Salmonella Typhimurium and Pseudomonas aeruginosa Respond Differently to the Fe Chelator Deferiprone and to Some Novel Deferiprone Derivatives
Abstract
1. Introduction
2. Results
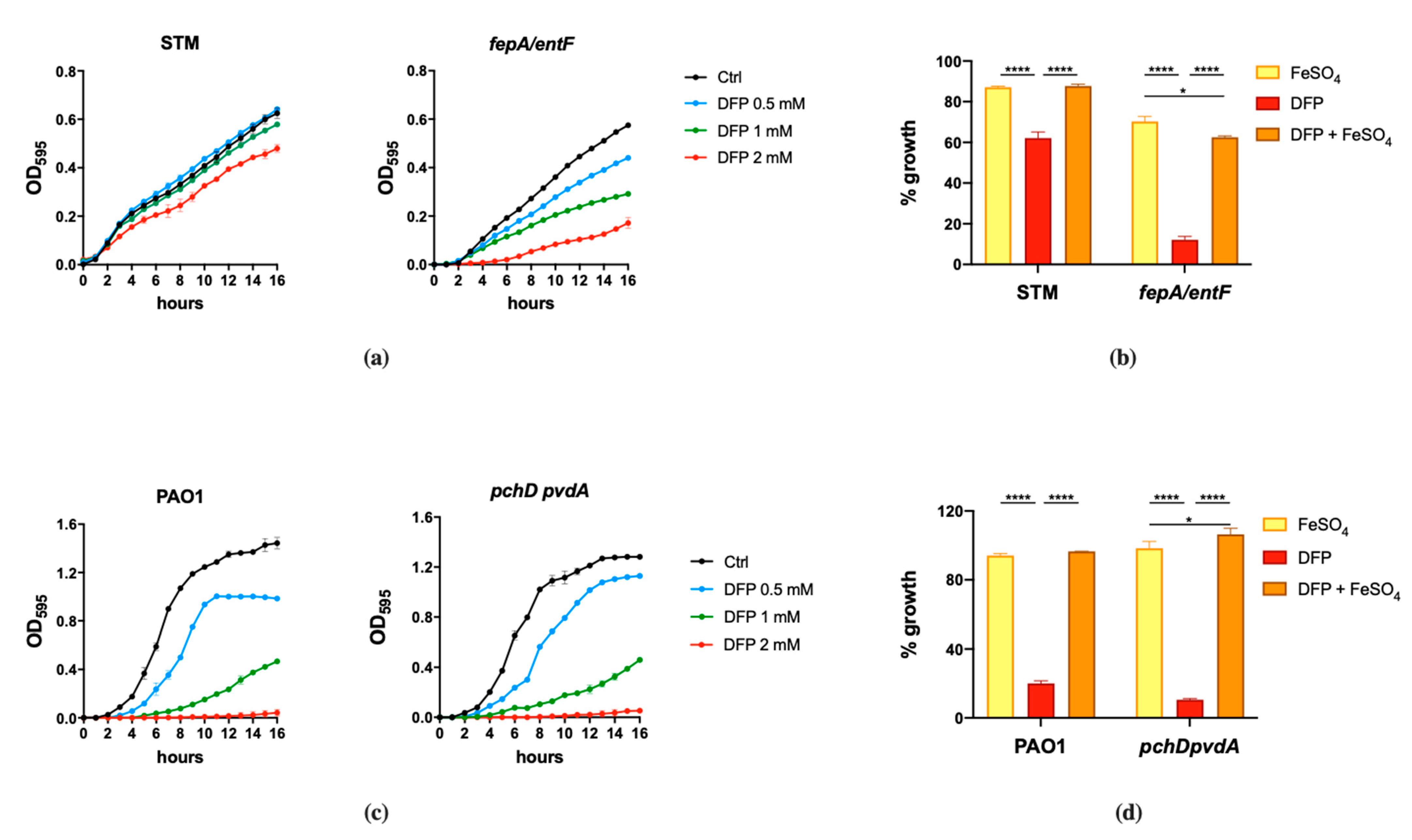
2.1. Salmonella Typhimurium and Pseudomonas aeruginosa Show Different Susceptibility to DFP
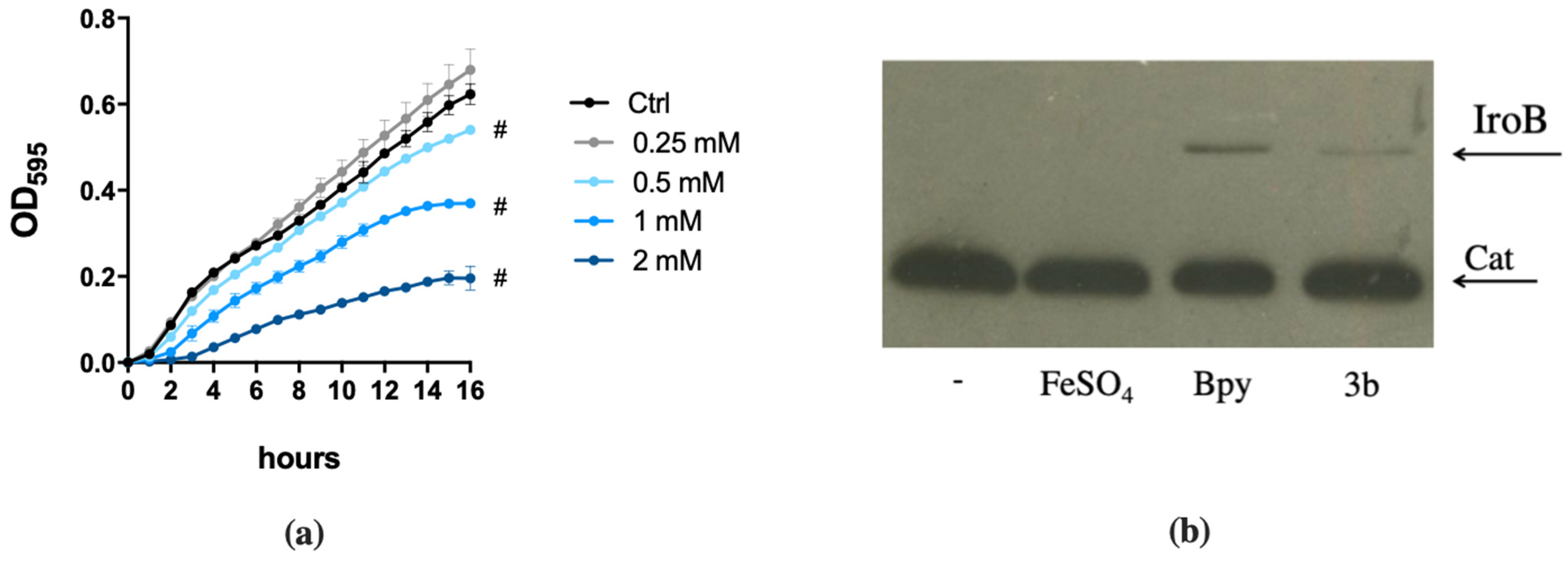
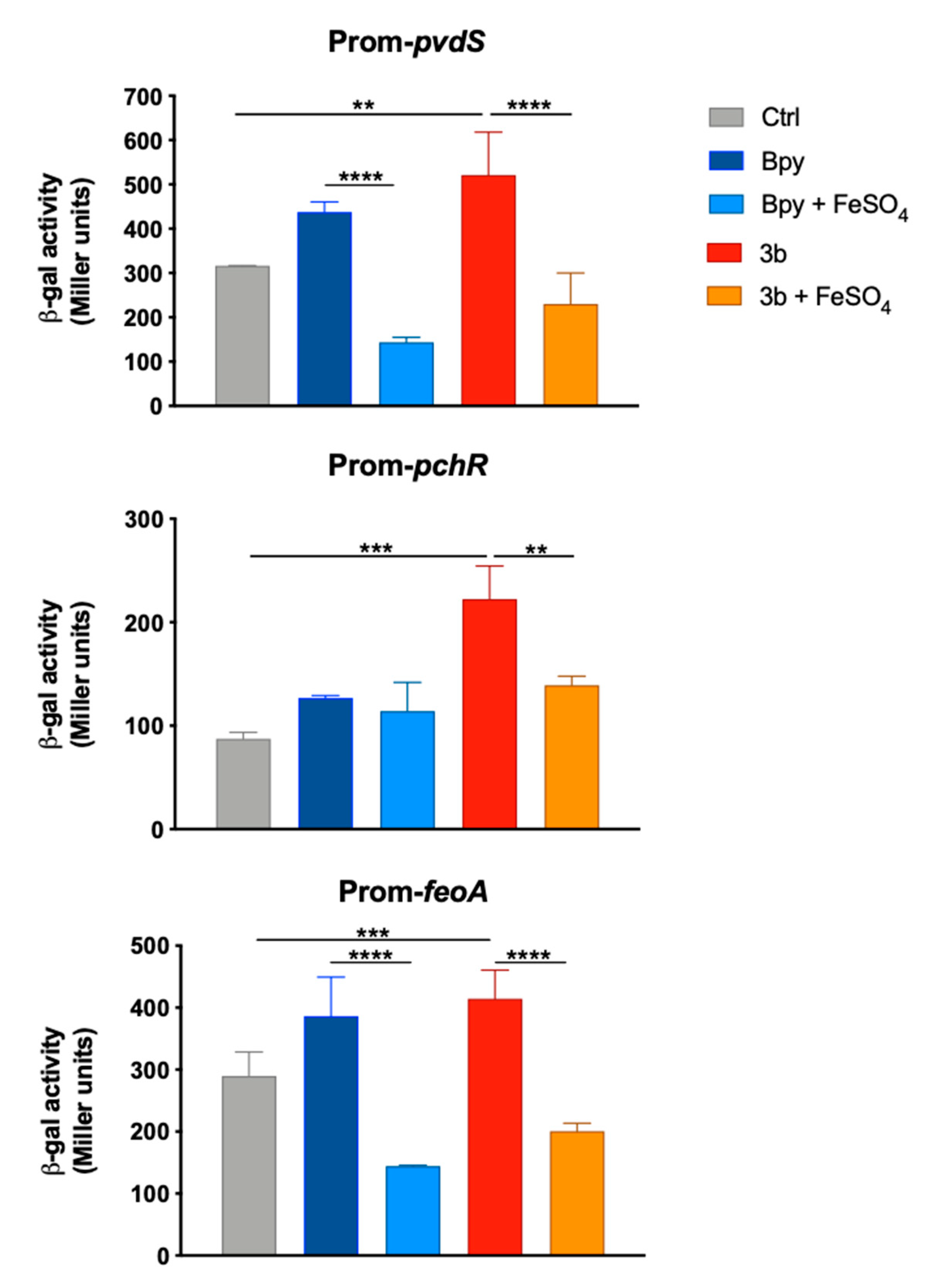
2.2. DFP Differently Modulates the Expression of Fe-Dependent Genes in P. aeruginosa and in S. Typhimurium
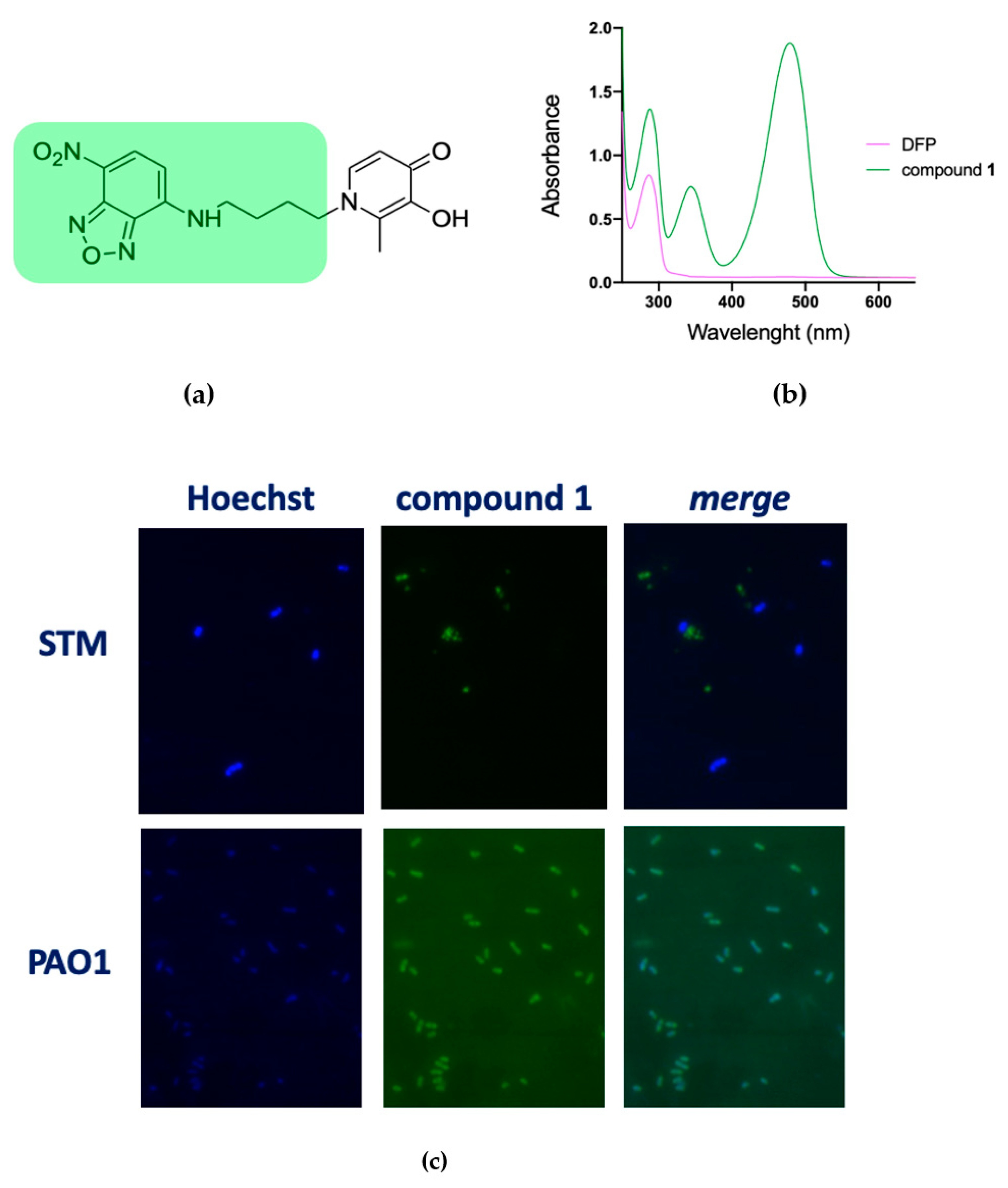
2.3. DFP Can Easily Penetrate in P. aeruginosa, but Not in S. Typhimurium
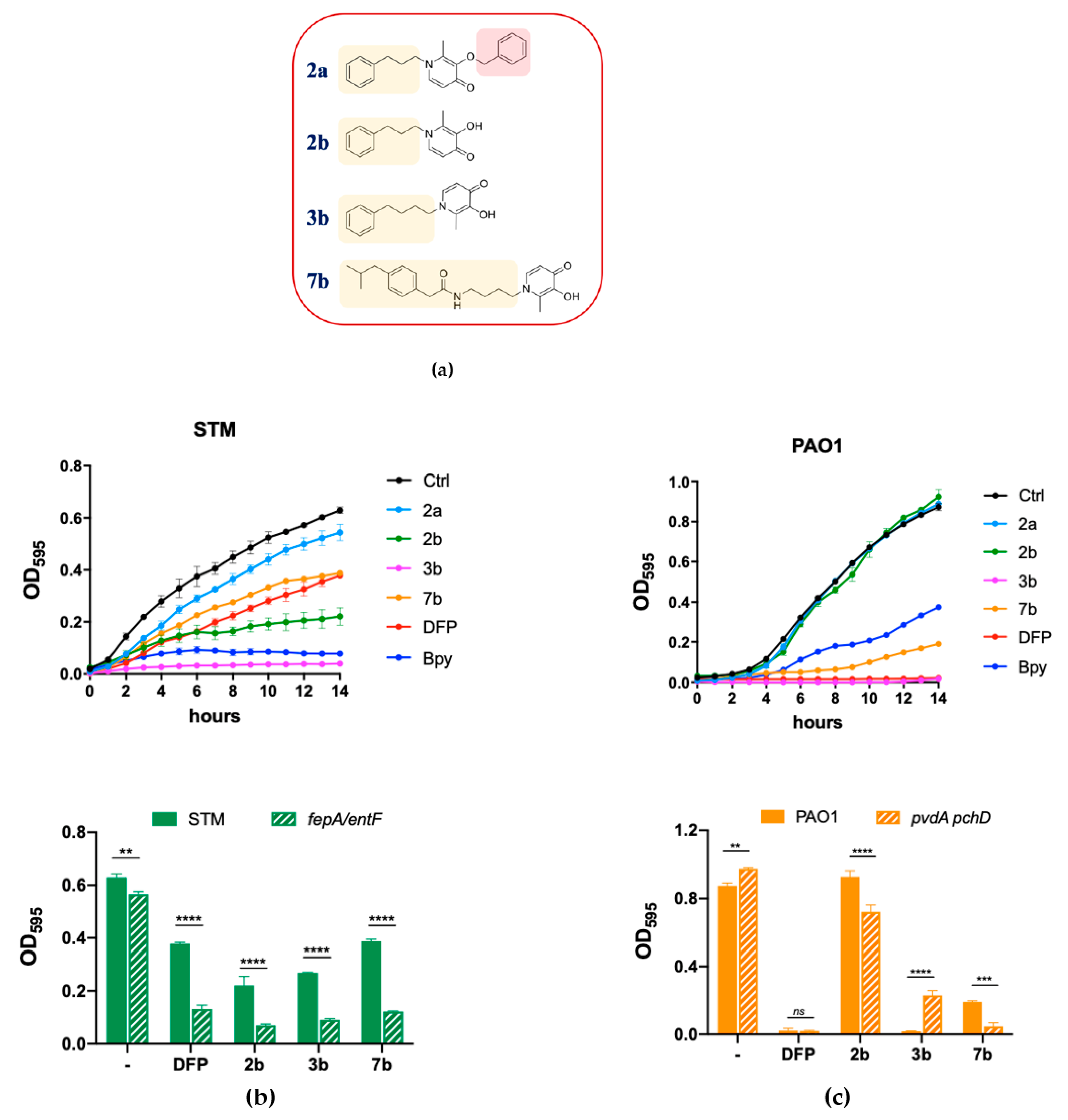
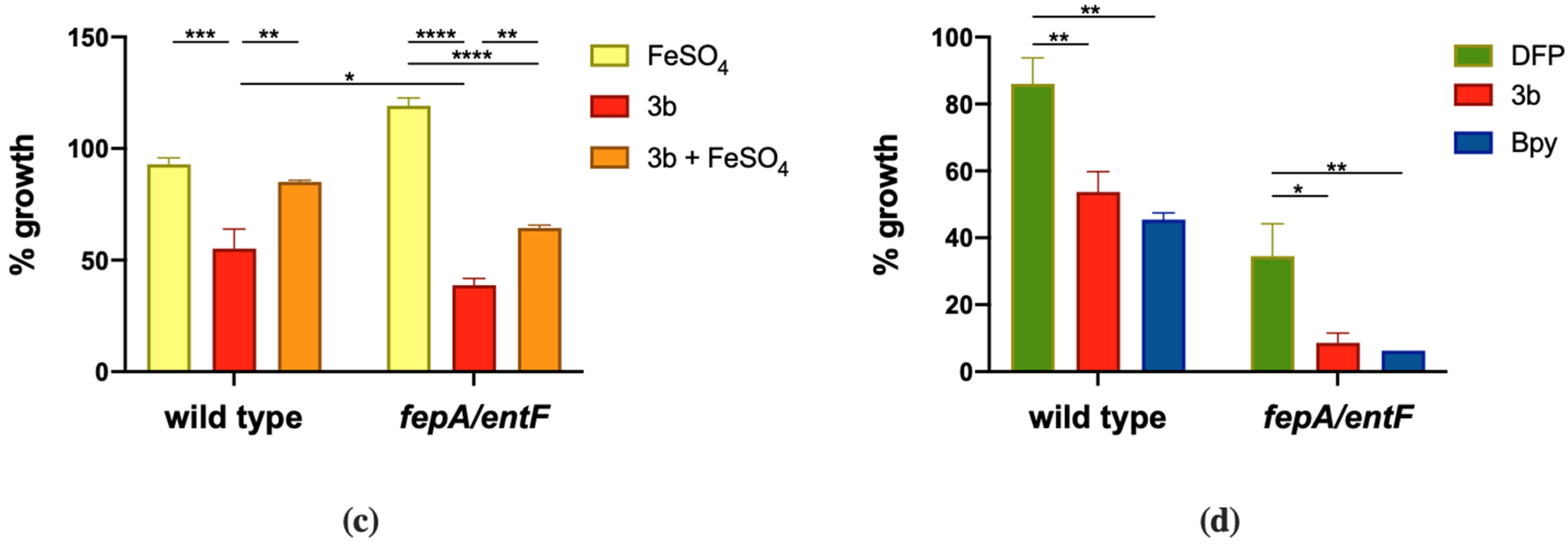
2.4. Antimicrobial Activity of DFP Derivatives
2.5. Detailed Analysis of Selected Compounds
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Media and Chemicals
4.2. DFP, Derivatives and Fluorescent Compounds
4.3. Analyses of Bacterial Growth
4.4. pchR, pvdS and feoA Promoters’ Activity Assay
4.5. SDS-PAGE, Western Blotting and Immunodetection
4.6. Fluorescence Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef] [PubMed]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta Bioenerg. 1999, 1413, 99–107. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Núñez, G.; Sakamoto, K.; Soares, M.P. Innate nutritional immunity. J. Immunol. 2018, 201, 11–18. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Lau, C.K.Y.; Krewulak, K.D.; Vogel, H.J. Bacterial ferrous iron transport: The feo system. FEMS Microbiol. Rev. 2016, 40, 273–298. [Google Scholar] [CrossRef]
