Effects of Growth Hormone Receptor Ablation in Corticotropin-Releasing Hormone Cells
Abstract
1. Introduction
2. Results
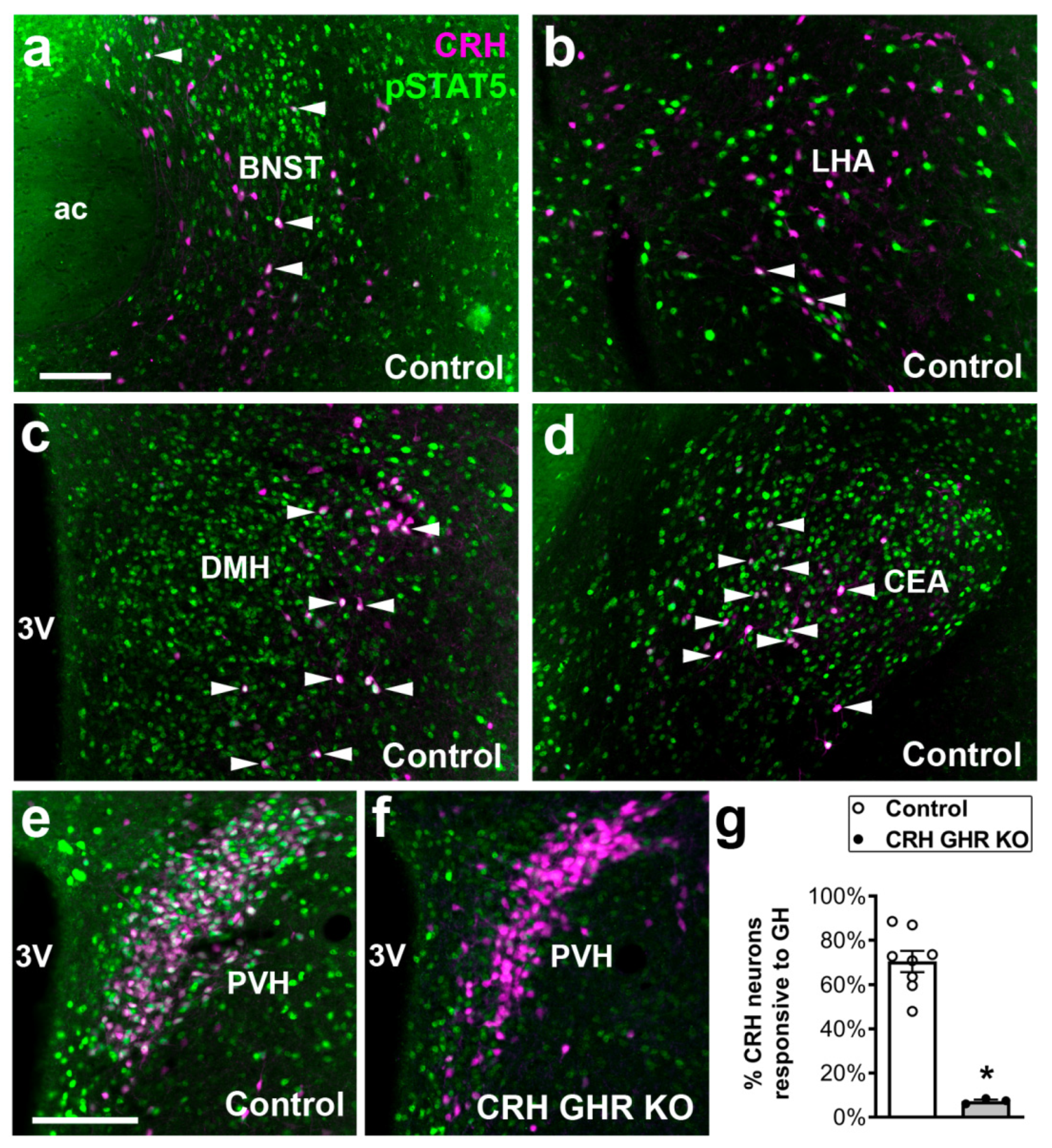
