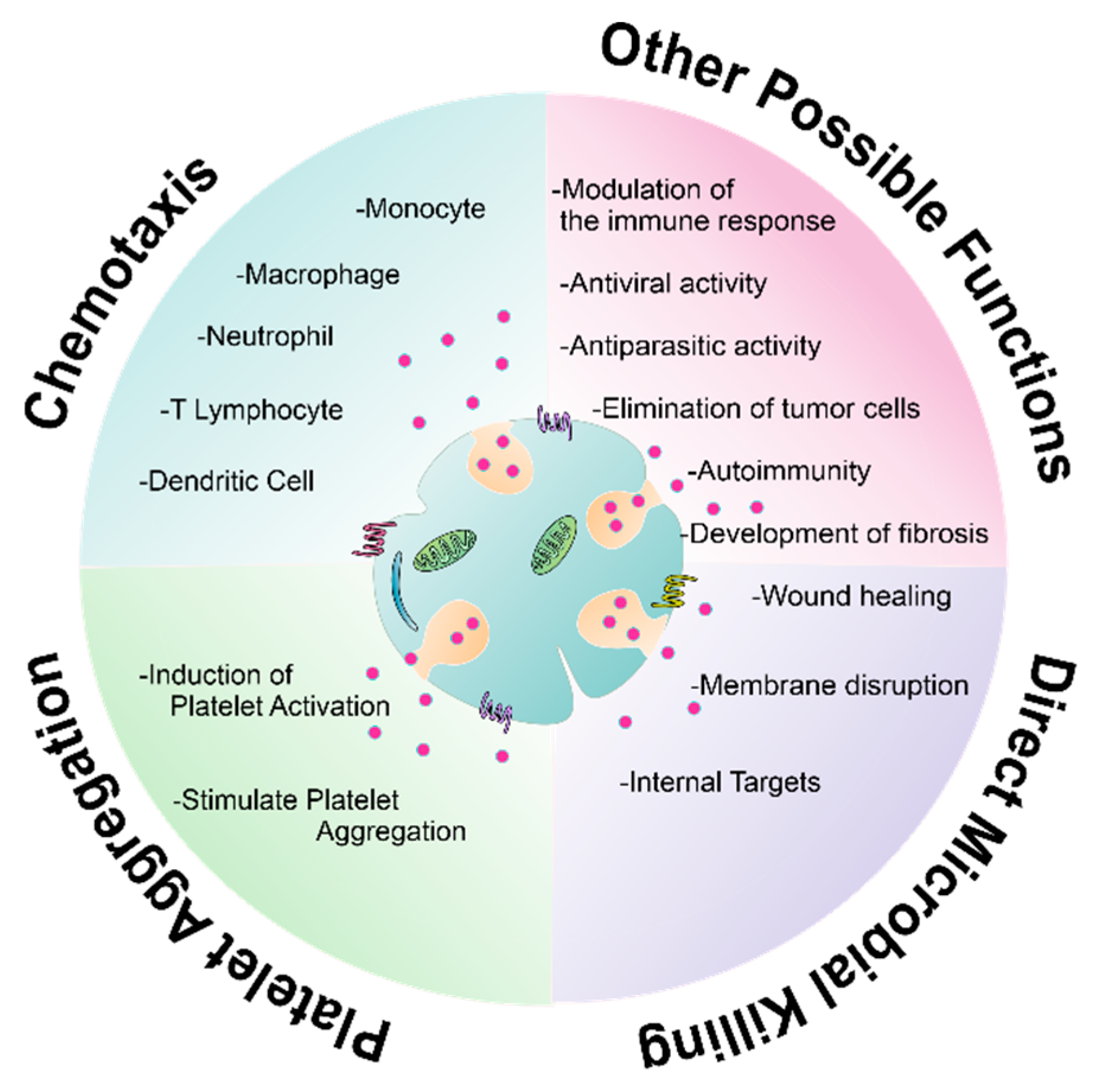
7. New Non-Microbicidal Pathways for Platelet Antimicrobial Molecules
7.1. Immunoregulation
7.2. Anticancer Activity
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets, and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger, A.L., III; Michelson, A.D. Platelet physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging roles for platelets as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef]
- Ciferri, S.; Emiliani, C.; Guglielmini, G.; Orlacchio, A.; Nenci, G.G.; Gresele, P. Platelets release their lysosomal content in vivo in humans upon activation. Thromb. Haemost. 2000, 83, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, A.S.; Schwertz, H.; Kraiss, L.W.; Zimmerman, G.A. Protein synthesis by platelets: Historical and new perspectives. J. Thromb. Haemost. 2009, 7, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jurk, K.; Kehrel, B.E. Platelets: Physiology and biochemistry. Semin. Thromb. Hemost. 2005, 31, 381–392. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Hally, K.; Fauteux-Daniel, S.; Hamzeh-Cognasse, H.; Larsen, P.; Cognasse, F. Revisiting platelets and toll-like receptors (TLRs): At the interface of vascular immunity and thrombosis. Int. J. Mol. Sci. 2020, 21, 6150. [Google Scholar] [CrossRef]
- Nording, H.; Langer, H.F. Complement links platelets to innate immunity. Semin. Immunol. 2018, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Al-Tamimi, M.; Baker, R.; Andrews, R.K.; Gardiner, E.E. The platelet Fc receptor, FcγRIIa. Immunol. Rev. 2015, 268, 241–252. [Google Scholar] [CrossRef]
- Brown, G.T.; Narayanan, P.; Li, W.; Silverstein, R.L.; McIntyre, T.M. Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop. J. Immunol. 2013, 191, 5196–5203. [Google Scholar] [CrossRef]
- Beaulieu, L.M.; Lin, E.; Mick, E.; Koupenova, M.; Weinberg, E.O.; Kramer, C.D.; Genco, C.A.; Tanriverdi, K.; Larson, M.G.; Benjamin, E.; et al. Interleukin 1 receptor 1 and interleukin 1β regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arter. Thromb. Vasc. Biol. 2014, 34, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Limb, G.A.; Webster, L.; Soomro, H.; Janikoun, S.; Shilling, J. Platelet expression of tumour necrosis factor-alpha (TNF-α), TNF receptors and intercellular adhesion molecule-1 (ICAM-1) in patients with proliferative diabetic retinopathy. Clin. Exp. Immunol. 1999, 118, 213–218. [Google Scholar] [CrossRef]
- Pignatelli, P.; de Biase, L.; Lenti, L.; Tocci, G.; Brunelli, A.; Cangemi, R.; Riondino, S.; Grego, S.; Volpe, M.; Violi, F. Tumor necrosis factor-α as trigger of platelet activation in patients with heart failure. Blood 2005, 106, 1992–1994. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Schmitt, M.M.N. Platelets and their chemokines in atherosclerosis—Clinical applications. Front. Physiol. 2014, 5, 294. [Google Scholar] [CrossRef] [PubMed]
- Dib, P.R.B.; Quirino-Teixeira, A.C.; Merij, L.B.; Pinheiro, M.B.M.; Rozini, S.V.; Andrade, F.B.; Hottz, E.D. Innate immune receptors in platelets and platelet-leukocyte interactions. J. Leukoc. Biol. 2020, 108, 1157–1182. [Google Scholar] [CrossRef]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.; Lentz, S.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-mediated modulation of adaptive immunity: A communication link between innate and adaptive immune compartments. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M.; Morrell, C.N. Platelets present antigen in the context of MHC class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef]
- Colberg, L.; Cammann, C.; Greinacher, A.; Seifert, U. Structure and function of the ubiquitin-proteasome system in platelets. J. Thromb. Haemost. 2020, 18, 771–780. [Google Scholar] [CrossRef]
- Klockenbusch, C.; Walsh, G.M.; Brown, L.M.; Hoffman, M.D.; Ignatchenko, V.; Kislinger, T.; Kast, J. Global proteome analysis identifies active immunoproteasome subunits in human platelets. Mol. Cell. Proteom. 2014, 13, 3308–3319. [Google Scholar] [CrossRef]
