Efficient Attenuation of Dextran Sulfate Sodium-Induced Colitis by Oral Administration of 5,6-Dihydroxy-8Z,11Z,14Z,17Z-eicosatetraenoic Acid in Mice
Abstract
:1. Introduction
2. Results
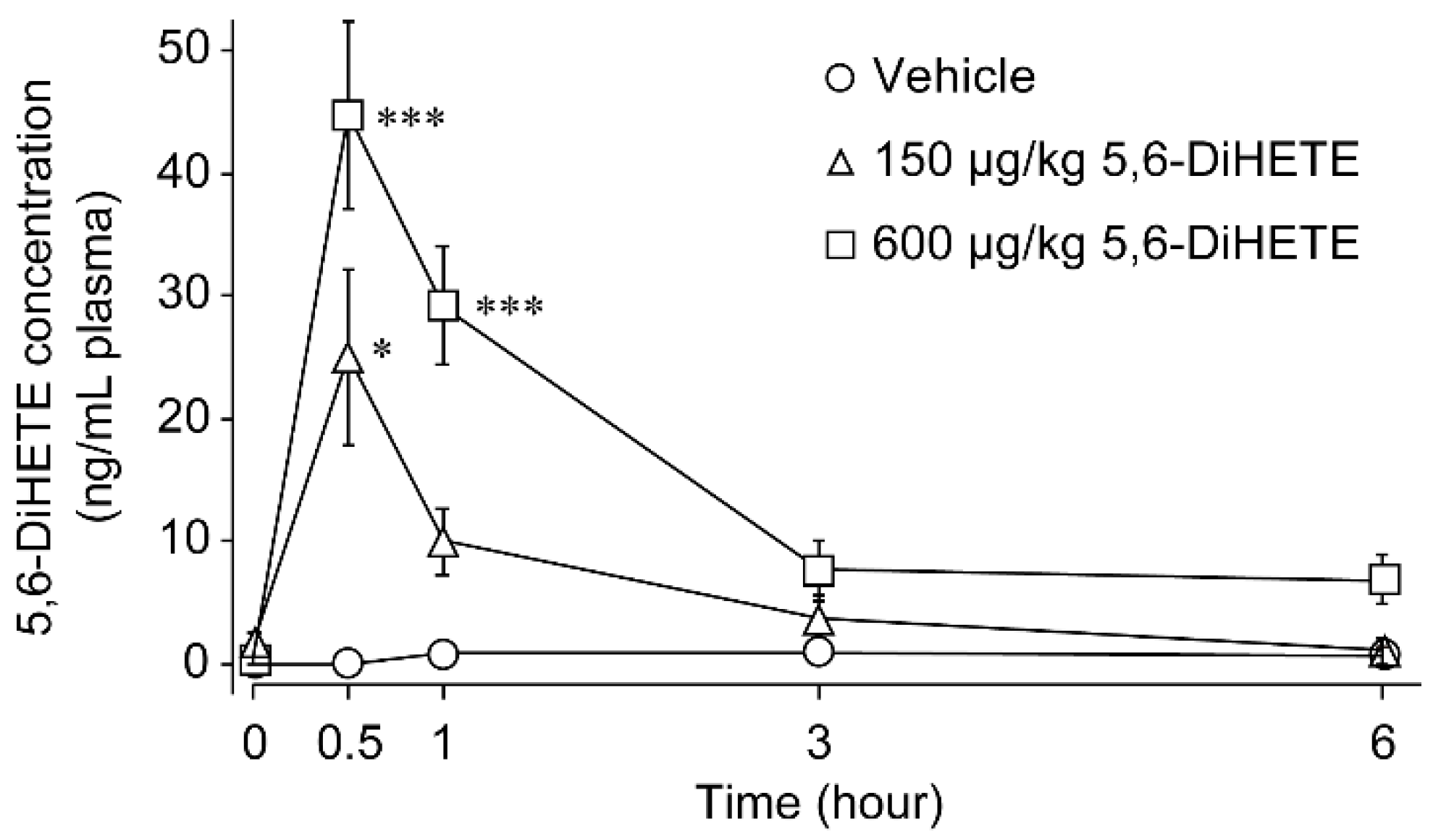
2.1. Pharmacokinetics of 5,6-DiHETE in Mice Plasma
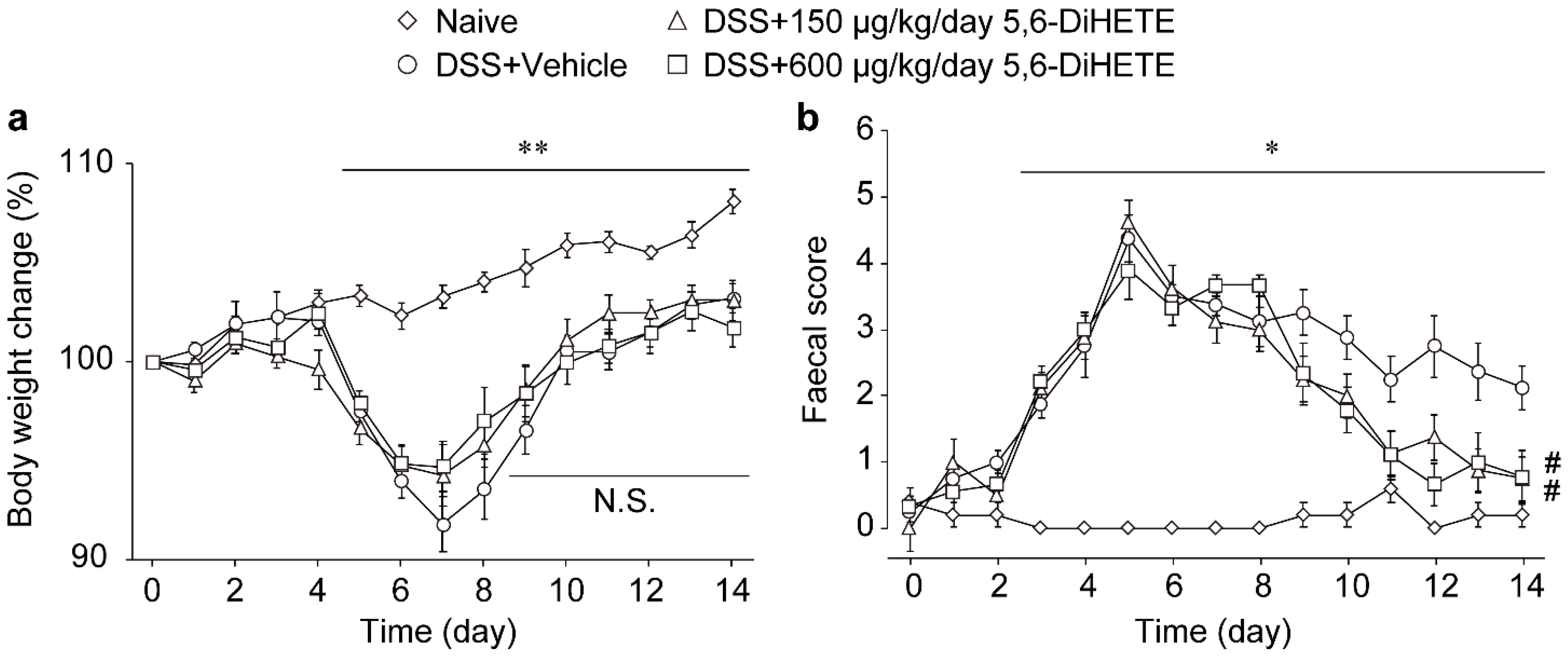
2.2. Oral Administration of 5,6-DiHETE Did Not Affect Body Weight Change but Improved Faecal Condition
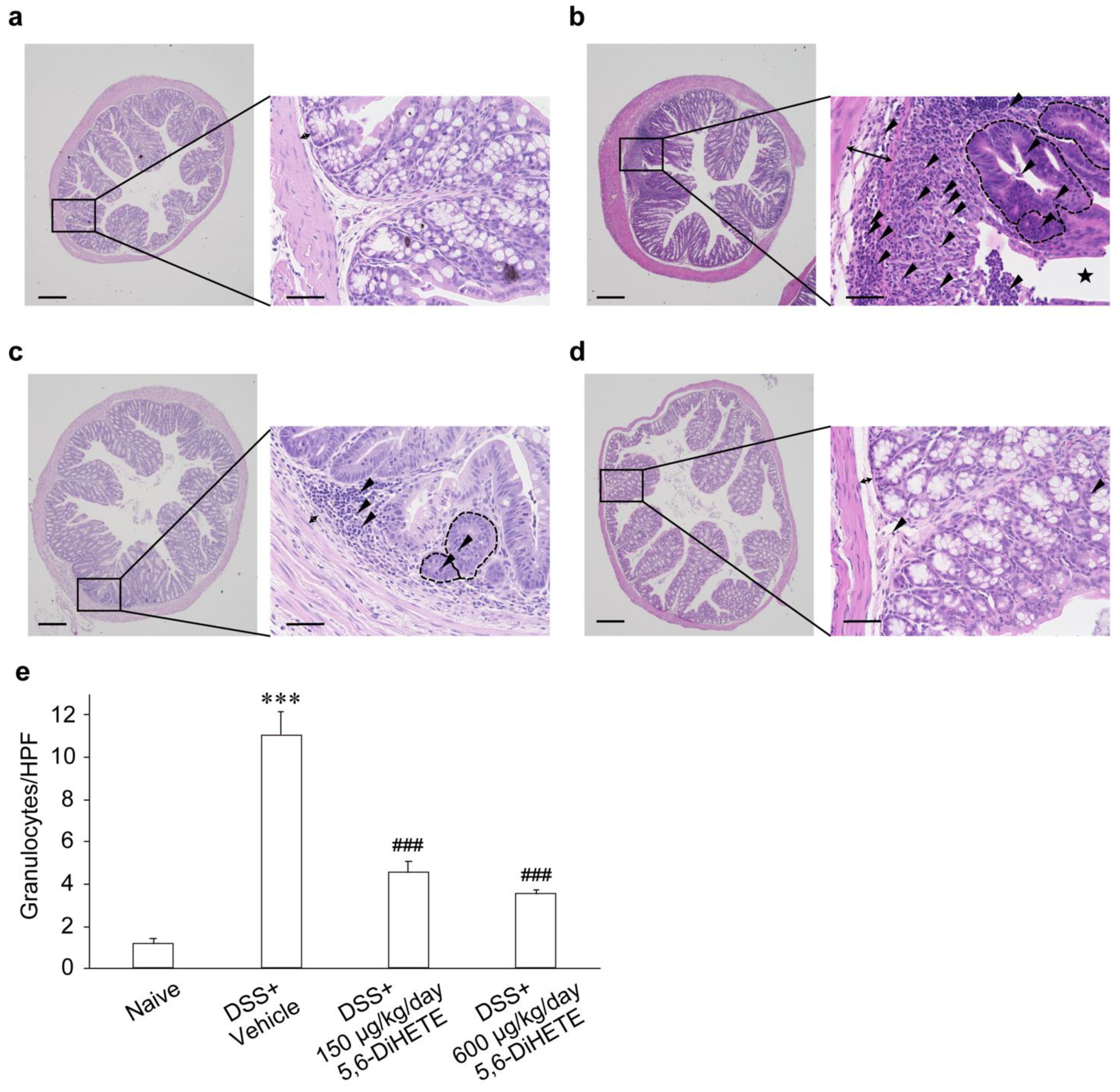
2.3. 5,6-DiHETE Administration Attenuated DSS-Induced Colon Inflammation in Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. Pharmacokinetic Study
4.3. Measurement of 5,6-DiHETE with LC/MS
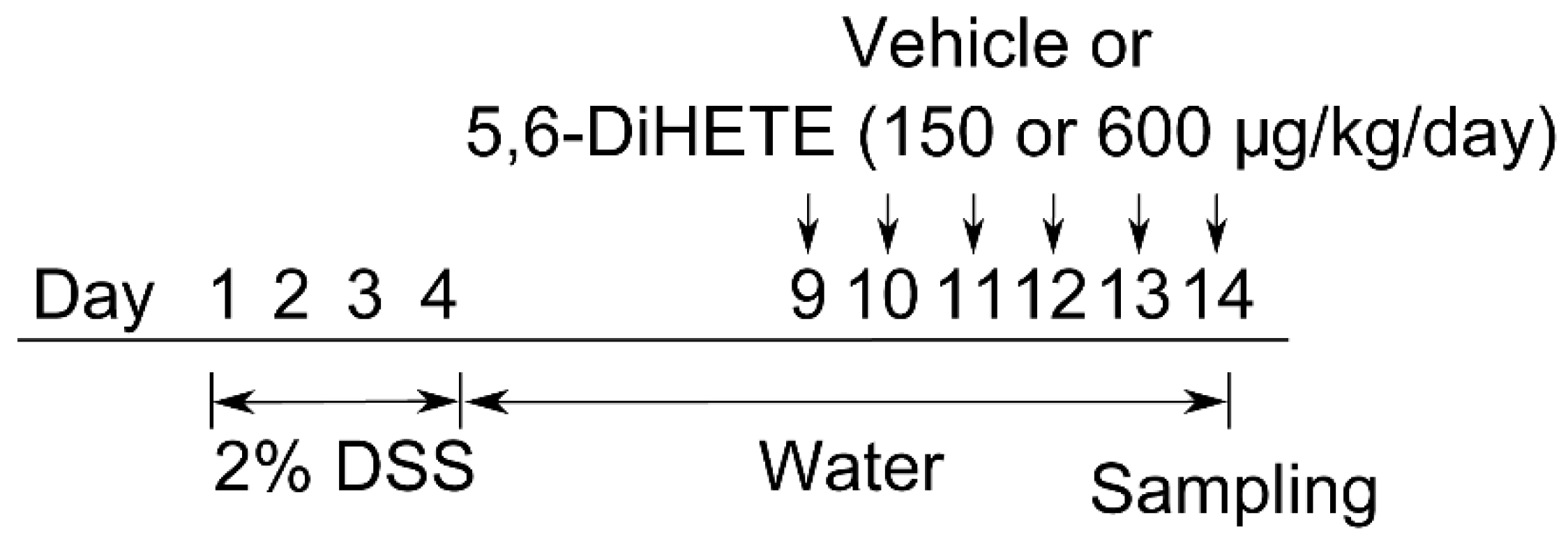
4.4. Colitis Models and 5,6-DiHETE Administration
4.5. Evaluation of Faecal Condition
4.6. Histological Assessment
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Schreiber, S. Diagnostics of Inflammatory Bowel Disease. Gastroenterology 2007, 133, 1670–1689. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, P.; Colombel, J.-F.; Aboubakr, A.; Narula, N. Systematic review: Safety of mesalazine in ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 1597–1609. [Google Scholar] [CrossRef] [Green Version]
- Buchman, A.L. Side effects of cortico-steroid therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, N.; Bai, A. Clinical use and mechanisms of infliximab treatment on inflammatory bowel disease: A recent update. Biomed. Res. Int. 2013, 2013, 581631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, E.; Romano, B.; Iannotti, F.A.; Parisi, O.A.; D’Armiento, M.; Pignatiello, S.; Coretti, L.; Lucafò, M.; Venneri, T.; Stocco, G.; et al. The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol. Res. 2019, 149, 104464. [Google Scholar] [CrossRef]
