Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease
Abstract
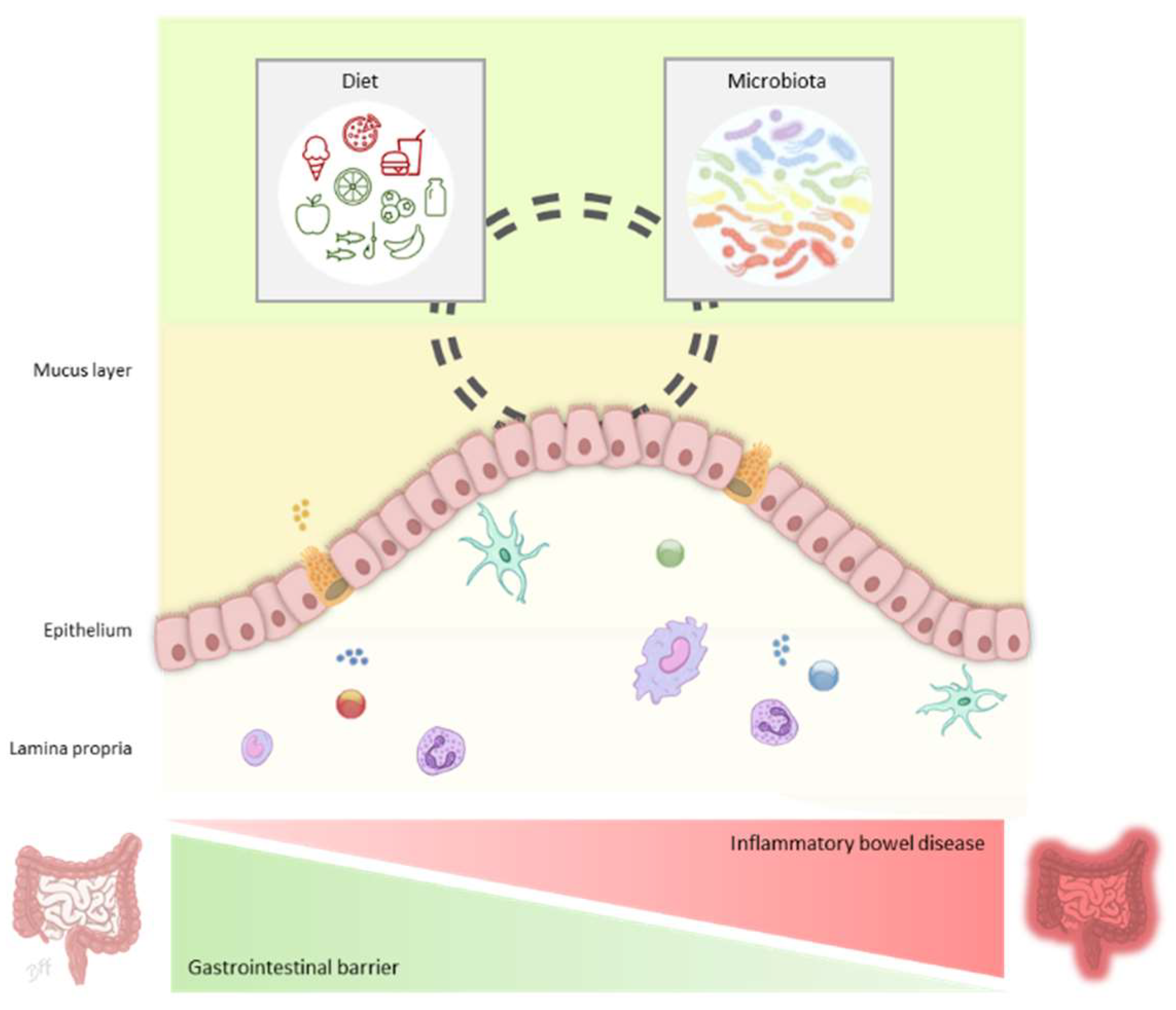
:1. Introduction to Inflammatory Bowel Disease
2. Gastrointestinal Barrier
2.1. Mucus Layer
2.2. Mucus Layer under Inflammatory Conditions
3. Gut Microbiota and the Mucus Layer in IBD
4. Dietary Compounds and the Mucus Layer in IBD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Mulder, D.J.; Noble, A.J.; Justinich, C.J.; Duffin, J.M. A tale of two diseases: The history of inflammatory bowel disease. J. Crohns Colitis 2014, 8, 341–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, G.G.; Ng, S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khor, B.; Gardet, A.; Ramnik, J.X. Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Aldars-García, L.; Chaparro, M.; Gisbert, J.P. Systematic review: The gut microbiome and its potential clinical application in inflammatory bowel disease. Microorganisms 2021, 9, 977. [Google Scholar] [CrossRef]
- Hoentjen, F.; Dieleman, L.A. Pathophysiology of inflammatory bowel diseases. Handb. Prebiotics 2008, 341–374. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Chamaillard, M.; Gonzalez, F.; Beclin, E.; Decourcelle, C.; Antunes, L.; Gay, J.; Neut, C.; Colombel, J.F.; Desreumaux, P. Mesenteric fat in Crohn’s disease: A pathogenetic hallmark or an innocent bystander? Gut 2007, 56, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Bonovas, S.; Pantavou, K.; Evripidou, D.; Bastiampillai, A.J.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Safety of biological therapies in ulcerative colitis: An umbrella review of meta-analyses. Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; ElJurdi, K.; Zmeter, N.; Rubin, D. Emerging therapies for inflammatory bowel diseases. Dig. Dis. 2016, 34, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisbert, J.P.; Chaparro, M. Predictors of primary response to biologic treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in patients with inflammatory bowel disease: From basic science to clinical practice. J. Crohns Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Digby-Bell, J.L.; Atreya, R.; Monteleone, G.; Powell, N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 9–20. [Google Scholar] [CrossRef] [PubMed]
- De Medina, F.S.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Sharpe, C.; Thornton, D.J.; Grencis, R.K. A sticky end for gastrointestinal helminths; the role of the mucus barrier. Parasite Immunol. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Salzman, N.H.; Underwood, M.A.; Bevins, C.L. Paneth cells, defensins, and the commensal microbiota: A hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007, 19, 70–83. [Google Scholar] [CrossRef]
- Aldars-García, L.; Marin, A.C.; Chaparro, M.; Gisbert, J.P. The interplay between immune system and microbiota in inflammatory bowel disease: A narrative review. Int. J. Mol. Sci. 2021, 22, 3706. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology 2010, 139, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Marin, A.C.; Moreno, L.O.; Baldan-Martin, M.; Mora-Gutiérrez, I.; Lanas-Gimeno, A.; Moreno-Monteagudo, J.A.; Santander, C.; Sánchez, B.; Chaparro, M.; et al. Immunomodulatory effect of gut microbiota-derived bioactive peptides on human immune system from healthy controls and patients with inflammatory bowel disease. Nutrients 2019, 11, 2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.; Marin, A.C.; Fernández-Tomé, S.; Montalban-Arques, A.; Carrasco, A.; Tristán, E.; Ortega-Moreno, L.; Mora-Gutiérrez, I.; Díaz-Guerra, A.; Caminero-Fernández, R.; et al. Human intestinal pro-inflammatory CD11chighCCR2+CX3CR1+ macrophages, but not their tolerogenic CD11c-CCR2-CX3CR1- counterparts, are expanded in inflammatory bowel disease article. Mucosal Immunol. 2018, 11, 1114–1126. [Google Scholar] [CrossRef]