- Velayudhan, J.; Hughes, N.J.; McColm, A.A.; Bagshaw, J.; Clayton, C.L.; Andrews, S.C.; Kelly, D.J. Iron acquisition and virulence in Helicobacter pylori: A major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 2000, 37, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Robey, M.; Cianciotto, N.P. Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect. Immun. 2002, 70, 5659–5669. [Google Scholar] [CrossRef] [PubMed]
- Pérez, N.; Johnson, R.; Sen, B.; Ramakrishnan, G. Two parallel pathways for ferric and ferrous iron acquisition support growth and virulence of the intracellular pathogen Francisella tularensis Schu S4. Microbiologyopen 2016, 5, 453–468. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Dale, S.E.; Doherty-Kirby, A.; Lajoie, G.; Heinrichs, D.E. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: Identification and characterization of genes involved in production of a siderophore. Infect. Immun. 2004, 72, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Olson, R.; MacDonald, U.; Metzger, D.; Maltese, L.M.; Drake, E.J.; Gulick, A.M. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect. Immun. 2014, 82, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Jones, S.A.; Mercer, L.E.; Meador, J.P.; Caughron, J.E.; Jordan, L.; Newton, S.M.; Conway, T.; Klebba, P.E. Role of catecholate siderophores in gram-negative bacterial colonization of the mouse gut. PLoS ONE 2012, 7, e50020. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Nitanai, H.; Hoshino, K.; Otani, T. Requirement of the Pseudomonas aeruginosa tonB gene for high-affinity iron acquisition and infection. Infect. Immun. 2000, 68, 4498–4504. [Google Scholar] [CrossRef]
- Runci, F.; Gentile, V.; Frangipani, E.; Rampioni, G.; Leoni, L.; Lucidi, M.; Visaggio, D.; Harris, G.; Chen, W.; Stahl, J.; et al. Contribution of active iron uptake to Acinetobacter baumannii pathogenicity. Infect Immun. 2019, 87, e00755-18. [Google Scholar] [CrossRef]
- Batista, B.B.; de Sousa Santos, R.E.R.; Ricci-Azevedo, R.; da Silva Neto, J.F. Production and uptake of distinct endogenous catecholate-type siderophores are required for iron acquisition and virulence in Chromobacterium violaceum. Infect Immun. 2019, 87, e00577-19. [Google Scholar] [CrossRef]
- Vinuesa, V.; McConnell, M.J. Recent Advances in iron chelation and gallium-based therapies for antibiotic resistant bacterial infections. Int. J. Mol. Sci. 2021, 22, 2876. [Google Scholar] [CrossRef]
- Visca, P.; Bonchi, C.; Minandri, F.; Frangipani, E.; Imperi, F. The dual personality of iron chelators: Growth inhibitors or promoters? Antimicrob. Agents Chemother. 2013, 57, 2432–2433. [Google Scholar] [CrossRef]