- Yeaman, M.R. Platelets in defense against bacterial pathogens. Cell. Mol. Life Sci. 2009, 67, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Karshovska, E.; Weber, C.; von Hundelshausen, P. Platelet chemokines in health and disease. Thromb. Haemost. 2013, 110, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.N.; Porto, W.F.; Ribeiro, S.; Batista, I.; Franco, O.L. Host-defense peptides and their potential use as biomarkers in human diseases. Drug Discov. Today 2018, 23, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.F.; Martinez, R.J. Antibacterial peptide from normal rabbit serum. 2. Compositional microanalysis. Biochemistry 1981, 20, 5981–5987. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Norman, D.C.; Bayer, A.S. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect. Immun. 1992, 60, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.P.; Yeaman, M.R.; Bayer, A.S. Staphylocidal action of thrombin-induced platelet microbicidal protein is influenced by microenvironment and target cell growth phase. Infect. Immun. 1996, 64, 3758–3764. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Norman, D.C.; Bayer, A.S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob. Agents Chemother. 1992, 36, 1665–1670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krijgsveld, J.; Zaat, S.A.; Meeldijk, J.; van Veelen, P.A.; Fang, G.; Poolman, B.; Brandt, E.; Ehlert, J.E.; Kuijpers, A.J.; Engbers, G.H.; et al. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 2000, 275, 20374–20381. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial peptides from human platelets. Infect. Immun. 2002, 70, 6524–6533. [Google Scholar] [CrossRef]
- Yount, N.Y.; Gank, K.D.; Xiong, Y.Q.; Bayer, A.S.; Pender, T.; Welch, W.H.; Yeaman, M.R. Platelet microbicidal protein 1: Structural themes of a multifunctional antimicrobial peptide. Antimicrob. Agents Chemother. 2004, 48, 4395–4404. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.; Waring, A.J.; Gank, K.D.; Welch, W.H.; Kupferwasser, D.; Yeaman, M.R. Structural correlates of antimicrobial efficacy in IL-8 and related human kinocidins. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Torres-Juarez, F.; Trejo-Martínez, L.A.; Layseca-Espinosa, E.; Leon-Contreras, J.C.; Enciso-Moreno, J.A.; Hernandez-Pando, R.; Rivas-Santiago, B. Platelets immune response against Mycobacterium tuberculosis infection. Microb. Pathog. 2021, 153, 104768. [Google Scholar] [CrossRef]
- Skarnes, R.C.; Watson, D.W. Antimicrobial factors of normal tissues and fluids. Bacteriol. Rev. 1957, 21, 273–294. [Google Scholar] [CrossRef]
- Donaldson, D.M.; Marcus, S. Studies on serum bactericidal activity; interrelationships of heparin, citrate, protamine and xirra-diation on serum and plasma bactericidal activity against Bacillus subtilis. J. Immunol. 1958, 81, 292–296. [Google Scholar]
- Hunder, G.G.; Jacox, R.F. Experimentally induced alterations of the bactericidal property of rabbit serum for Bacillus subtilis: I. Effects of total body X-ray irradiation, intravenous administration of immune platelet serum, thorotrast and intraperitoneal injection of endotoxin. J. Immunol. 1963, 90, 540–547. [Google Scholar]
- Hirsch, J.G. Comparative bactericidal activities of blood serum and plasma serum. J. Exp. Med. 1960, 112, 15–22. [Google Scholar] [CrossRef]
- Myrvik, Q.N.; Leake, E.S. Studies on antibacterial factors in mammalian tissues and fluids: IV. Demonstration of two nondialyzable components in the serum bactericidin system for Bacillus subtilis. J. Immunol. 1960, 84, 247. [Google Scholar]
- Jago, R.; Jacox, R.F. Cellular source and character of a heat-stable bactericidal property associated with rabbit and rat platelets. J. Exp. Med. 1961, 113, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.F.; Martinez, R.J. Antibacterial peptide from normal rabbit serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry 1981, 20, 5973–5981. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.F.; Martinez, R.J. Antibacterial peptide from normal rabbit serum. 3. Inhibition of microbial electron transport. Biochemistry 1981, 20, 5988–5994. [Google Scholar] [CrossRef]