- Isidro, R.A.; Appleyard, C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G59–G73. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C.; et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Pineiro, A.M.; Bergstrom, J.H.; Ermund, A.; Gustafsson, J.K.; Schutte, A.; Johansson, M.E.V.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. AJP Gastrointest. Liver Physiol. 2013, 305, G348–G356. [Google Scholar] [CrossRef]
- Ambort, D.; Johansson, M.E.V.; Gustafsson, J.K.; Ermund, A.; Hansson, G.C. Perspectives on mucus properties and formation-lessons from the biochemical world. Cold Spring Harb. Perspect. Med. 2012, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; Mcguckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klomp, L.W.J.; Rens, L.V.A.N.; Stroust, G.J. Cloning and analysis of human gastric mucin cDNA reveals two types of conserved cysteine-rich domains. Biochem. J. 1995, 838, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Ambort, D.; Johansson, M.E.V.; Gustafsson, J.K.; Nilsson, H.E.; Ermund, A. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. USA 2012, 109, 5645–5650. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Muller, A.; Young, V.B.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2017, 167, 1339–1353. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.K.; Miyoshi, H.; Beatty, W.L.; Head, R.D.; Malvin, N.P.; Cadwell, K.; Guan, J.; Saitoh, T.; Akira, S.; Seglen, P.O.; et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 2013, 32, 3130–3144. [Google Scholar] [CrossRef] [Green Version]
- Wlodarska, M.; Thaiss, C.A.; Nowarski, R.; Henao-Mejia, J.; Zhang, J.P.; Brown, E.M.; Frankel, G.; Levy, M.; Katz, M.N.; Philbrick, W.M.; et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 2014, 156, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heazlewood, C.K.; Cook, M.C.; Eri, R.; Price, G.R.; Tauro, S.B.; Taupin, D.; Thornton, D.J.; Chin, W.P.; Crockford, T.L.; Cornall, R.J.; et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008, 5, 0440–0460. [Google Scholar] [CrossRef] [Green Version]
- Shkoda, A.; Ruiz, P.A.; Daniel, H.; Kim, S.C.; Rogler, G.; Sartor, R.B.; Haller, D. Interleukin-10 Blocked endoplasmic reticulum stress in intestinal epithelial cells: Impact on chronic inflammation. Gastroenterology 2007, 132, 190–207. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Tauro, S.; Das, I.; Tong, H.; Chen, A.C.H.; Jeffery, P.L.; McDonald, V.; Florin, T.H.; McGuckin, M.A. IL-10 Promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 2013, 144, 357–368.e9. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Wang, H.; Ghia, J.E.; Haq, N.; Deng, Y.; Velcich, A.; Grencis, R.K.; Thornton, D.J.; Khan, W.I. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 2010, 138, 1763–1771.e5. [Google Scholar] [CrossRef]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [Green Version]
- Finnie, I.A.; Dwarakanath, A.D.; Taylor, B.A.; Rhodes, J.M. Colonic mucin synthesis is increased by sodium butyrate. Gut 1995, 36, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, H.; Kettlewell, M.G.W.; Jewell, D.P.; Kent, P.W. Glycosylation and sulphation of colonic mucus glycoproteins in patients with ulcerative colitis and in healthy subjects. Gut 1993, 34, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Einerhand, A.W.C.; Renes, I.B.; Makkink, M.K.; Van Der Sluis, M.; Büller, H.A.; Dekker, J. Role of mucins in inflammatory bowel disease: Important lessons from experimental models. Eur. J. Gastroenterol. Hepatol. 