Self-Assembly Nanoparticles of Natural Bioactive Abietane Diterpenes
Abstract
:1. Introduction
2. Results and Discussion
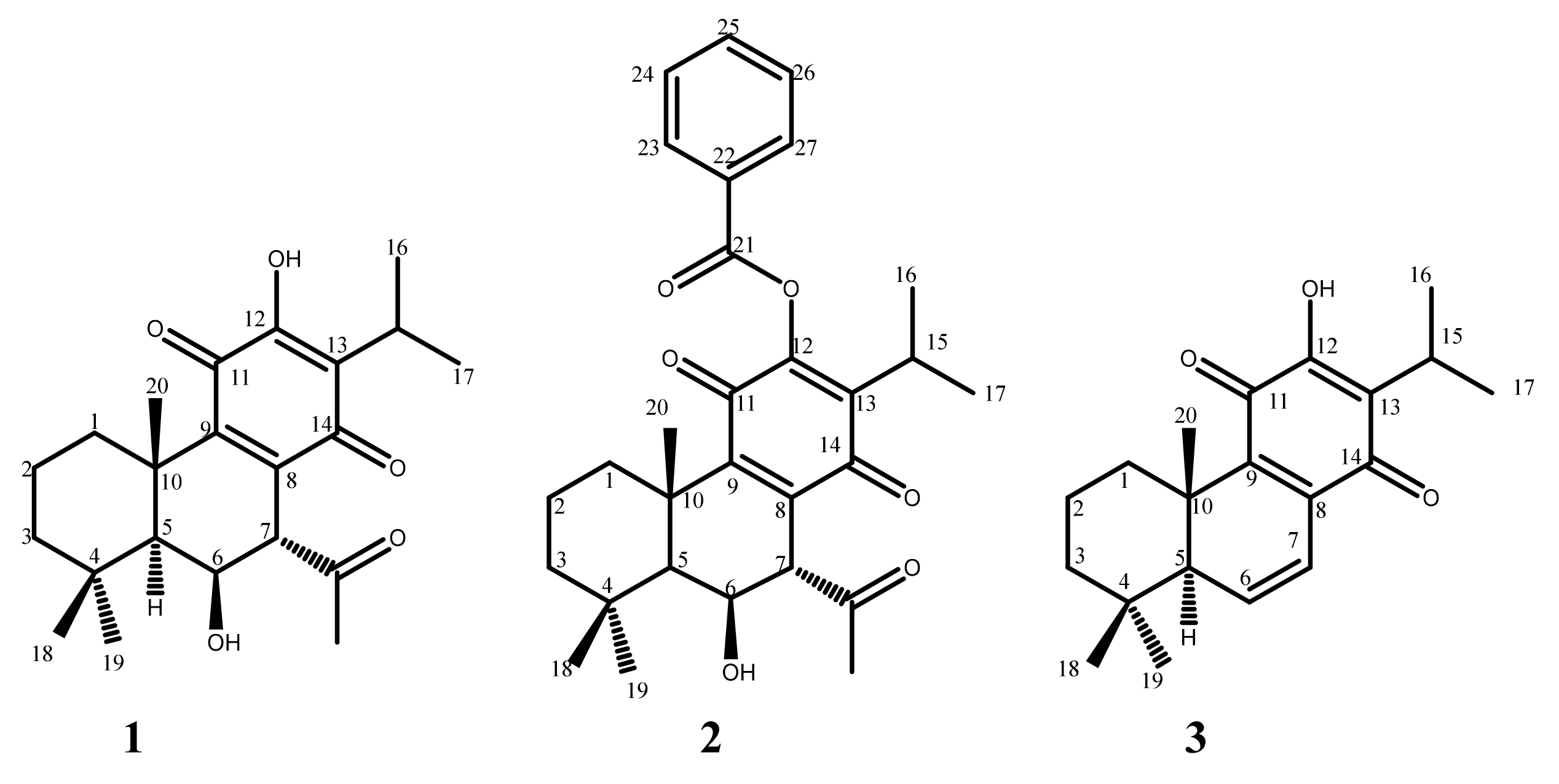
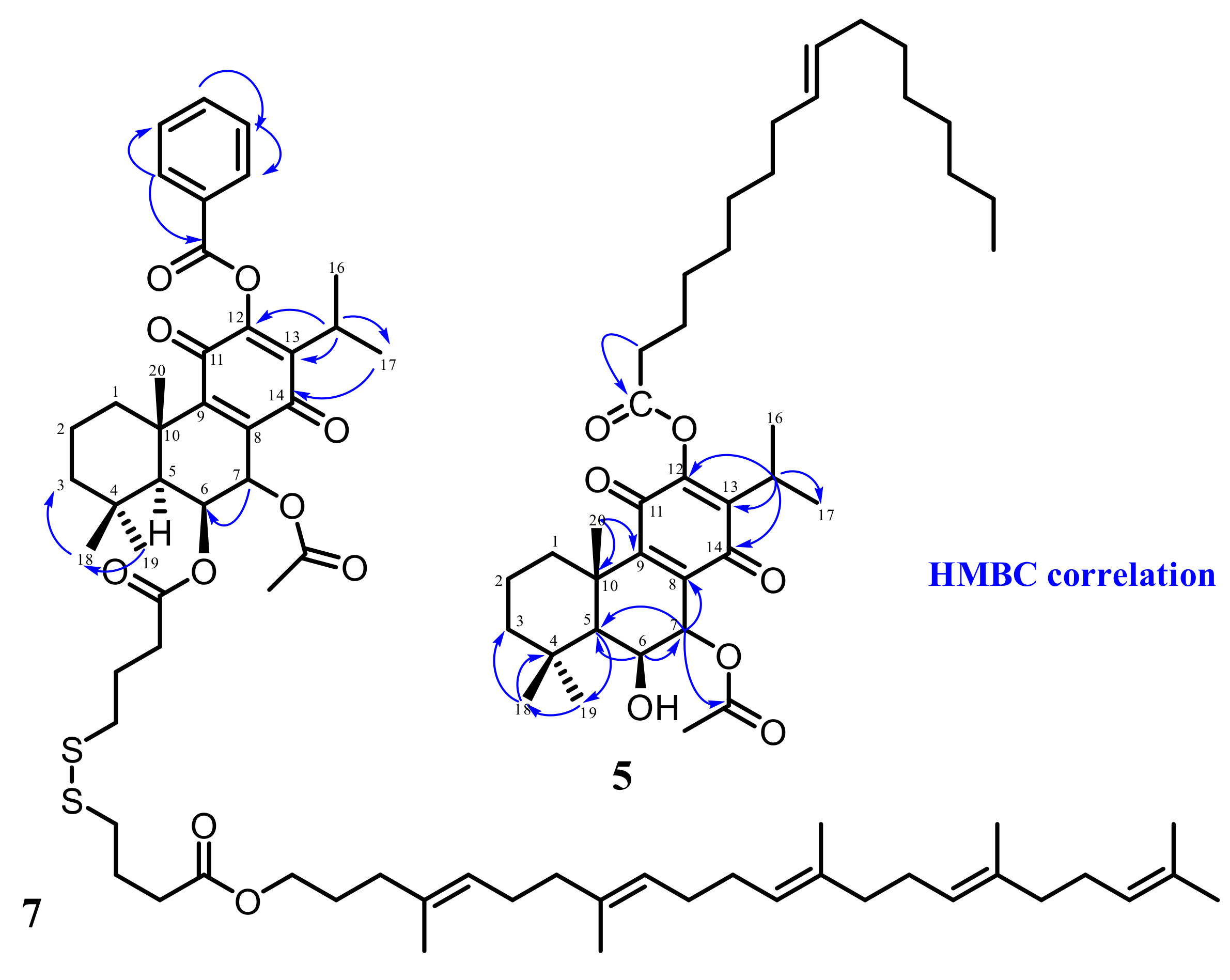
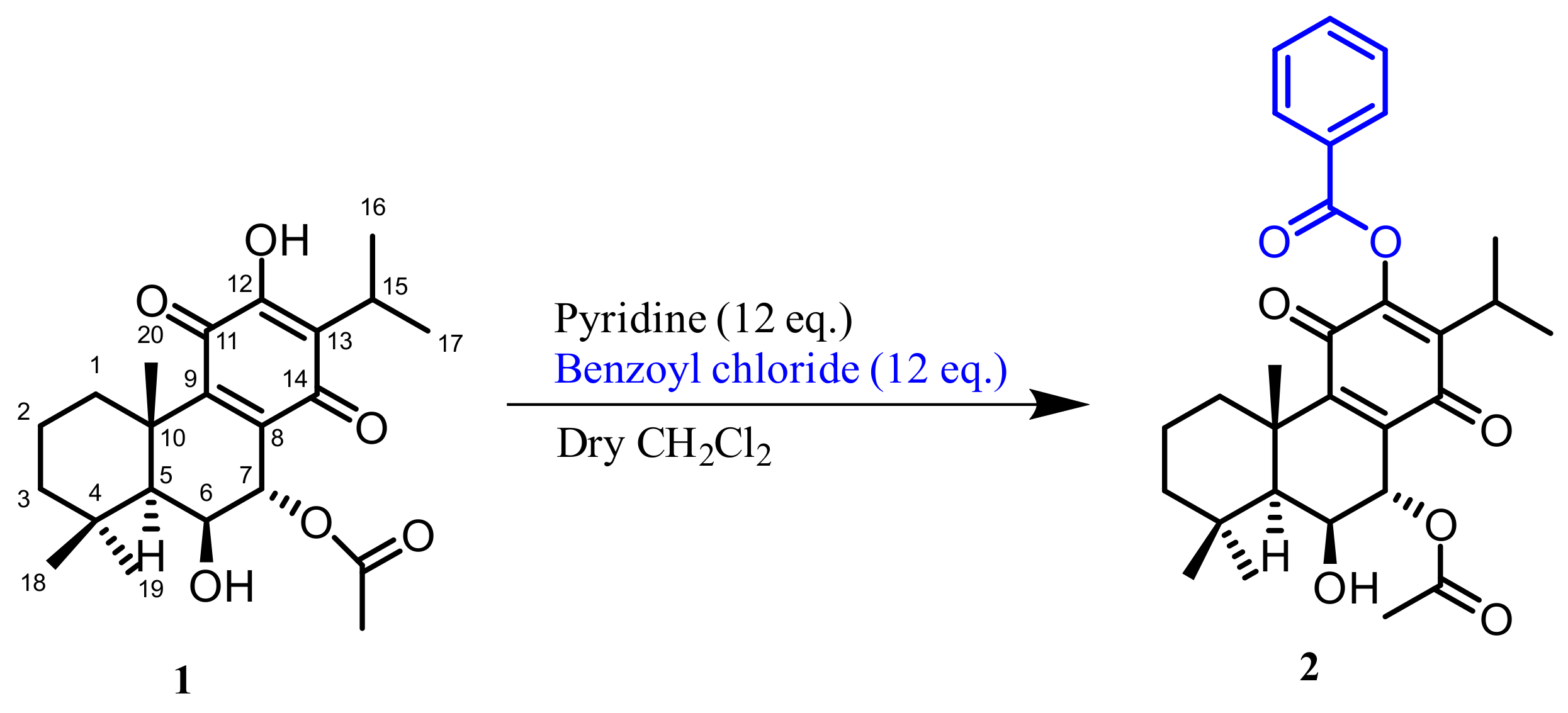
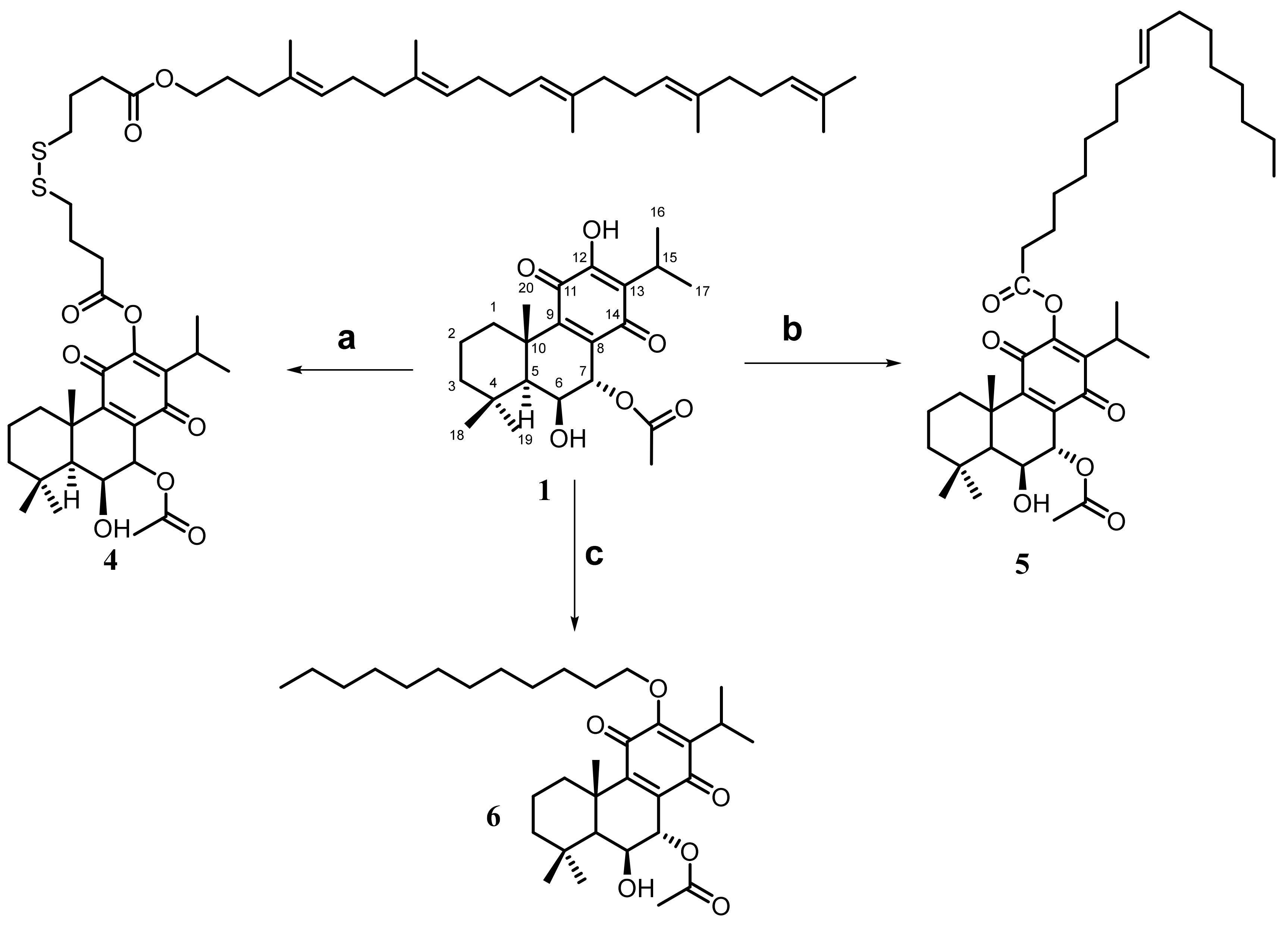
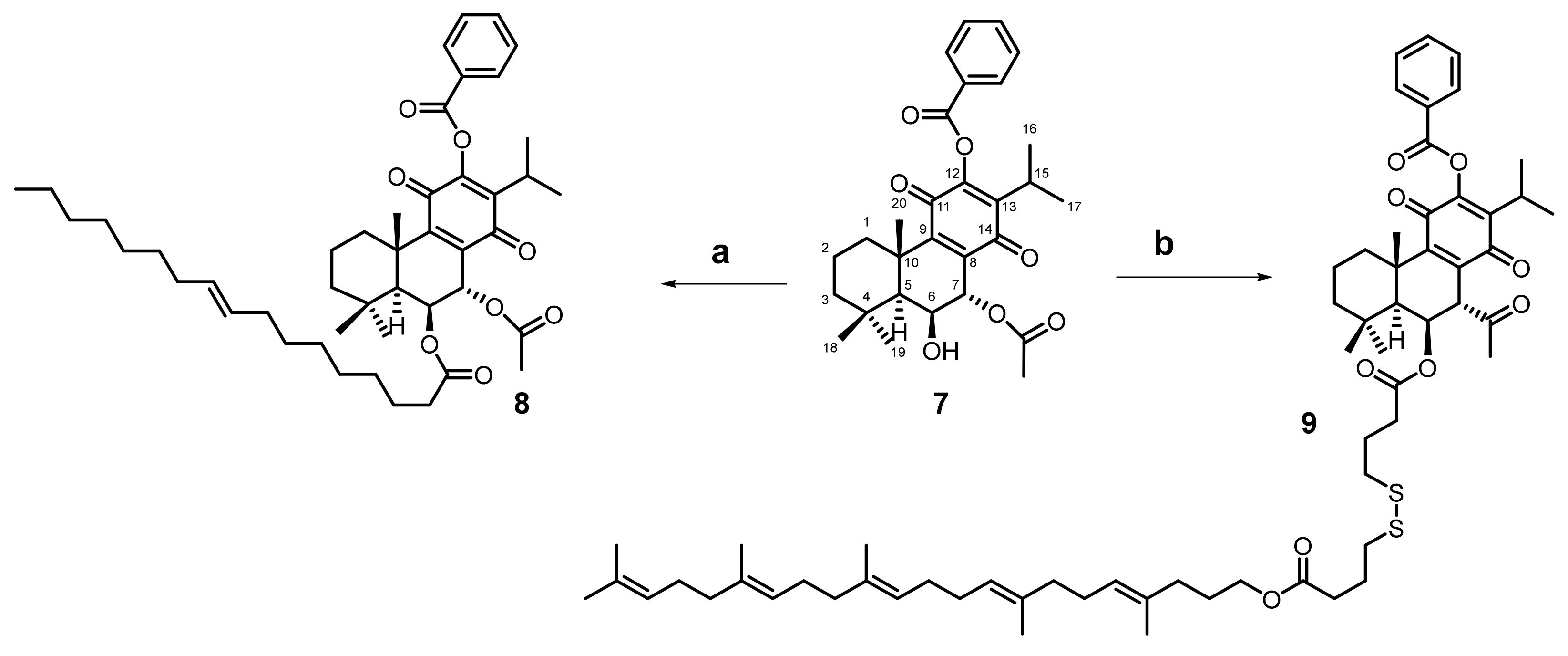
2.1. Hemisynthesis of Self-Assembly Nanoparticles Conjugates
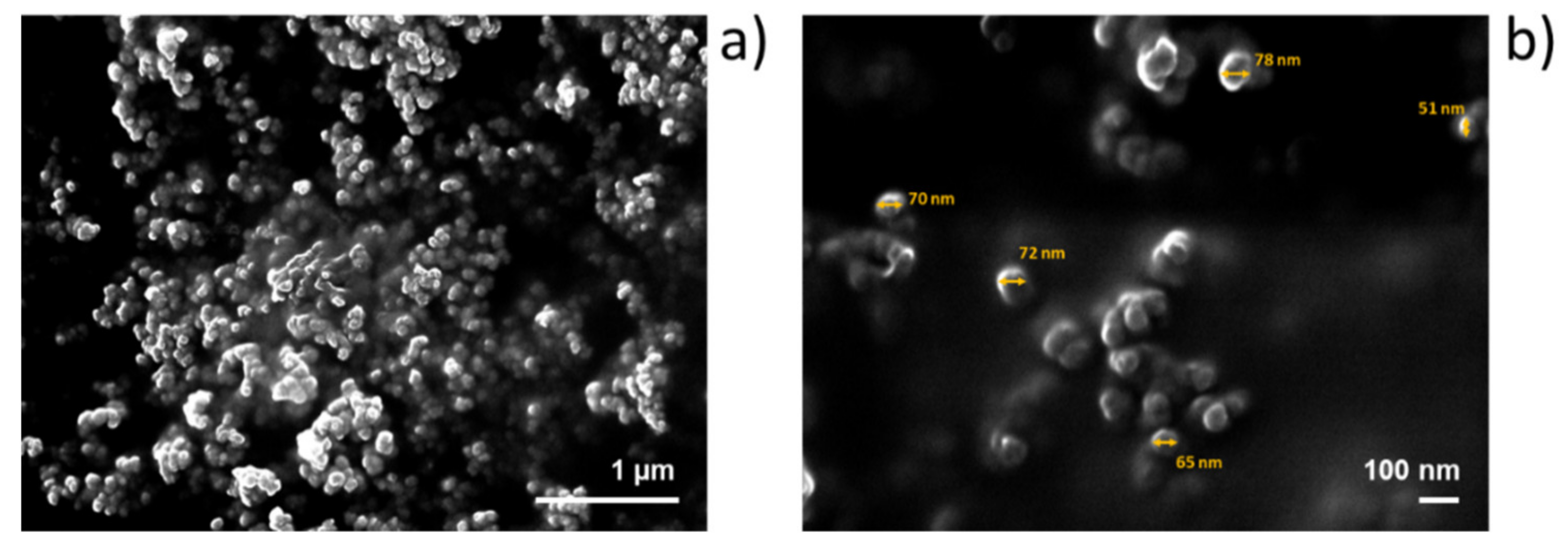
2.2. Nanoassemblies: Preparation and Characterization
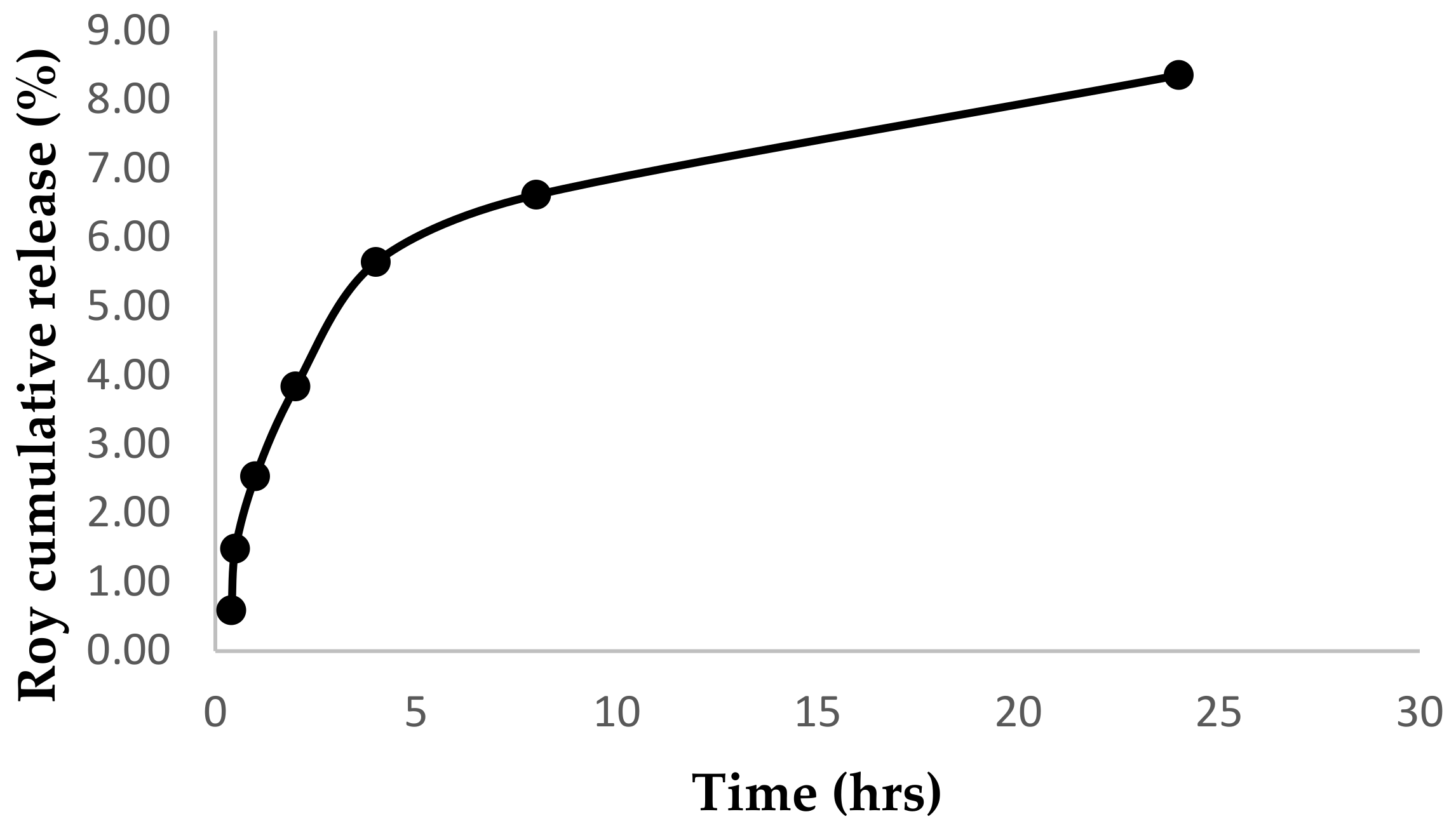
2.3. In Vitro Release Studies
2.4. Biological Activity Study
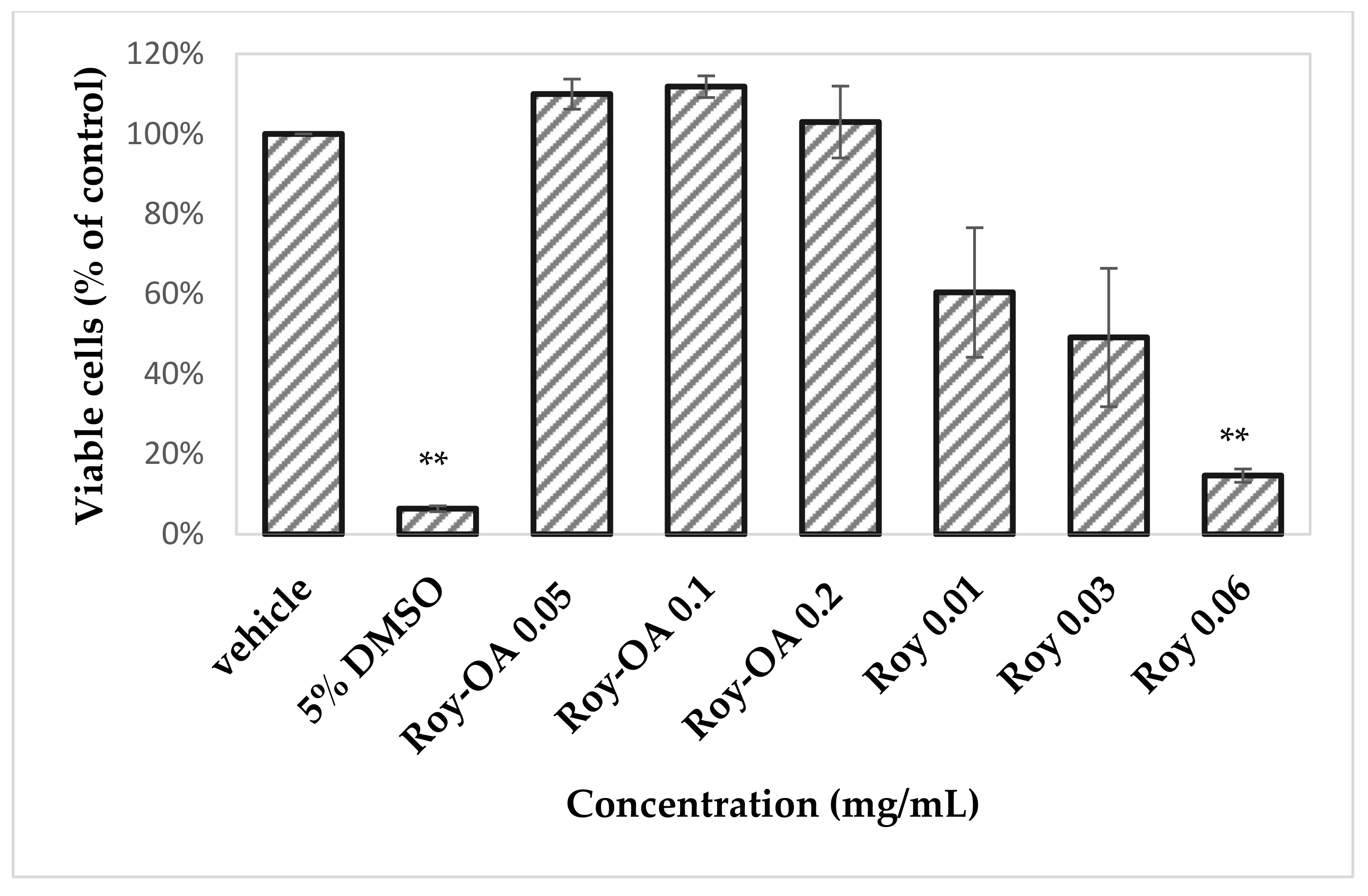
2.4.1. Preliminary Toxicity Assay
2.4.2. Cytotoxicity Study
3. Materials and Methods
3.1. Plant Material
3.2. Reaction Procedure
3.3. Hemisynthesis of Drug Conjugates for Self-Assembly Nanoparticles
3.3.1. Synthesis of Drug Conjugates Using Squalene as a Self-Assembly Inducer [7]
3.3.2. Synthesis of Drug Conjugates Using Oleic Acid as a Self-Assembly Inducer [6]
3.3.3. Synthesis of Roy-Dodecane Conjugate Using 1-Bromododecane as Self-Assembly Inducer [14]
3.4. Characterization of Synthesized Molecules
3.4.1. 12BzRoy-Squalene Conjugate