- Weksler, B.B.; Nachman, R.L. Rabbit platelet bactericidal protein. J. Exp. Med. 1971, 134, 1114–1130. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Ibrahim, A.S.; Edwards, J.E.; Bayer, A.S.; Ghannoum, M.A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob. Agents Chemother. 1993, 37, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Soldan, S.S.; Ghannoum, M.A.; Edwards, J.E., Jr.; Filler, S.G.; Bayer, A.S. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect. Immun. 1996, 64, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Sullam, P.M.; Dazin, P.F.; Bayer, A.S. Platelet microbicidal protein alone and in combination with antibiotics reduces Staphylococcus aureus adherence to platelets in vitro. Infect. Immun. 1994, 62, 3416–3423. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Sullam, P.M.; Dazin, P.F.; Ghannoum, M.A.; Edwards, J.E., Jr.; Bayer, A.S. Fluconazole and platelet microbicidal protein inhibit Candida adherence to platelets in vitro. Antimicrob. Agents Chemother. 1994, 38, 1460–1465. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Puentes, S.M.; Norman, D.C.; Bayer, A.S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect. Immun. 1992, 60, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Tang, Y.Q.; Shen, A.J.; Bayer, A.S.; Selsted, M.E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 1997, 65, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.-P.; Bayer, A.S.; Kagan, B.L.; Yeaman, M.R. Membrane permeabilization by thrombin-induced platelet microbicidal protein 1 is modulated by transmembrane voltage polarity and magnitude. Infect. Immun. 1999, 67, 2475–2481. [Google Scholar] [CrossRef]
- Koo, S.-P.; Yeaman, M.R.; Nast, C.C.; Bayer, A.S. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect Immun. 1997, 65, 4795–4800. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Bayer, A.S.; Koo, S.P.; Foss, W.; Sullam, P.M. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Investig. 1998, 101, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-Q.; Yeaman, M.R.; Bayer, A.S. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob. Agents Chemother. 1999, 43, 1111–1117. [Google Scholar] [CrossRef]
- Kupferwasser, L.I.; Skurray, R.A.; Brown, M.; Firth, N.; Yeaman, M.R.; Bayer, A.S. Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in staphylococci: Role of the qacA locus. Antimicrob. Agents Chemother. 1999, 43, 2395–2399. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Whitmire, W.; Xiong, Y.Q.; Molden, J.; Jones, T.; Peschel, A.; Staubitz, P.; Adler-Moore, J.; McNamara, P.J.; Proctor, R.A.; et al. In vitro susceptibility of Staphylococcus aureus to thrombin-induced platelet microbicidal protein-1 (tPMP-1) is influenced by cell membrane phospholipid composition and asymmetry. Microbiology 2007, 153, 1187–1197. [Google Scholar] [CrossRef]
- Dankert, J. Role of Platelets in Early Pathogenesis of Viridans Group Streptococcal Endocarditis: A Study on Thrombodefensins: A Study in Which Penicillin Tolerance is Shown to be Associated with Increased Virulence. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 1988. [Google Scholar]
- Zaat, S.A.J.; Koper, M.; Grasselier, H.; Meeldijk, J.; Krijgsveld, J.; Dankert, J. Cell-adherent glucan does not protect endocarditis-causing viridans streptococci against bactericidal proteins from human blood platelets. Cancer Biol. Nucl. Envel. 1997, 418, 709–712. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Bancroft, D.P.; Lai, C.K.; Maione, T.E. Crystal structure of recombinant human platelet factor 4. Biochemistry 1994, 33, 8361–8366. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.; Waring, A.J.; Gank, K.D.; Kupferwasser, D.; Wiese, R.; Bayer, A.S.; Welch, W.H. Modular determinants of antimicrobial activity in platelet factor-4 family kinocidins. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 609–619. [Google Scholar] [CrossRef]
- Trier, D.A.; Gank, K.D.; Kupferwasser, D.; Yount, N.Y.; French, W.J.; Michelson, A.D.; Kupferwasser, L.I.; Xiong, Y.Q.; Bayer, A.S.; Yeaman, M.R. Platelet antistaphylococcal responses occur through P2X 1 and P2Y 12 receptor-induced activation and kinocidin release. Infect. Immun. 2008, 76, 5706–5713. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Ganz, T.; Liese, A.M.; Burdick, M.D.; Liu, L.; Strieter, R.M. Cutting edge: IFN-inducible ELR−CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 2001, 167, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Hoover, D.M.; Staley, P.; Tucker, K.D.; Lubkowski, J.; Oppenheim, J.J. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J. Leukoc. Biol. 2003, 74, 448–455. [Google Scholar] [CrossRef]
- Power, C.A.; Clemetson, J.M.; Clemetson, K.J.; Wells, T.N. Chemokine and chemokine receptor mRNA expression in human platelets. Cytokine 1995, 7, 479–482. [Google Scholar] [CrossRef]