2002, 14, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Varsha, S.; Kelli, J.; Jianyi, Y.; Sun, L.; Ruxian, L.; Huimin, Y.; Julie, I.; Jennifer, F.-A.; Nicholas, Z.C.; Mark, D.; et al. Chronic inflammation in ulcerative colitis causes long term changes in goblet cell function. Cell. Mol. Gastroenterol. Hepatol. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Pullan, R.D.; Thomas, G.A.O.; Rhodes, M.; Newcombe, R.G.; Williams, G.T.; Allen, A.; Rhodes, J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994, 35, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Van Klinken, B.J.W.; Van Der Wal, J.W.G.; Einerhand, A.; Büller, H.A.; Dekker, J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut 1999, 44, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.M.H.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.V.; Eklund, L.; Sjövall, H.; Hansson, G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 2011, 17, 2299–2307. [Google Scholar] [CrossRef]
- Shaoul, R.; Okada, Y.; Cutz, E.; Marcon, M.A. Colonic Expression of MUC2, MUC5AC, and TFF1 in Inflammatory Bowel Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 488–493. [Google Scholar] [CrossRef]
- Forgue-Lafitte, M.E.; Fabiani, B.; Levy, P.P.; Maurin, N.; Flejou, J.F.; Bara, J. Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. Int. J. Cancer 2007, 121, 1543–1549. [Google Scholar] [CrossRef]
- Borralho, P.; Vieira, A.; Freitas, J.; Chaves, P.; Soares, J. Aberrant gastric apomucin expression in ulcerative colitis and associated neoplasia. J. Crohns Colitis 2007, 1, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Olli, K.E.; Rapp, C.; Connell, L.O.; Collins, C.B.; McNamee, E.N.; Jensen, O.; Jedlicka, P.; Allison, K.C.; Goldberg, M.S.; Gerich, M.E.; et al. Muc5ac expression protects the colonic barrier in experimental colitis. Inflamm. Bowel Dis. 2020, 26, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Van Passel, M.W.J.; Van De Bovenkamp, J.H.B.; Schipper, R.G.; De Vos, W.M.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 2010, 1, 254–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.V.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Sicard, J.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Laval, L.; Martin, R.; Natividad, J.N.; Chain, F.; Miquel, S.; De Maredsous, C.D.; Capronnier, S.; Sokol, H.; Verdu, E.; van Hylckama Vlieg, J.; et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wrzosek, L.; Miquel, S.; Noordine, M.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Crossm Bifidobacterium dentium fortifies the intestinal mucus layer. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Van Der Lugt, B.; Van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.P.; Brandt, R.M.C.; De Vos, W.M.; Savelkoul, H.F.J.; Steegenga, W.T.; et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 −/Δ 7 mice. Immun. Ageing 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.P. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahl, D.; Liu, H.; Schreiber, O.; Roos, S.; Phillipson, M.; Holm, L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016, 317, 300–310. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, Y.; Lyu, X.; Wang, J.; Wang, Y.; Yang, R. Prevention and alleviation of dextran sulfate sodium salt-induced inflammatory bowel disease in mice with bacillus subtilis-fermented milk via inhibition of the inflammatory responses and regulation of the intestinal flora. Front. Microbiol. 2021, 11, 622354. [Google Scholar] [CrossRef]