3.4.2. Roy-Oleic Acid Conjugate
3.5. Preparation of Self-Assembled Nanoparticles
3.6. Physical-Chemical and Morphological Characterization of Self-Assembly Nanoparticles
3.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.6.2. Dynamic Light Scattering (DLS)
3.6.3. Scanning Electron Microscopy (SEM) Analysis
3.7. In Vitro Release Studies
3.8. Preliminary Toxicity Assay
3.9. Cytotoxicity Study
3.9.1. Cell Culture
3.9.2. Cell Viability
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, R.; Xu, Z.P. Nanoparticle-Based Nanomedicines to Promote Cancer Immunotherapy: Recent Advances and Future Directions. Small 2019, 15, 1–21. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Althubiti, M.A. Cancer Nanomedicine: A New Era of Successful Targeted Therapy. J. Nanomater. 2019, 2019, 4927312. [Google Scholar] [CrossRef] [Green Version]
- Sim, T.; Lim, C.; Hoang, N.H.; Joo, H.; Lee, J.W.; Kim, D.W.; Lee, E.S.; Youn, Y.S.; Kim, J.O.; Oh, K.T. Nanomedicines for oral administration based on diverse nanoplatform. J. Pharm. Investig. 2016, 46, 351–362. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Wang, L.; Guo, T.; Wu, J.; Wan, J.; Zhou, L.; Li, H.; Li, Z.; Jiang, D.; et al. New generation nanomedicines constructed from self-assembling small-molecule prodrugs alleviate cancer drug toxicity. Cancer Res. 2017, 77, 6963–6974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, T.; Yao, X.; Zhang, S.; Guo, Y.; Duan, X.C.; Ren, W.; Dan, H.; Yin, Y.F.; Zhang, X. A self-assembling nanomedicine of conjugated linoleic acid-paclitaxel conjugate (CLA-PTX) with higher drug loading and carrier-free characteristic. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Fumagalli, G.; Christodoulou, M.S.; Riva, B.; Revuelta, I.; Marucci, C.; Collico, V.; Prosperi, D.; Riva, S.; Perdicchia, D.; Bassanini, I.; et al. Self-assembled 4-(1,2-diphenylbut-1-en-1-yl)aniline based nanoparticles: Podophyllotoxin and aloin as building blocks. Org. Biomol. Chem. 2017, 15, 1106–1109. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Zhang, T.; Shu, Y.; He, Z.; Ma, Y.; Liu, D.; Wang, Y. Redox-sensitive prodrug nanoassemblies based on linoleic acid-modified docetaxel to resist breast cancers. Acta Pharm. Sin. B 2019, 9, 421–432. [Google Scholar] [CrossRef]