- Da Silva, V.C.; De Araújo, A.A.; Araújo, D.F.D.S.; Lima, M.C.J.S.; Vasconcelos, R.C.; de Araújo Júnior, R.F.; Langasnner, S.M.Z.; Pedrosa, M.D.F.F.; De Medeiros, C.A.C.X.; Guerra, G.C.B. Intestinal Anti-Inflammatory Activity of the Aqueous Extract from Ipomoea asarifolia in DNBS-Induced Colitis in Rats. Int. J. Mol. Sci. 2018, 19, 4016. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Hanai, H.; Tozawa, K.; Aoshi, T.; Uchijima, M.; Nagata, T.; Koide, Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 2002, 123, 1912–1922. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.; Salomon, N.; Wu, J.C.Y.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.L.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449.e1. [Google Scholar] [CrossRef]
- Scaioli, E.; Sartini, A.; Bellanova, M.; Campieri, M.; Festi, D.; Bazzoli, F.; Belluzzi, A. Eicosapentaenoic Acid Reduces Fecal Levels of Calprotectin and Prevents Relapse in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1268–1275.e2. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Chi, S.G.; Chun, H.S. Oral administration of docosahexaenoic acid attenuates colitis induced by dextran sulfate sodium in mice. Mol. Nutr. Food Res. 2011, 55, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Blier, P.U.; Fortin, S. MAG-EPA reduces severity of DSS-induced colitis in rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G808–G821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, S.J.; Naylor, S.; Woolner, J.; Hunter, J.O. A double-blind, randomized, placebo-controlled trial of essential fatty acid supplementation in the maintenance of remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2002, 16, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, H.L.; McCaskey, S.J.; Duriancik, D.M.; Clinthorne, J.F.; Langohr, I.M.; Gardner, E.M.; Fenton, J.I. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010, 70, 7960–7969. [Google Scholar] [CrossRef] [Green Version]
- Oliw, E.H.; Bylund, J.; Herman, C. Bisallylic hydroxylation and epoxidation of polyunsaturated fatty acids by cytochrome P450. Lipids 1996, 31, 1003–1021. [Google Scholar] [CrossRef]
- Hamabata, T.; Nakamura, T.; Masuko, S.; Maeda, S.; Murata, T. Production of lipid mediators across different disease stages of dextran sodium sulfate-induced colitis in mice. J. Lipid Res. 2018, 59, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Ashina, K.; Derouiche, S.; Hamabata, T.; Nakamura, T.; Nagata, N.; Takenouchi, S.; Tominaga, M.; Murata, T. 5,6-dihydroxy-8Z,11Z,14Z,17Z-eicosatetraenoic acid accelerates the healing of colitis by inhibiting transient receptor potential vanilloid 4-mediated signaling. FASEB J. 2021, 35, e21238. [Google Scholar] [CrossRef]
- Hamabata, T.; Nakamura, T.; Tachibana, Y.; Horikami, D.; Murata, T. 5,6-DiHETE attenuates vascular hyperpermeability by inhibiting Ca2+ elevation in endothelial cells. J. Lipid Res. 2018, 59, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.; Steinhart, A.H.; Griffiths, A.M. Omega 3 fatty acids (fish oil) for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Griffiths, A.M.; Leder, O.; Turner, D. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aldebert, E.; Cenac, N.; Rousset, P.; Martin, L.; Rolland, C.; Chapman, K.; Selves, J.; Alric, L.; Vinel, J.; Vergnolle, N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 2011, 140, 275–285.e3. [Google Scholar] [CrossRef] [PubMed]