- Collinson, R.J.; Mazza-Parton, A.; Fuller, K.A.; Linden, M.D.; Erber, W.N.; Guo, B.B. Gene expression of CXCL1 (GRO-α) and EGF by platelets in myeloproliferative neoplasms. HemaSphere 2020, 4, e490. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.W.; Oler, A.J.; Tolley, N.D.; Hunter, B.N.; Low, E.N.; Nix, D.A.; Yost, C.C.; Zimmerman, G.A.; Weyrich, A. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 2011, 118, e101–e111. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.P.; Wohl, H. Human platelet factor 4: Purification and characterization by affinity chromatography. Purification of human platelet factor 4. J. Biol. Chem. 1976, 251, 324–328. [Google Scholar] [CrossRef]
- Youssef, A.; Barkhan, P. Release of platelet factor 4 by adenosine diphosphate and other platelet-aggregating agents. BMJ 1968, 1, 746–747. [Google Scholar] [CrossRef][Green Version]
- Collin, M.; Linge, H.; Bjartell, A.; Giwercman, A.; Malm, J.; Egesten, A. Constitutive expression of the antibacterial CXC chemokine GCP-2/CXCL6 by epithelial cells of the male reproductive tract. J. Reprod. Immunol. 2008, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Jovic, S.; Linge, H.; Shikhagaie, M.M.; Olin, A.I.; Lannefors, L.; Erjefält, J.S.; Mörgelin, M.; Egesten, A. The neutrophil-recruiting chemokine GCP-2/CXCL6 is expressed in cystic fibrosis airways and retains its functional properties after binding to extracellular DNA. Mucosal Immunol. 2015, 9, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; Krijgsveld, J.; de Boer, L.; Nguyen, L.T.; Boszhard, L.; Vreede, J.; Dekker, H.L.; Speijer, D.; Drijfhout, J.W.; te Velde, A.A.; et al. Native thrombocidin-1 and unfolded thrombocidin-1 exert antimicrobial activity via distinct structural elements. J. Biol. Chem. 2011, 286, 43506–43514. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Konrad, I.; Schürzinger, K.; Lorenz, M.; Schneider, S.; Zohlnhoefer, D.; Hoppe, K.; Schiemann, M.; Kennerknecht, E.; Sauer, S.; et al. Platelets secrete stromal cell–derived factor 1α and recruit bone marrow–derived progenitor cells to arterial thrombi in vivo. J. Exp. Med. 2006, 203, 1221–1233. [Google Scholar] [CrossRef]
- Stellos, K.; Langer, H.; Daub, K.; Schoenberger, T.; Gauss, A.; Geisler, T.; Bigalke, B.; Mueller, I.; Schumm, M.; Schaefer, I.; et al. Platelet-derived stromal cell–derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 2008, 117, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.; Chatterjee, M.; Lang, F.; Gawaz, M. Platelets as a novel source of pro-inflammatory chemokine CXCL14. Cell. Physiol. Biochem. 2017, 41, 1684–1696. [Google Scholar] [CrossRef]
- Maerki, C.; Meuter, S.; Liebi, M.; Mühlemann, K.; Frederick, M.J.; Yawalkar, N.; Moser, B.; Wolf, M. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J. Immunol. 2008, 182, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Kameyoshi, Y.; Dörschner, A.; Mallet, A.I.; Christophers, E.; Schröder, J.M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J. Exp. Med. 1992, 176, 587–592. [Google Scholar] [CrossRef]
- Jonnalagadda, D.; Izu, L.T.; Whiteheart, S. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood 2012, 120, 5209–5216. [Google Scholar] [CrossRef]
- Fujisawa, T.; Fujisawa, R.; Kato, Y.; Nakayama, T.; Morita, A.; Katsumata, H.; Nishimori, H.; Iguchi, K.; Kamiya, H.; Gray, P.W.; et al. Presence of high contents of thymus and activation-regulated chemokine in platelets and elevated plasma levels of thymus and activation-regulated chemokine and macrophage-derived chemokine in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2002, 110, 139–146. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Reiling, S.; Zarena, D.; Wang, G.S. Host defense antimicrobial peptides as antibiotics: Design and application strategies. Curr. Opin. Chem. Biol. 2017, 38, 87–96. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Nolan, E.M. Human α-defensin 6: A small peptide that self-assembles and protects the host by entangling microbes. Acc. Chem. Res. 2017, 50, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Pace, B.T.; Lackner, A.A.; Porter, E.; Pahar, B. The role of defensins in HIV pathogenesis. Mediat. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Varoga, D.; Wruck, C.J.; Podschun, R.; Sachweh, B.H.; Bornemann, J.; Bovi, M.; Sönmez, T.T.; Slowik, A.D.; Houben, A.; et al. Platelets display potent antimicrobial activity and release human beta-defensin 2. Platelets 2011, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Varoga, D.; Podschun, R.; Wruck, C.J.; Seekamp, A.; Brandenburg, L.