- Souza, L.; Elian, S.D.; Paula, M.; Garcia, C.C.; Vieira, T.; Teixeira, M.M.; Arantes, R.M.; Nicoli, J.R.; Martins, F.S. Escherichia coli strain Nissle 1917 ameliorates experimental colitis by modulating intestinal permeability, the inflammatory response and clinical signs in a faecal transplantation model. J. Med. Microbiol. 2016, 65, 201–210. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Backhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Singh, T.P.; Malik, R.K. In vivo implications of potential probiotic Lactobacillus reuteri lr6 on the gut and immunological parameters as an adjuvant against protein energy malnutrition. Probiotics Antimicrob. Proteins 2020, 12, 517–534. [Google Scholar] [CrossRef]
- Kumar, M.; Kissoon-Singh, V.; Coria, A.L.; Moreau, F.; Chadee, K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, 34–45. [Google Scholar] [CrossRef]
- Liu, X.; Yu, R.; Zou, K. Probiotic mixture VSL # 3 alleviates dextran sulfate sodium-induced colitis in mice by downregulating T follicular helper cells. Curr. Med. Sci. 2019, 39, 371–378. [Google Scholar]
- Je, I.; Lee, D.; Jeong, D.; Hong, D.; Yoon, J.; Moon, J.S.; Park, S. The probiotic, ID-JPL934, Attenuates dextran sulfate sodium-induced colitis in mice through inhibition of proinflammatory cytokines expression. J. Med. Food 2018, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The immune system bridges the gut microbiota with systemic energy homeostasis: Focus on TLRs, mucosal barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [Green Version]
- Bilotta, A.J.; Cong, Y. Gut microbiota metabolite regulation of host defenses at mucosal surfaces: Implication in precision medicine. Precis. Clin. Med. 2019, 2, 110–119. [Google Scholar] [CrossRef]
- Camilleri, M. Human intestinal barrier: Effects of stressors, diet, prebiotics, and probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef]
- Nguyen, N.; Zhang, B.; Holubar, S.; Pardi, D.; Singh, S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis (Review). Cochrane Database Syst. Rev. 2019, 5, CD001176. [Google Scholar] [CrossRef] [Green Version]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Koretz, R.L. Probiotics in gastroenterology: How pro is the evidence in adults? Am. J. Gastroenterol. 2018, 113, 1125–1136. [Google Scholar] [CrossRef]
- Alemao, C.A.; Budden, K.F.; Gomez, H.M.; Rehman, S.F.; Marshall, J.E.; Shukla, S.D.; Donovan, C.; Forster, S.C.; Yang, I.A.; Keely, S.; et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy 2021, 76, 714–734. [Google Scholar] [CrossRef]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the westernized diet in the onset and progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Tomé, S.; Hernández-Ledesma, B.; Chaparro, M.; Indiano-Romacho, P.; Bernardo, D.; Gisbert, J.P. Role of food proteins and bioactive peptides in inflammatory bowel disease. Trends Food Sci. Technol. 2019, 88, 194–206. [Google Scholar] [CrossRef]
- Hussain, M.; Ijaz, M.U.; Ahmad, M.I.; Khan, I.A.; Brohi, S.A.; Shah, A.U.; Shinwari, K.I.; Zhao, D.; Xu, X.; Zhou, G.; et al. Meat proteins in a high-fat diet have a substantial impact on intestinal barriers through mucus layer and tight junction protein suppression in C57BL/6J mice. Food Funct. 2019, 10, 6903–6914. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef]
- Chen, X.; Song, P.; Fan, P.; He, T.; Jacobs, D.; Levesque, C.L.; Johnston, L.J.; Ji, L.; Ma, N.; Chen, Y.; et al. Moderate dietary protein restriction optimized gut microbiota and mucosal barrier in growing pig model. Front. Cell. Infect. Microbiol. 2018, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Zhou, G.; Liu, X.; Song, S.; Xu, X.; Hooiveld, G.; Müller, M.; Liu, L.; Kristiansen, K.; Li, C. Dietary protein sources differentially affect the growth of akkermansia muciniphila and maintenance of the gut mucus barrier in mice. Mol. Nutr. Food Res. 2019, 63, 1900589. [Google Scholar] [CrossRef]