- Fichna, J.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; Malecka-Panas, E.; Janecka, A.; Krajewska, W.M.; Storr, M.A. Transient Receptor Potential Vanilloid 4 blockade protects against experimental colitis in mice: A new strategy for inflammatory bowel diseases treatment? Neurogastroenterol. Motil. 2012, 24, e557–e560. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamaba, R.; Inoue, K.; Utsumi, D.; Tsukahara, T.; Amagase, K.; Tominaga, M.; Kato, S. Transient receptor potential vanilloid 4 channel regulates vascular endothelial permeability during colonic inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2018, 175, 84–99. [Google Scholar] [CrossRef] [Green Version]
- Gajos, G.; Zalewski, J.; Rostoff, P.; Nessler, J.; Piwowarska, W.; Undas, A. Reduced thrombin formation and altered fibrin clot properties induced by polyunsaturated omega-3 fatty acids on top of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention (OMEGA-PCI Clot). Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.Z.S.; Jianguo, J.; Kunapuli, S.P. Molecular mechanism of thromboxane A2-induced platelet aggregation. Essential role for P2T(AC) and α(2A) receptors. J. Biol. Chem. 1999, 274, 29108–29114. [Google Scholar] [CrossRef] [Green Version]
- Nau, H. Species differences in pharmacokinetics and drug teratogenesis. Environ. Health Perspect. 1986, 70, 113–129. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M.F.; Fuss, I.; Kelsall, B.L.; Stüber, E.; Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995, 182, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kida, M.; Nakamura, T.; Murata, T. A novel eicosapentaenoic acid-derived anti-inflammatory lipid