- Marsella, M.; Borgna-Pignatti, C. Transfusional iron overload and iron chelation therapy in Thalassemia major and sickle cell disease. Hematol. Oncol. Clin. N. Am. 2014, 28, 703–727. [Google Scholar] [CrossRef]
- Nikseresht, S.; Bush, A.I.; Ayton, S. Treating Alzheimer’s disease by targeting iron. Br. J. Pharmacol. 2019, 176, 3622–3635. [Google Scholar] [CrossRef]
- Lesic, B.; Foulon, J.; Carniel, E. Comparison of the effects of deferiprone versus deferoxamine on growth and virulence of Yersinia enterocolitica. Antimicrob. Agents Chemother. 2002, 46, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Corey, B.W.; Si, Y.; Craft, D.W.; Zurawski, D.V. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob. Agents Chemother. 2012, 56, 5419–5421. [Google Scholar] [CrossRef] [PubMed]
- Neupane, G.P.; Kim, D.M. Comparison of the effects of deferasirox, deferiprone, and deferoxamine on the growth and virulence of Vibrio vulnificus. Transfusion 2009, 49, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Thomas, N.; Zhang, G.; Prestidge, C.A.; Coenye, T.; Wormald, P.-J.; Vreugde, S. Deferiprone and gallium-protoporphyrin have the capacity to potentiate the activity of antibiotics in Staphylococcus aureus small colony variants. Front. Cell. Infect. Microbiol. 2017, 7, 280. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Dichtl, S.; Steixner, S.; Nogler, M.; Weiss, G. Iron chelation destabilizes bacterial biofilms and potentiates the antimicrobial activity of antibiotics against coagulase-negative staphylococci. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- Houshmandyar, S.; Eggleston, I.M.; Bolhuis, A. Biofilm-specific uptake of a 4-pyridone-based iron chelator by Pseudomonas aeruginosa. BioMetals 2021, 34, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Bortolami, M.; Pandolfi, F.; Messore, A.; Rocco, D.; Feroci, M.; Di Santo, R.; De Vita, D.; Costi, R.; Cascarino, P.; Simonetti, G.; et al. Design, synthesis and biological evaluation of a series of iron and copper chelating deferiprone derivatives as new agents active against candida albicans. Bioorganic Med. Chem. Lett. 2021, 42, 128087. [Google Scholar] [CrossRef] [PubMed]
- Saccoliti, F.; Madia, V.M.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Design, synthesis, and biological evaluation of New1-(Aryl-1H-pyrrolyl) (phenyl) methyl-1H-imidazole derivatives as antiprotozoal agents. J. Med. Chem. 2019, 62, 1330–1347. [Google Scholar] [CrossRef]
- Ammendola, S.; Cerasi, M.; Battistoni, A. Deregulation of transition metals homeostasis is a key feature of cadmium toxicity in Salmonella. BioMetals 2014, 27, 703–714. [Google Scholar] [CrossRef][Green Version]