-O.; Pufe, T.; Lippross, S. Thrombocytes are effectors of the innate immune system releasing human beta defensin-3. Injury 2011, 42, 682–686. [Google Scholar] [CrossRef]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.A.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Valle-Jiménez, X.; Ramírez-Cosmes, A.; Aquino-Domínguez, A.S.; Sánchez-Peña, F.; Bustos-Arriaga, J.; Romero-Tlalolini, M.D.; Torres-Aguilar, H.; Serafín-López, J.; Aguilar Ruíz, S.R. Human platelets and megakaryocytes express defensin alpha 1. Platelets 2019, 31, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, G.H.; Agerberth, B.; Odeberg, J.; Bergman, T.; Olsson, B.; Salcedo, R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 1996, 238, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Lopez-Garcia, B.; Braff, M.; Dorschner, R.A.; Gallo, R.L. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J. Immunol. 2004, 172, 3070–3077. [Google Scholar] [CrossRef]
- Bandurska, K.; Berdowska, A.; Barczynska, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- Salamah, M.F.; Ravishankar, D.; Kodji, X.; Moraes, L.A.; Williams, H.F.; Vallance, T.; Albadawi, D.A.; Vaiyapuri, R.; Watson, K.; Gibbins, J.; et al. The endogenous antimicrobial cathelicidin LL37 induces platelet activation and augments thrombus formation. Blood Adv. 2018, 2, 2973–2985. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Bertling, A.; Brodde, M.F.; Müller, A.; Roth, J.; van Aken, H.; Jurk, K.; Heilmann, C.; Peters, G.; Kehrel, B.E. Human neutrophil alpha-defensins induce formation of fibrinogen and thrombospondin-1 amyloid-like structures and activate platelets via glycoprotein IIb/IIIa. J. Thromb. Haemost. 2012, 10, 647–661. [Google Scholar] [CrossRef]
- Miyawaki, K.; Iwasaki, H.; Jiromaru, T.; Kusumoto, H.; Yurino, A.; Sugio, T.; Uehara, Y.; Odawara, J.; Daitoku, S.; Kunisaki, Y.; et al. Identification of unipotent megakaryocyte progenitors in human hematopoiesis. Blood 2017, 129, 3332–3343. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E. Human megakaryocytes: Finding the root. Blood 2017, 129, 3277–3279. [Google Scholar] [CrossRef]
- Pircher, J.; Czermak, T.; Ehrlich, A.; Eberle, C.; Gaitzsch, E.; Margraf, A.; Grommes, J.; Saha, P.; Titova, A.; Ishikawa-Ankerhold, H.; et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Rademacher, F.; Dreyer, S.; Kopfnagel, V.; Gläser, R.; Werfel, T.; Harder, J. The antimicrobial and immunomodulatory function of RNase 7 in skin. Front. Immunol. 2019, 10, 2553. [Google Scholar] [CrossRef]
- Oka, Y.; Orth, D.N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J. Clin. Investig. 1983, 72, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Aybay, C.; Karakus, R.; Yucel, A. Characterization of human epidermal growth factor in human serum and urine under native conditions. Cytokine 2006, 35, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Raadsen, M.; du Toit, J.; Langerak, T.; van Bussel, B.; van Gorp, E.; Goeijenbier, M. Thrombocytopenia in virus infections. J. Clin. Med. 2021, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Bozza, F.A.; Bozza, P. Platelets in immune response to virus and immunopathology of viral infections. Front. Med. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A. Platelets and infection—An emerging role of platelets in viral infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R. Platelets: At the nexus of antimicrobial defence. Nat. Rev. Microbiol. 2014, 12, 426–437. [Google Scholar] [CrossRef]
- Tsegaye, T.S.; Gnirß, K.; Rahe-Meyer, N.; Kiene, M.; Krämer-Kühl, A.; Behrens, G.; Münch, J.; Pöhlmann, S. Platelet activation suppresses HIV-1 infection of T cells. Retrovirology 2013, 10, 48. [Google Scholar] [CrossRef]
- Auerbach, D.J.; Lin, Y.; Miao, H.; Cimbro, R.; DiFiore, M.J.; Gianolini, M.E.; Furci, L.; Biswas, P.; Fauci, A.S.; Lusso, P. Identification of the platelet-derived chemokine CXCL4/PF-4 as a broad-spectrum HIV-1 inhibitor. Proc. Natl. Acad. Sci. USA 2012, 109, 9569–9574. [Google Scholar] [CrossRef]
- Schols, D.; Proost, P.; van Damme, J.; de Clercq, E. RANTES and MCP-3 inhibit the replication of T-cell-tropic human immunodeficiency virus type 1 strains (SF-2, MN, and HE). J. Virol. 1997, 71, 7300–7304. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.; le Gall, S.; Schwartz, O.; Salamero, J.; Montes, M.; Loetscher, P.; Baggiolini, M.; Virelizier, J.-L.; Arenzana-Seisdedos, F. HIV coreceptor downregulation as antiviral principle: SDF-1α–dependent Internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 1997, 186, 139–146. [Google Scholar] [CrossRef]