- Han, K.-S.; Deglaire, A.; Sengupta, R.; Moughan, P.J. Hydrolyzed casein influences intestinal mucin gene expression in the rat. J. Agric. Food Chem. 2008, 56, 5572–5576. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Martínez-Maqueda, D.; Tabernero, M.; Largo, C.; Recio, I.; Miralles, B. Effect of the long-term intake of a casein hydrolysate on mucin secretion and gene expression in the rat intestine. J. Funct. Foods 2017, 33, 176–180. [Google Scholar] [CrossRef]
- Plaisancié, P.; Claustre, J.; Estienne, M.; Henry, G.; Boutrou, R.; Paquet, A.; Léonil, J. A novel bioactive peptide from yoghurts modulates expression of the gel-forming MUC2 mucin as well as population of goblet cells and Paneth cells along the small intestine. J. Nutr. Biochem. 2013, 24, 213–221. [Google Scholar] [CrossRef]
- Bessette, C.; Benoit, B.; Sekkal, S.; Bruno, J.; Estienne, M.; Léonil, J.; Ferrier, L.; Théodorou, V.; Plaisancié, P. Protective effects of β-casofensin, a bioactive peptide from bovine β-casein, against indomethacin-induced intestinal lesions in rats. Mol. Nutr. Food Res. 2016, 60, 823–833. [Google Scholar] [CrossRef]
- Araújo, D.F.S.; Guerra, G.C.B.; Pintado, M.M.E.; Sousa, Y.R.F.; Algieri, F.; Rodriguez-Nogales, A.; Araújo, R.F.; Gálvez, J.; Queiroga, R.C.R.E.; Rodriguez-Cabezas, M.E. Intestinal anti-inflammatory effects of goat whey on DNBS-induced colitis in mice. PLoS ONE 2017, 12, e0185382. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Agric. Food Chem. 2009, 57, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 2012, 142, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Handa, O.; Naito, Y.; Takayama, S.; Suyama, Y.; Ushiroda, C.; Majima, A.; Hirai, Y.; Mizushima, K.; Okayama, T.; et al. High-fat diet causes constipation in mice via decreasing colonic mucus. Dig. Dis. Sci. 2020, 65, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Wang, S.; Tu, Z.; Zhang, L.; Wang, X.; Hou, Y.; Wang, C.; Chen, J.; Liu, Y. Flaxseed oil attenuates intestinal damage and inflammation by regulating necroptosis and TLR4/NOD signaling pathways following lipopolysaccharide challenge in a piglet model. Mol. Nutr. Food Res. 2018, 62, 1700814. [Google Scholar] [CrossRef]
- Zou, J.; Chassaing, B.; Singh, V.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 2018, 23, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Ishisono, K.; Mano, T.; Yabe, T.; Kitaguchi, K. Dietary fiber pectin ameliorates experimental colitis in a neutral sugar side chain-dependent manner. Front. Immunol. 2019, 10, 2979. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Zheng, M.; Hao, S.; Lum, J.S.; Chen, X.; Huang, X.; Yu, Y.; Zheng, K. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J. Neuroinflammation 2020, 17, 1–21. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.; Sue, A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. NIH public access. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Fu, J.; Dijkstra, G.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.-H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.; Sung, J.J.; et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology 2021, 27, S0016-5085(21)03439-9. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Belzer, C. Nutritional strategies for mucosal health: The interplay between microbes and mucin glycans. Trends Microbiol. 2021, 30, S0966-842X(21)00135-9. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Abreu, M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef]