- Ammendola, S.; Lembo, A.; Battistoni, A.; Tagliatesta, P.; Ghisalberti, C.; Desideri, A. 10-Undecanhydroxamic acid, a hydroxamate derivative of the undecanoic acid, has strong antimicrobial activity through a mechanism that limits iron availability. FEMS Microbiol. Lett. 2009, 294, 61–67. [Google Scholar] [CrossRef]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and chelation in biochemistry and medicine: New approaches to controlling iron metabolism and treating related diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef]
- Albrecht-Gary, A.M.; Blanc, S.; Rochel, N.; Ocacktan, A.Z.; Abdallah, M.A. Bacterial iron transport: Coordination properties of pyoverdin PaA, a peptidic siderophore of Pseudomonas aeruginosa. Inorg. Chem. 1994, 33, 6391–6402. [Google Scholar] [CrossRef]
- Brandel, J.; Humbert, N.; Elhabiri, M.; Schalk, I.J.; Mislin, G.L.A.; Albrecht-Gary, A.-M. Pyochelin, a siderophore of Pseudomonas aeruginosa: Physicochemical characterization of the iron(iii), copper(ii) and zinc(ii) complexes. Dalton Trans. 2012, 41, 2820–2834. [Google Scholar] [CrossRef]
- Schlegel, K.; Lex, J.; Taraz, K.; Budzikiewicz, H. The X-ray structure of the pyochelin Fe3+ complex. Z. Naturforsch. C 2006, 61, 263–266. [Google Scholar] [CrossRef]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell. Infect. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Ammendola, S.; Ciavardelli, D.; Consalvo, A.; Battistoni, A. Cobalt can fully recover the phenotypes related to zinc deficiency in: Salmonella typhimurium. Metallomics 2020, 12, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Bortolami, M.; Pandolfi, F.; De Vita, D.; Carafa, C.; Messore, A.; Di Santo, R.; Feroci, M.; Costi, R.; Chiarotto, I.; Bagetta, D.; et al. New deferiprone derivatives as multi-functional cholinesterase inhibitors: Design, synthesis and in vitro evaluation. Eur. J. Med. Chem. 2020, 198, 112350. [Google Scholar] [CrossRef]
- D’Orazio, M.; Mastropasqua, M.C.; Cerasi, M.; Pacello, F.; Consalvo, A.; Chirullo, B.; Mortensen, B.; Skaar, E.P.; Ciavardelli, D.; Pasquali, P.; et al. The capability of Pseudomonas aeruginosa to recruit zinc under conditions of limited metal availability is affected by inactivation of the ZnuABC transporter. Metallomics 2015, 7, 1023–1035. [Google Scholar] [CrossRef]
- Miller, J. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1972. [Google Scholar]
- Ammendola, S.; Pasquali, P.; Pistoia, C.; Petrucci, P.; Petrarca, P.; Rotilio, G.; Battistoni, A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect. Immun. 2007, 75, 5867–5876. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammendola, S.; Secli, V.; Pacello, F.; Bortolami, M.; Pandolfi, F.; Messore, A.; Di Santo, R.; Scipione, L.; Battistoni, A. Salmonella Typhimurium and Pseudomonas aeruginosa Respond Differently to the Fe Chelator Deferiprone and to Some Novel Deferiprone Derivatives. Int. J. Mol. Sci. 2021, 22, 10217. https://doi.org/10.3390/ijms221910217