mediator 5,6-DiHETE is abundant in blue back fish intestines. J. Food Sci. 2020, 85, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.M.; Ali, M.A.M.; Churchill, D.N. Effect of n-3 fatty acids from fish oil on hemostasis, blood pressure, and lipid profile of dialysis patients. J. Am. Soc. Nephrol. 1992, 2, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.M.; Green, A.T.; Teare, J.P.; Jenkins, A.P.; Punchard, N.A.; Ainley, C.C.; H.Thompson, R.P. A randomized controlled study of evening primrose oil and fish oil in ulcerative colitis. Aliment. Pharmacol. Ther. 1993, 7, 159–166. [Google Scholar] [CrossRef]
- Salomon, P.; Kornbluth, A.A.; Janowitz, H.D.; Janowitz, H.D. Treatment of Ulcerative Colitis with Fish Oil n-3-w-Fatty Acid: An Open Trial. J. Clin. Gasfroenferol. 1990, 12, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Kruschewski, M.; Foitzik, T.; Perez-Canto´, A.; Canto´, C.; Hu¨botter, A.; Hu¨botter, H.; Buhr, H.J. Changes of Colonic Mucosal Microcirculation and Histology in Two Colitis Models An Experimental Study Using Intravital Microscopy and a New Histological Scoring System. Dig. Dis. Sci. 2001, 46, 2336–2343. [Google Scholar] [CrossRef]
| Groups | Mucosal Integrity (0~6) | Cell Infiltration (0~3) | Cryptitis (0~2) | Edema (0~1) | Total (0~12) |
|---|---|---|---|---|---|
| Naive | 0.4 ± 0.4 | 0.4 ± 0.2 | 0.0 ± 0.0 | 0.4 ± 0.2 | 1.2 ± 0.5 |
| DSS + Vehicle | 4.0 ± 0.5 ** | 2.6 ± 0.2 ** | 1.8 ± 0.1 *** | 0.8 ± 0.1 | 9.4 ± 0.6 *** |
| DSS + 5,6-DiHETE (150 μg/kg/day) | 3.0 ± 0.6 | 1.1 ± 0.1 ## | 1.3 ± 0.3 | 0.5 ± 0.2 | 5.9 ± 0.9 # |
| DSS + 5,6-DiHETE (600 μg/kg/day) | 3.1 ± 0.3 | 1.0 ± 0.0 ## | 0.6 ± 0.3 # | 0.4 ± 0.2 | 5.1 ± 0.6 # |
| Index | Criterion | Score |