- Holme, P.A.; Müller, F.; Solum, N.O.; Brosstad, F.; Frøland, S.S.; Aukrust, P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998, 12, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Shirane, J.; Hieshima, K.; Shibano, M.; Watanabe, M.; Jin, Z.; Nagakubo, D.; Saito, T.; Shimomura, Y.; Yoshie, O. Novel antiviral activity of chemokines. Virology 2006, 350, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Mackewicz, C.E.; Yuan, J.; Tran, P.; Diaz, L.; Mack, E.; Selsted, M.E.; Levy, J.A. α-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. Aids 2003, 17, F23–F32. [Google Scholar] [CrossRef] [PubMed]
- Mattar, E.H.; Almehdar, H.A.; Uversky, V.N.; Redwan, E.M. Virucidal activity of human α- and β-defensins against hepatitis C virus genotype 4. Mol. BioSyst. 2016, 12, 2785–2797. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.; Day, P.M.; Thompson, C.D.; Lubkowski, J.; Lu, W.; Lowy, D.R.; Schiller, J.T. Human -defensins block papillomavirus infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1516–1521. [Google Scholar] [CrossRef]
- Quiñones-Mateu, M.E.; Lederman, M.M.; Feng, Z.; Chakraborty, B.; Weber, J.; Rangel, H.R.; Marotta, M.L.; Mirza, M.; Jiang, B.; Kiser, P.; et al. Human epithelial β-defensins 2 and 3 inhibit HIV-1 replication. Aids 2003, 17, F39–F48. [Google Scholar] [CrossRef]
- Howell, M.D.; Streib, J.E.; Leung, D.Y. Antiviral activity of human β-defensin 3 against vaccinia virus. J. Allergy Clin. Immunol. 2007, 119, 1022–1025. [Google Scholar] [CrossRef]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef]
- Matsumura, T.; Sugiyama, N.; Murayama, A.; Yamada, N.; Shiina, M.; Asabe, S.; Wakita, T.; Imawari, M.; Kato, T. Antimicrobial peptide LL-37 attenuates infection of hepatitis C virus. Hepatol. Res. 2015, 46, 924–932. [Google Scholar] [CrossRef]
- Alagarasu, K.; Patil, P.; Shil, P.; Seervi, M.; Kakade, M.; Tillu, H.; Salunke, A. In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides 2017, 92, 23–30. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Ali, P.S.; Sheeza, A.; Hemalatha, K. COVID-19 (novel coronavirus 2019)—Recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2006–2011. [Google Scholar] [PubMed]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Kho, S.; Barber, B.E.; Johar, E.; Andries, B.; Poespoprodjo, J.R.; Kenangalem, E.; Piera, K.A.; Ehmann, A.; Price, R.N.; William, T.; et al. Platelets kill circulating parasites of all major plasmodium species in human malaria. Blood 2018, 132, 1332–1344. [Google Scholar] [CrossRef]
- McMorran, B.J.; Wieczorski, L.; Drysdale, K.E.; Chan, J.-A.; Huang, H.M.; Smith, C.; Mitiku, C.; Beeson, J.G.; Burgio, G.; Foote, S.J. Platelet factor 4 and duffy antigen required for platelet killing of Plasmodium falciparum. Science 2012, 338, 1348–1351. [Google Scholar] [CrossRef]
- Crauwels, P.; Bank, E.; Walber, B.; Wenzel, U.A.; Agerberth, B.; Chanyalew, M.; Abebe, M.; König, R.; Ritter, U.; Reiling, N.; et al. Cathelicidin contributes to the restriction of leishmania in human host macrophages. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Rico-Mata, R.; de Leon-Rodriguez, L.M.; Avila, E.E. Effect of antimicrobial peptides derived from human cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp. Parasitol. 2013, 133, 300–306. [Google Scholar] [CrossRef]
- Carryn, S.; Schaefer, D.A.; Imboden, M.; Homan, E.J.; Bremel, R.D.; Riggs, M.W. Phospholipases and cationic peptides inhibit Cryptosporidium parvum sporozoite infectivity by parasiticidal and non-parasiticidal mechanisms. J. Parasitol. 2012, 98, 199–204. [Google Scholar] [CrossRef]
- Alecu, M.; Coman, G.; Mușetescu, A.; Coman, O.A. Antimicrobial peptides as an argument for the involvement of innate immunity in psoriasis (review). Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Amenomori, M.; Mukae, H.; Ishimatsu, Y.; Sakamoto, N.; Kakugawa, T.; Hara, A.; Hara, S.; Fujita, H.; Ishimoto, H.; Hayashi, T.; et al. Differential effects of human neutrophil peptide-1 on growth factor and interleukin-8 production by human lung fibroblasts and epithelial cells. Exp. Lung Res. 2010, 36, 411–419. [Google Scholar] [CrossRef][Green Version]