Ammendola S, Secli V, Pacello F, Bortolami M, Pandolfi F, Messore A, Di Santo R, Scipione L, Battistoni A. Salmonella Typhimurium and Pseudomonas aeruginosa Respond Differently to the Fe Chelator Deferiprone and to Some Novel Deferiprone Derivatives. International Journal of Molecular Sciences. 2021; 22(19):10217. https://doi.org/10.3390/ijms221910217
Chicago/Turabian StyleAmmendola, Serena, Valerio Secli, Francesca Pacello, Martina Bortolami, Fabiana Pandolfi, Antonella Messore, Roberto Di Santo, Luigi Scipione, and Andrea Battistoni. 2021. "Salmonella Typhimurium and Pseudomonas aeruginosa Respond Differently to the Fe Chelator Deferiprone and to Some Novel Deferiprone Derivatives" International Journal of Molecular Sciences 22, no. 19: 10217. https://doi.org/10.3390/ijms221910217
APA StyleAmmendola, S., Secli, V., Pacello, F., Bortolami, M., Pandolfi, F., Messore, A., Di Santo, R., Scipione, L., & Battistoni, A. (2021). Salmonella Typhimurium and Pseudomonas aeruginosa Respond Differently to the Fe Chelator Deferiprone and to Some Novel Deferiprone Derivatives. International Journal of Molecular Sciences, 22(19), 10217. https://doi.org/10.3390/ijms221910217