2.1. Distribution of CRH Neurons That Are Responsive to GH
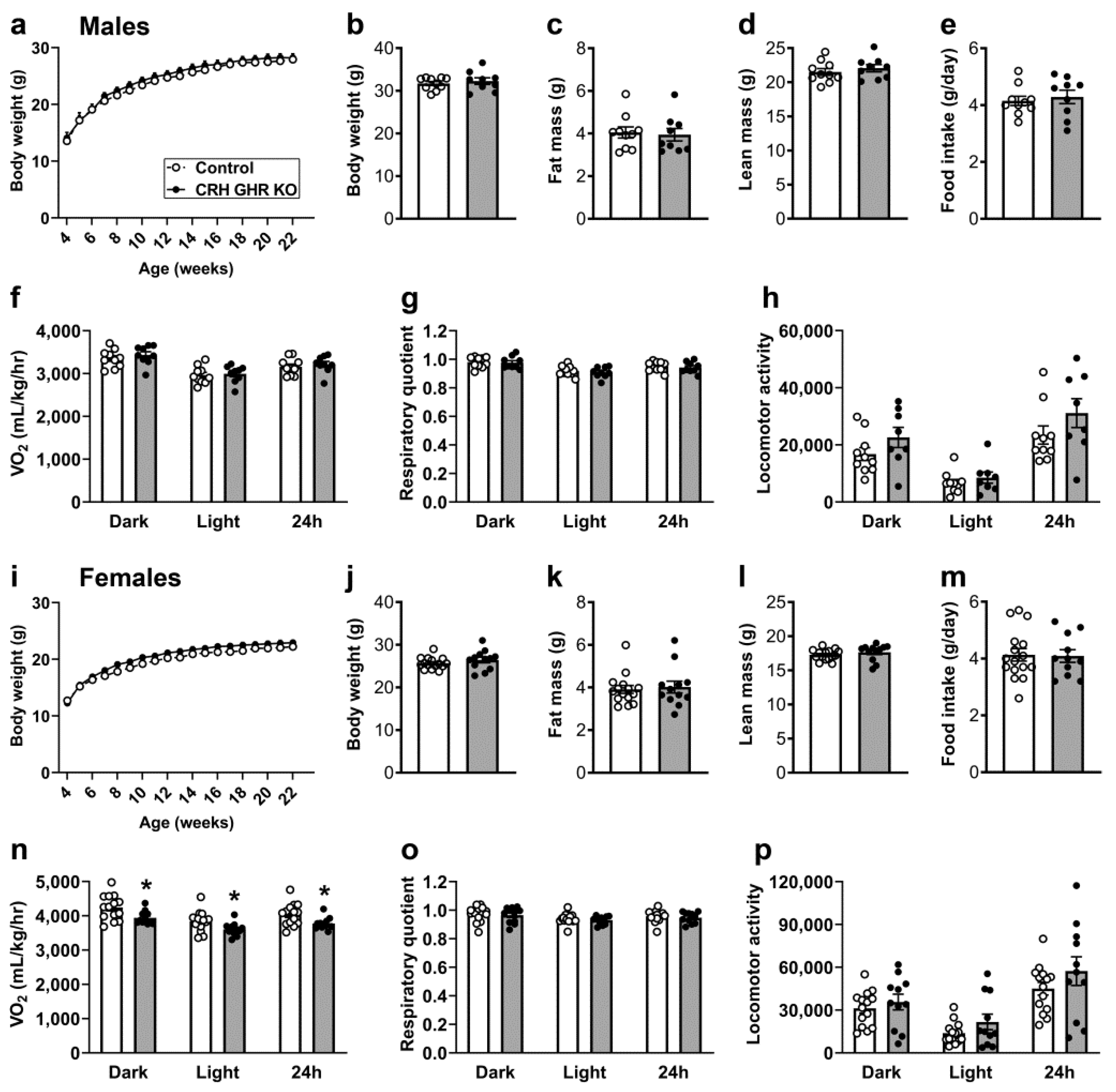
2.2. Reduced Energy Expenditure in CRH GHR KO Female Mice