- Kundra, P.; Rachmühl, C.; Lacroix, C.; Geirnaert, A. Role of dietary micronutrients on gut microbial dysbiosis and modulation in inflammatory bowel disease. Mol. Nutr. Food Res. 2021, 65, 1901271. [Google Scholar] [CrossRef]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Cao, Y.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1321–1345. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, P.; Noureddine, N.; Wawrzyniak, M.; Lucchinetti, E.; Krämer, S.D.; Rogler, G.; Zaugg, M.; Hersberger, M. Nutritional lipids and mucosal inflammation. Mol. Nutr. Food Res. 2021, 65, 1901269. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Leech, B.; Mcintyre, E.; Steel, A.; Sibbritt, D. Risk factors associated with intestinal permeability in an adult population: A systematic review. Int. J. Clin. Pract. 2019, 73, e13385. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.M.; Pan, C.; Cantor, R.M.; Tang, W.H.W.; Garcia-Garcia, J.C.; Kurtz, I.; Hazen, S.L.; Bergeron, N.; Krauss, R.M.; Lusis, A.J. Impact of individual traits, saturated fat, and protein source on the gut microbiome. mBio 2018, 9, e01604–e01618. [Google Scholar] [CrossRef] [Green Version]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [Green Version]
| Bacterial Strain | Animal Model | Experimental Administration | Study Period | Outcomes and Mechanisms of Action | Reference |
|---|---|---|---|---|---|
| Lactobacillus rhamnosus CNCM I-3690 and L. paracasei CNCM I-3689 | DNBS-induced colitis in C57BL/6J mice | Intragastric administration with 1 × 109 CFU/mL | 10 days |
| [66] |
| Lactobacillus rhamnosus CNCM I-3690 | DNBS-induced colitis in C57BL/6J mice | Intragastric administration with 5 × 109 CFU/mL | 10 days |
| [73] |
| Lactobacillus reuteri R2LC and Lactobacillus reuteri 4659 | DSS-induced colitis in C57BL/6J mice | Oral gavage with 1 × 108 live bacteria | 14 days |
| [74] |
| Bacillus subtilis JNFE0126 | DSS-induced colitis in C57BL/6J mice | B. subtilis-fermented milk oral gavage (6 × 108 CFU/mL) | 21 days |
| [75] |
| Escherichia coli strain Nissle 1917 | DSS-induced colitis in BALB/c mice | Intragastric administration with 1 × 109 CFU/mL | 17 days |
| [76] |
| Bifidobacterium longum NCC 2705 | Western style diet-induced obesity in C57BL/6J mice | Supplementation of the drinking water with 2 × 106 CFU/mL | 4 weeks |
| [77] |
| Bifidobacterium dentium ATCC 27678 | Swiss Webster germfree mice | Oral gavage with 2 × 108 CFU/mL | 1–2 weeks |
| [69] |
| Lactobacillus reuteri LR6 | Protein and energy malnutrition in Swiss mice | Diet with fermented product or bacterial suspension at 1 × 109 CFU/day | 1 week |
| [78] |
| Akkermansia muciniphila MucT BAA-835 | Accelerated aging Ercc1-/Δ7 mice | Oral gavage with 2 × 108 CFU/200 µL | 10 weeks |
| [71] |
| VSL#3 probiotic mixture | DSS-induced colitis in Muc2−/− mice | Oral gavage with 2.25 × 109 CFU/day | 2 weeks |
| [79] |
| VSL#3 probiotic mixture | DSS-induced colitis in C57BL/6J mice | Oral gavage with 3 × 109 live bacteria | 60 days |
| [80] |
| Lactobacillus johnsonii IDCC9203, Lactobacillus plantarum IDCC3501 and Bifidobacterium animalis subspecies lactis IDCC4301 (ID-JPL934 probiotic mixture) | DSS-induced colitis in BALB/c mice | Oral gavage with probiotic mixture (1 × 106–1 × 109 CFU/day) | 8 days |
| [81] |
| Lactobacillus rhamnosus, L. acidophilus and Bifidobacterium bifidumi | High fat diet-induced obesity in Swiss mice | Oral gavage with probiotic mixture (6 × 108 CFU of each strain; final concentration of 1.8 × 109 CFU of bacteria) | 5 weeks |
| [82] |
| Food Group/Compounds | Animal Model | Experimental Administration | Study Period | Outcomes and Mechanisms of Action | Reference |
|---|---|---|---|---|---|
| Proteins | |||||