- Borrelli, S.; Christodoulou, M.S.; Ficarra, I.; Silvani, A.; Cappelletti, G.; Cartelli, D.; Damia, G.; Ricci, F.; Zucchetti, M.; Dosio, F.; et al. New class of squalene-based releasable nanoassemblies of paclitaxel, podophyllotoxin, camptothecin and epothilone A. Eur. J. Med. Chem. 2014, 85, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. Linkers: The key elements for the creation of efficient nanotherapeutics. J. Control. Release 2018, 270, 260–267. [Google Scholar] [CrossRef]
- Garcia, C.; Isca, V.M.S.; Pereira, F.; Monteiro, C.M.; Ntungwe, E.; Sousa, F.; Dinic, J.; Holmstedt, S.; Roberto, A.; Díaz-Lanza, A.; et al. Royleanone Derivatives From Plectranthus spp. as a Novel Class of P-Glycoprotein Inhibitors. Front. Pharmacol. 2020, 11, 1711. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Vladimir, S.; Bakandritsos, A. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Dosio, F.; Reddy, L.H.; Ferrero, A.; Stella, B.; Cattel, L.; Couvreur, P. Novel nanoassemblies composed of squalenoyl-paclitaxel derivatives: Synthesis, characterization, and biological evaluation. Bioconj. Chem. 2010, 21, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Bisi, A.; Mokhtar Mahmoud, A.; Allará, M.; Naldi, M.; Belluti, F.; Gobbi, S.; Ligresti, A.; Rampa, A. Polycyclic Maleimide-based Scaffold as New Privileged Structure for Navigating the Cannabinoid System Opportunities. ACS Med. Chem. Lett. 2019, 10, 596–600. [Google Scholar] [CrossRef]