|---|---|---|
| Mucosal integrity | Single cell death | 2 |
| Erosions | 4 | |
| Florid ulcerations | 6 | |
| Infiltration of granulocytes in the lamina propria mucosae | Number of cells at 400× magnification | |
| =1~5 | 1 | |
| =6~10 | 2 | |
| >10 | 3 | |
| Cryptitis (granulocytes in cryptal epithelium) | Inflammatory cells in crypts | 2 |
| Cryptal abscess | 1 | |
| Mucosal/Submucosal edema | Existing | 1 |
| Total score | 0~12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenouchi, S.; Imai, D.; Nakamura, T.; Murata, T. Efficient Attenuation of Dextran Sulfate Sodium-Induced Colitis by Oral Administration of 5,6-Dihydroxy-8Z,11Z,14Z,17Z-eicosatetraenoic Acid in Mice. Int. J. Mol. Sci. 2021, 22, 9295. https://doi.org/10.3390/ijms22179295
Takenouchi S, Imai D, Nakamura T, Murata T. Efficient Attenuation of Dextran Sulfate Sodium-Induced Colitis by Oral Administration of 5,6-Dihydroxy-8Z,11Z,14Z,17Z-eicosatetraenoic Acid in Mice. International Journal of Molecular Sciences. 2021; 22(17):9295. https://doi.org/10.3390/ijms22179295
Chicago/Turabian StyleTakenouchi, Shinya, Daiki Imai, Tatsuro Nakamura, and Takahisa Murata. 2021. "Efficient Attenuation of Dextran Sulfate Sodium-Induced Colitis by Oral Administration of 5,6-Dihydroxy-8Z,11Z,14Z,17Z-eicosatetraenoic Acid in Mice" International Journal of Molecular Sciences 22, no. 17: 9295. https://doi.org/10.3390/ijms22179295
APA StyleTakenouchi, S., Imai, D., Nakamura, T., & Murata, T. (2021). Efficient Attenuation of Dextran Sulfate Sodium-Induced Colitis by Oral Administration of 5,6-Dihydroxy-8Z,11Z,14Z,17Z-eicosatetraenoic Acid in Mice. International Journal of Molecular Sciences, 22(17), 9295. https://doi.org/10.3390/ijms22179295