- Von Hundelshausen, P.; Duchene, J. Platelet-derived chemokines in atherosclerosis. Hämostaseologie 2015, 35, 137–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, A.; Bisht, P.; Bhattacharya, S.; Guchhait, P. Role of platelet cytokines in dengue virus infection. Front. Cell. Infect. Microbiol. 2020, 10, 561366. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, N.; Zhu, L.; Ersoy, M.; Hermansson, A.; Hjemdahl, P.; Hu, H.; Hansson, G.K.; Li, N. Platelets regulate CD4+ T-cell differentiation via multiple chemokines in humans. Thromb. Haemost. 2011, 106, 353–362. [Google Scholar] [CrossRef]
- Chertov, O.; Michiel, D.F.; Xu, L.; Wang, J.M.; Tani, K.; Murphy, W.J.; Longo, D.L.; Taub, D.D.; Oppenheim, J.J. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 1996, 271, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Chertov, O.; Oppenheim, J.J. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 2000, 68, 9–14. [Google Scholar]
- Yang, D.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef]
- Tjabringa, G.S.; Ninaber, D.K.; Drijfhout, J.W.; Rabe, K.F.; Hiemstra, P. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int. Arch. Allergy Immunol. 2006, 140, 103–112. [Google Scholar] [CrossRef]
- Scott, M.G.; Davidson, D.J.; Gold, M.; Bowdish, D.; Hancock, R. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Biragyn, A.; Ruffini, P.A.; Leifer, C.A.; Klyushnenkova, E.; Shakhov, A.; Chertov, O.; Shirakawa, A.K.; Farber, J.M.; Segal, D.M.; Oppenheim, J.J.; et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science 2002, 298, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Currie, A.; Reid, G.; Bowdish, D.; Macdonald, K.L.; Ma, R.C.; Hancock, R.E.W.; Speert, D.P. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004, 172, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.; Lederman, M.M.; Feng, Z.; Drage, M.G.; Jadlowsky, J.; Harding, C.V.; Weinberg, A.; Sieg, S.F. Human β-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc. Natl. Acad. Sci. USA 2007, 104, 18631–18635. [Google Scholar] [CrossRef]
- Tewary, P.; de la Rosa, G.; Sharma, N.; Rodriguez, L.G.; Tarasov, S.G.; Howard, O.M.Z.; Shirota, H.; Steinhagen, F.; Klinman, D.M.; Yang, D.; et al. β-defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-α production by human plasmacytoid dendritic cells, and promote inflammation. J. Immunol. 2013, 191, 865–874. [Google Scholar] [CrossRef]
- Antonio-Santos, A.; Pérez-Campos, E.; Hernández-Cruz, P.A.; Solórzano-Mata, C.; Narváez-Morales, J.; Torres-Aguilar, H.; Villegas-Sepúlveda, N.; Aguilar-Ruiz, S.R. Human platelets express Toll-like receptor 3 and respond to poly I: C. Hum. Immunol. 2014, 75, 1244–1251. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S.; Niyonsaba, F.; Hirata, M.; Adachi, Y.; Tamura, H.; Heumann, D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-α by blocking the binding of LPS to CD14+ cells. J. Immunol. 2001, 167, 3329–3338. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Papo, N.; Shai, Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. J. Biol. Chem. 2006, 281, 1636–1643. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, N.; Yang, J.; Meng, X.; Yang, R.; Li, J.; Sun, T. Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cell. Physiol. Biochem. 2013, 32, 614–623. [Google Scholar] [CrossRef]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Bergnach, C.; Orlando, F.; Silvestri, C.; Mocchegiani, F.; Licci, A.; Skerlavaj, B.; Rocchi, M.; et al. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2006, 50, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Semple, F.; MacPherson, H.; Webb, S.; Cox, S.L.; Mallin, L.J.; Tyrrell, C.; Grimes, G.R.; Semple, C.A.; Nix, M.A.; Millhauser, G.L.; et al. Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 2011, 41, 3291–3300. [Google Scholar] [CrossRef] [PubMed]
- Panyutich, A.V.; Panyutich, E.A.; Krapivin, V.A.; Baturevich, E.A.; Ganz, T. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J. Lab. Clin. Med. 1993, 122, 202–207. [Google Scholar] [PubMed]
- Chen, Q.; Hakimi, M.; Wu, S.; Jin, Y.; Cheng, B.; Wang, H.; Xie, G.; Ganz, T.; Linzmeier, R.M.; Fang, X. Increased genomic copy number of DEFA1/DEFA3 is associated with susceptibility to severe sepsis in Chinese Han population. Anesthesiology 2010, 112, 1428–1434. [Google Scholar] [CrossRef]
- Lippross, S.; Klueter, T.; Steubesand, N.; Oestern, S.; Mentlein, R.; Hildebrandt, F.; Podschun, R.; Pufe, T.; Seekamp, A.; Varoga, D. Multiple trauma induces serum production of host defence peptides. Injury 2012, 43, 137–142. [Google Scholar] [CrossRef]