- Luo, C.; Sun, J.; Liu, D.; Sun, B.; Miao, L.; Musetti, S.; Li, J.; Han, X.; Du, Y.; Li, L.; et al. Self-Assembled Redox Dual-Responsive Prodrug-Nanosystem Formed by Single Thioether-Bridged Paclitaxel-Fatty Acid Conjugate for Cancer Chemotherapy. Nano Lett. 2016, 16, 5401–5408. [Google Scholar] [CrossRef] [Green Version]
- Ntungwe, E.; Mar, E.; Teod, C.; Teixid, S.; Capote, N.A.; Saraiva, L.; Mar, A. Preliminary Biological Activity Screening of Plectranthus spp. Extracts for the Search of Anticancer Lead Molecules. Pharmaceuticals 2021, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.E.S.; Garcia, C.; Pereira, F.; Mota, J.; Pereira, P.; Cebola, M.J.; Reis, C.P.; Correia, I.; Piedade, M.F.M.; Minas Da Piedade, M.E.; et al. Extraction Optimization and Structural and Thermal Characterization of the Antimicrobial Abietane 7α-Acetoxy-6β-hydroxyroyleanone. Mol. Pharm. 2018, 15, 1412–1419. [Google Scholar] [CrossRef]
- Matias, D.; Nicolai, M.; Saraiva, L.; Pinheiro, R.; Faustino, C.; Diaz Lanza, A.; Pinto Reis, C.; Stankovic, T.; Dinic, J.; Pesic, M.; et al. Cytotoxic Activity of Royleanone Diterpenes from Plectranthus madagascariensis Benth. ACS Omega 2019, 4, 8094–8103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isca, V.M.S.; Ferreira, R.J.; Garcia, C.; Monteiro, C.M.; Dinic, J.; Holmstedt, S.; André, V.; Pesic, M.; Dos Santos, D.J.V.A.; Candeias, N.R.; et al. Molecular Docking Studies of Royleanone Diterpenoids from Plectranthus spp. as P-Glycoprotein Inhibitors. ACS Med. Chem. Lett. 2020, 11, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Silva, C.O.; Monteiro, C.M.; Nicolai, M.; Viana, A.; Andrade, J.M.; Barasoain, I.; Stankovic, T.; Quintana, J.; Hernández, I.; et al. Anticancer properties of the abietane diterpene 6,7-dehydroroyleanone obtained by optimized extraction. Future Med. Chem. 2018, 1, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Garcia, C.A. Isolation, Synthesis and Nanoencapsulation of Cytotoxic Compounds from Plectranthus spp. Ph.D. Thesis, Universidad de Alcalá, Alcalá de Henares, Madrid, 2019. [Google Scholar]
- The Plant List. Version 1.1. 2013. Available online: http://www.theplantlist.org/ (accessed on 1 January 2021).