| Total proteins | Adult finishing pigs | Three study groups (16%, normal dietary protein concentration; 13%, low dietary protein concentration; 10%, extremely low dietary protein concentration) | 50 days |
| [94] |
| Total proteins | Growing pigs | Three study groups (18%, normal dietary protein concentration; 15%, low dietary protein concentration; 12%, extremely low dietary protein concentration) | 30 days |
| [95] |
| Chicken and soy proteins | C57BL/6 mice | Chicken or soy protein-based diets | 4 weeks |
| [96] |
| Milk casein | Rats | Milk casein hydrolysate | 8 days |
| [97] |
| Milk casein | Zucker rats | Milk casein hydrolysate | 8 weeks |
| [98] |
| Milk β-casein | Rats pups | Milk β-casein peptide f(94–123) | 9 days |
| [99] |
| Milk β-casein | Indomethacin-induced jejunal injury in rats | Milk β-casein peptide f(94–123) | 8 days |
| [100] |
| Goat whey | DNBS-induced colitis in CD1 mice | Goat whey proteins, fatty acids and oligosaccharides | 16 days |
| [101] |
| Hen egg | DSS-induced colitis in piglets | Egg white lysozyme | 5 days |
| [102] |
| Soybean protein | DSS-induced colitis in piglets | Soybean protein derived di- and tri-peptides | 5 days |
| [103] |
| Pea protein | DSS-induced colitis in C57BL/6J mice | Pea seed protein extracts | 23 days |
| [104] |
| Lipids | |||||
| High- and low-fat diets | C57BL/6J mice | Chicken, soy or pork protein-based administration either with low fat (12% kcal) or high fat (60% kcal) diets | 12 weeks |
| [93] |
| High-fat diet | C57BL/6 mice | High-fat diet (56.7 Fat kcal %), in comparison with normal chow diet (12.0 Fat kcal %) | 8 weeks |
| [105] |
| High-fat diet | Spontaneous colitis in Winnie mice | High-fat diet (46% available energy as fat), in comparison with normal chow diet (11% available energy as fat) | 9 weeks |
| [106] |
| Flaxseed oil | LPS-induced intestinal injury in weaned piglets | Supplementation of diets with flaxseed oil in comparison with corn oil (5% weight:weight) | 3 weeks |
| [107] |
| Fiber | |||||
| Inulin | Western style diet-induced obesity in C57BL/6J mice | 1% oligofructose-enriched inulin supplementation in the drinking water | 4 weeks |
| [77] |
| Inulin and cellulose | Western style diet-induced obesity in C57BL/6J mice | Supplementation of high-fat diets (60 kcal% fat) with 20 % fiber | 4 weeks |
| [108] |
| Pectin | TNBS- and DSS-induced colitis in C57BL/6J mice | Diet supplemented with characteristically high (5% orange pectin) in comparison to low (5% citrus pectin) side chain content of pectin | 10–14 days |
| [109] |
| Microbiota-accessible carbohydrates | High-fat and fiber-deficient diet in C57BL/6J mice | Supplementation of high-fat (31.5% fat by weight) and fiber-deficient (5% fiber by weight) diet with microbiota-accessible carbohydrates | 15 weeks |
| [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Tomé, S.; Ortega Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 10224. https://doi.org/10.3390/ijms221910224
Fernández-Tomé S, Ortega Moreno L, Chaparro M, Gisbert JP. Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2021; 22(19):10224. https://doi.org/10.3390/ijms221910224
Chicago/Turabian StyleFernández-Tomé, Samuel, Lorena Ortega Moreno, María Chaparro, and Javier P. Gisbert. 2021. "Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease" International Journal of Molecular Sciences 22, no. 19: 10224. https://doi.org/10.3390/ijms221910224
APA StyleFernández-Tomé, S., Ortega Moreno, L., Chaparro, M., & Gisbert, J. P. (2021). Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. International Journal of Molecular Sciences, 22(19), 10224. https://doi.org/10.3390/ijms221910224