- Berkestedt, I.; Herwald, H.; Ljunggren, L.; Nelson, A.; Bodelsson, M. Elevated plasma levels of antimicrobial polypeptides in patients with severe sepsis. J. Innate Immun. 2010, 2, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Carcillo, J.A.; Doughty, L.A.; Sasser, H.; Heine, R.P. Plasma concentrations of defensins and lactoferrin in children with severe sepsis. Pediatr. Infect. Dis. J. 2002, 21, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Raque, V.-J.X.; Carlos, S.-G.J.; Eduardo, R.-R.; Rafael, B.-H.; Ángeles, R.-T.M.D.L.; Adriana, R.-C.; Honorio, T.-A.; José, B.-A.; Roberto, A.-R.S. Modification of immunological features in human platelets during sepsis. Immunol. Investig. 2018, 47, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, G.; Walborn, A.; Rondina, M.; Fareed, J.; Hoppensteadt, D. Biomarkers of platelet activation and their prognostic value in patients with sepsis-associated disseminated intravascular coagulopathy. Clin. Appl. Thromb. 2021, 27, 1076029620943300. [Google Scholar] [CrossRef]
- Baxter, A.A.; Lay, F.T.; Poon, I.K.H.; Kvansakul, M.; Hulett, M.D. Tumor cell membrane-targeting cationic antimicrobial peptides: Novel insights into mechanisms of action and therapeutic prospects. Cell. Mol. Life Sci. 2017, 74, 3809–3825. [Google Scholar] [CrossRef]
- Galeotti, T.; Borrello, S.; Minotti, G.; Masotti, L. Membrane alterations in cancer cells. Ann. N. Y. Acad. Sci. 1986, 488, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr. Top. Med. Chem. 2015, 16, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Freire, J.M.; Pacheco, T.R.; Barata, J.T.; Castanho, M.A. Apoptotic human neutrophil peptide-1 anti-tumor activity revealed by cellular biomechanics. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 308–316. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Y.-S.; Pan, W.-B.; Xiao, B.; Wen, Y.-J.; Chen, X.-C.; Chen, L.-J.; Deng, H.-X.; You, J.; Kan, B.; et al. Human α-defensin-1 inhibits growth of human lung adenocarcinoma xenograft in nude mice. Mol. Cancer Ther. 2008, 7, 1588–1597. [Google Scholar] [CrossRef]
- Phan, T.K.; Lay, F.; Poon, I.; Hinds, M.; Kvansakul, M.; Hulett, M.D. Human β-defensin 3 contains an oncolytic motif that binds PI (4, 5) P2 to mediate tumour cell permeabilisation. Oncotarget 2016, 7, 2054–2069. [Google Scholar] [CrossRef]
- Bullard, R.S.; Gibson, W.; Bose, S.K.; Belgrave, J.K.; Eaddy, A.C.; Wright, C.J.; Hazen-Martin, D.J.; Lage, J.M.; Keane, T.E.; Ganz, T.A.; et al. Functional analysis of the host defense peptide human beta defensin-1: New insight into its potential role in cancer. Mol. Immunol. 2008, 45, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Itoh, A.; Isogai, E.; Hirose, K.; Hosokawa, Y.; Abiko, Y.; Shibata, T.; Hirata, M.; Isogai, H. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett. 2004, 212, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.S.; Mookherjee, N.; Hancock, R.; Bleackley, R.C. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving bax activity. Mol. Cancer Res. 2009, 7, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Cheng, A.; To, K.F.; Tong, J.H.; Li, M.S.; Shen, J.; Wong, C.C.; Zhang, L.; Chan, R.L.; Wang, X.J.; et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012, 72, 6512–6523. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino-Domínguez, A.S.; Romero-Tlalolini, M.d.l.A.; Torres-Aguilar, H.; Aguilar-Ruiz, S.R. Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets. Int. J. Mol. Sci. 2021, 22, 10230. https://doi.org/10.3390/ijms221910230
Aquino-Domínguez AS, Romero-Tlalolini MdlA, Torres-Aguilar H, Aguilar-Ruiz SR. Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets. International Journal of Molecular Sciences. 2021; 22(19):10230. https://doi.org/10.3390/ijms221910230
Chicago/Turabian StyleAquino-Domínguez, Alba S., María de los A. Romero-Tlalolini, Honorio Torres-Aguilar, and Sergio R. Aguilar-Ruiz. 2021. "Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets" International Journal of Molecular Sciences 22, no. 19: 10230. https://doi.org/10.3390/ijms221910230
APA StyleAquino-Domínguez, A. S., Romero-Tlalolini, M. d. l. A., Torres-Aguilar, H., & Aguilar-Ruiz, S. R. (2021). Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets. International Journal of Molecular Sciences, 22(19), 10230. https://doi.org/10.3390/ijms221910230