- Van Jaarsveld, E.J. The Southern African Plectranthus and the Art of Turning Shade to Glade, 1st ed.; Fernwood Press: Cape Town, South Africa, 2006. [Google Scholar]
- Silva, C.O.; Molpeceres, J.; Batanero, B.; Fernandes, A.S.; Saraiva, N.; Costa, J.G.; Rijo, P.; Figueiredo, I.V.; Faísca, P.; Reis, C.P. Functionalized diterpene parvifloron D-loaded hybrid nanoparticles for targeted delivery in melanoma therapy. Ther. Deliv. 2016, 7, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Ntungwe, E., N.; Domínguez-Martín, E.M.; Roberto, A.; Tavares, J.; Isca, V.M.S.; Pereira, P.; Cebola, M.-J.; Rijo, P. Artemia species: An Important Tool to Screen General Toxicity Samples. Curr. Pharm. Des. 2020, 26, 2892–2908. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Gaspar, J.; Cabral, M.F.; Rueff, J.; Castro, M.; Batinic-Haberle, I.; Costa, J.; Oliveira, N.G. Protective role of ortho-substituted Mn(III) N-alkylpyridylporphyrins against the oxidative injury induced by tert-butylhydroperoxide. Free Radic. Res. 2010, 44, 430–440. [Google Scholar] [CrossRef] [PubMed]
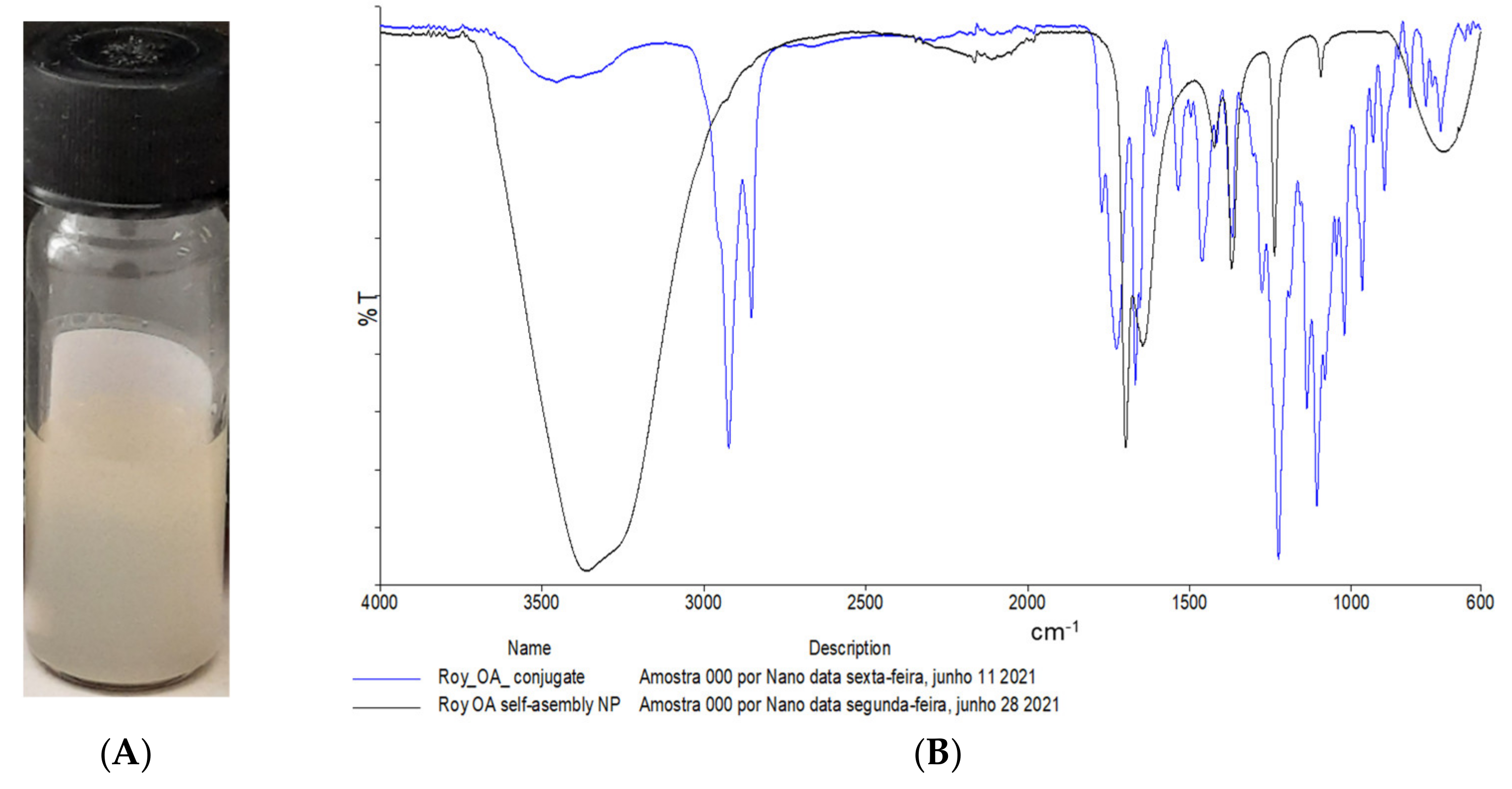
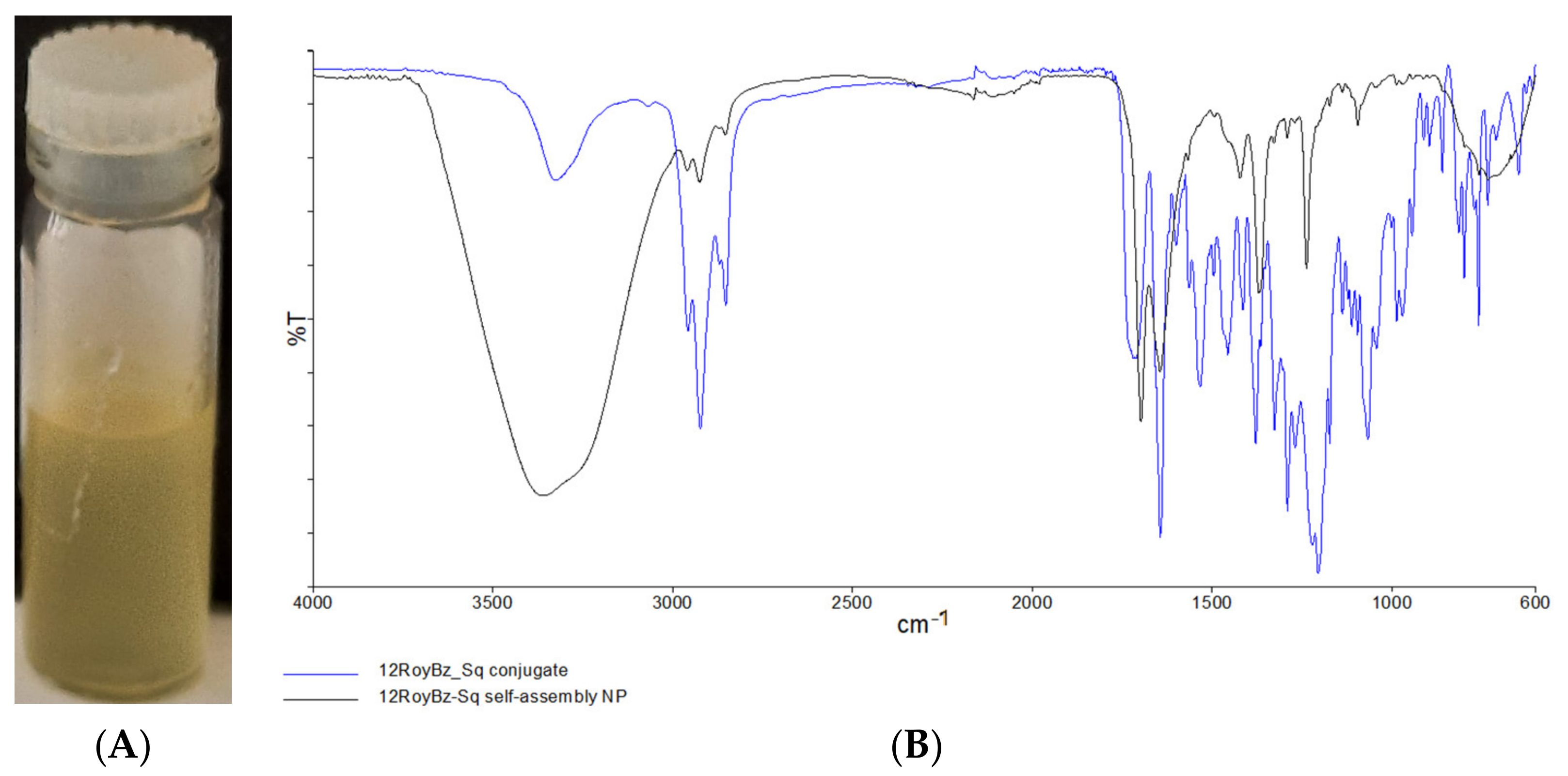


| Sample | Size (nm) | Pdl | Zeta Potential (mV) |
|---|---|---|---|
| ROY-OA NP Assembly | 509.33 ± 4.29 | 0.249 ± 0.012 | −46.2 ± 0.4 |
| 12BzRoy-Sq NP Assembly | 2739.33 ± 100.50 | 0.731 ± 0.187 | −28.9 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntungwe, E.; Domínguez-Martín, E.M.; Bangay, G.; Garcia, C.; Guerreiro, I.; Colombo, E.; Saraiva, L.; Díaz-Lanza, A.M.; Rosatella, A.; Alves, M.M.; et al. Self-Assembly Nanoparticles of Natural Bioactive Abietane Diterpenes. Int. J. Mol. Sci. 2021, 22, 10210. https://doi.org/10.3390/ijms221910210
Ntungwe E, Domínguez-Martín EM, Bangay G, Garcia C, Guerreiro I, Colombo E, Saraiva L, Díaz-Lanza AM, Rosatella A, Alves MM, et al. Self-Assembly Nanoparticles of Natural Bioactive Abietane Diterpenes. International Journal of Molecular Sciences. 2021; 22(19):10210. https://doi.org/10.3390/ijms221910210
Chicago/Turabian StyleNtungwe, Epole, Eva María Domínguez-Martín, Gabrielle Bangay, Catarina Garcia, Iris Guerreiro, Eleonora Colombo, Lucilia Saraiva, Ana María Díaz-Lanza, Andreia Rosatella, Marta M. Alves, and et al. 2021. "Self-Assembly Nanoparticles of Natural Bioactive Abietane Diterpenes" International Journal of Molecular Sciences 22, no. 19: 10210. https://doi.org/10.3390/ijms221910210
APA StyleNtungwe, E., Domínguez-Martín, E. M., Bangay, G., Garcia, C., Guerreiro, I., Colombo, E., Saraiva, L., Díaz-Lanza, A. M., Rosatella, A., Alves, M. M., Reis, C. P., Passarella, D., & Rijo, P. (2021). Self-Assembly Nanoparticles of Natural Bioactive Abietane Diterpenes. International Journal of Molecular Sciences, 22(19), 10210. https://doi.org/10.3390/ijms221910210