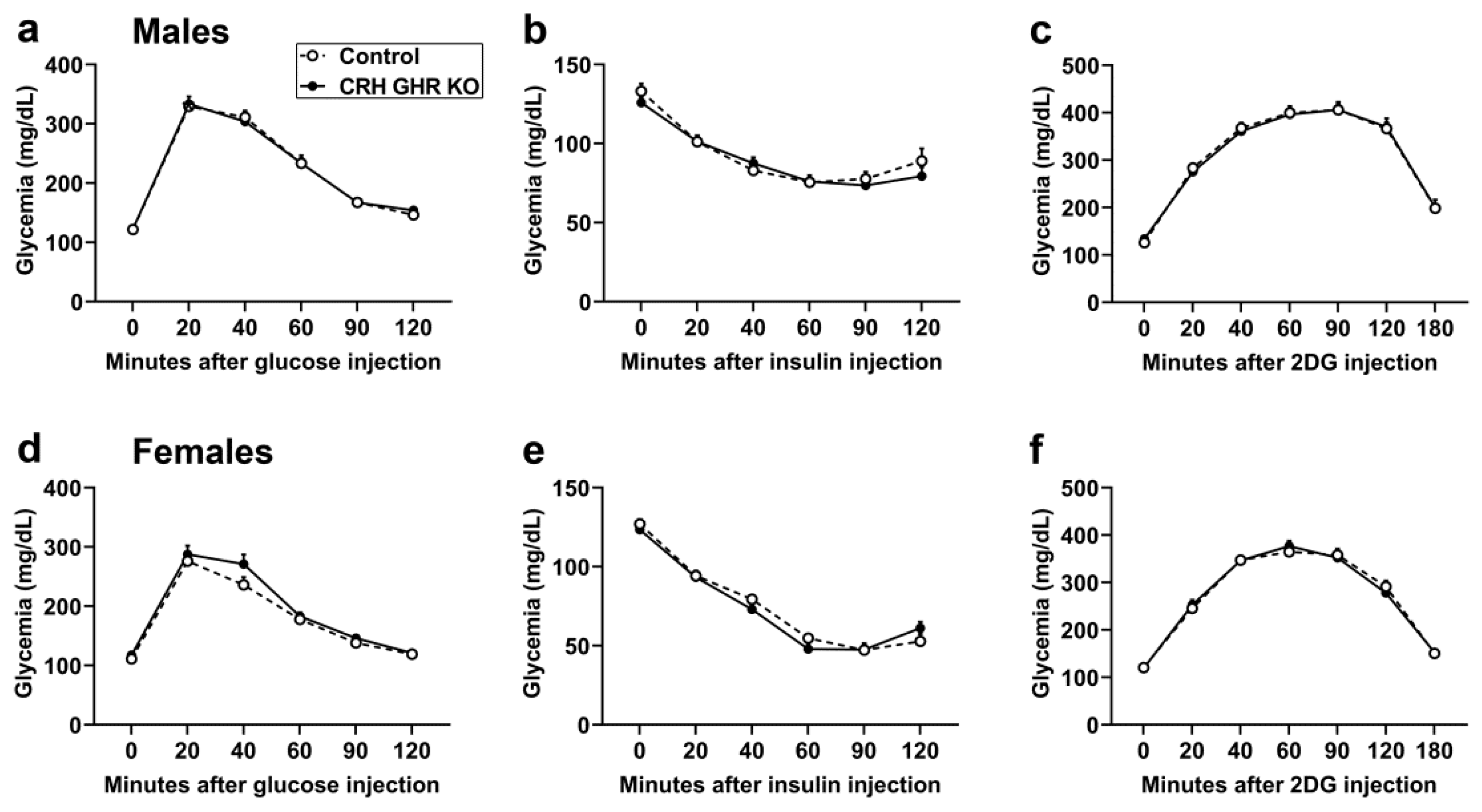
2.3. GHR Ablation in CRH Cells Does Not Affect Glucose Homeostasis and Counterregulatory Response
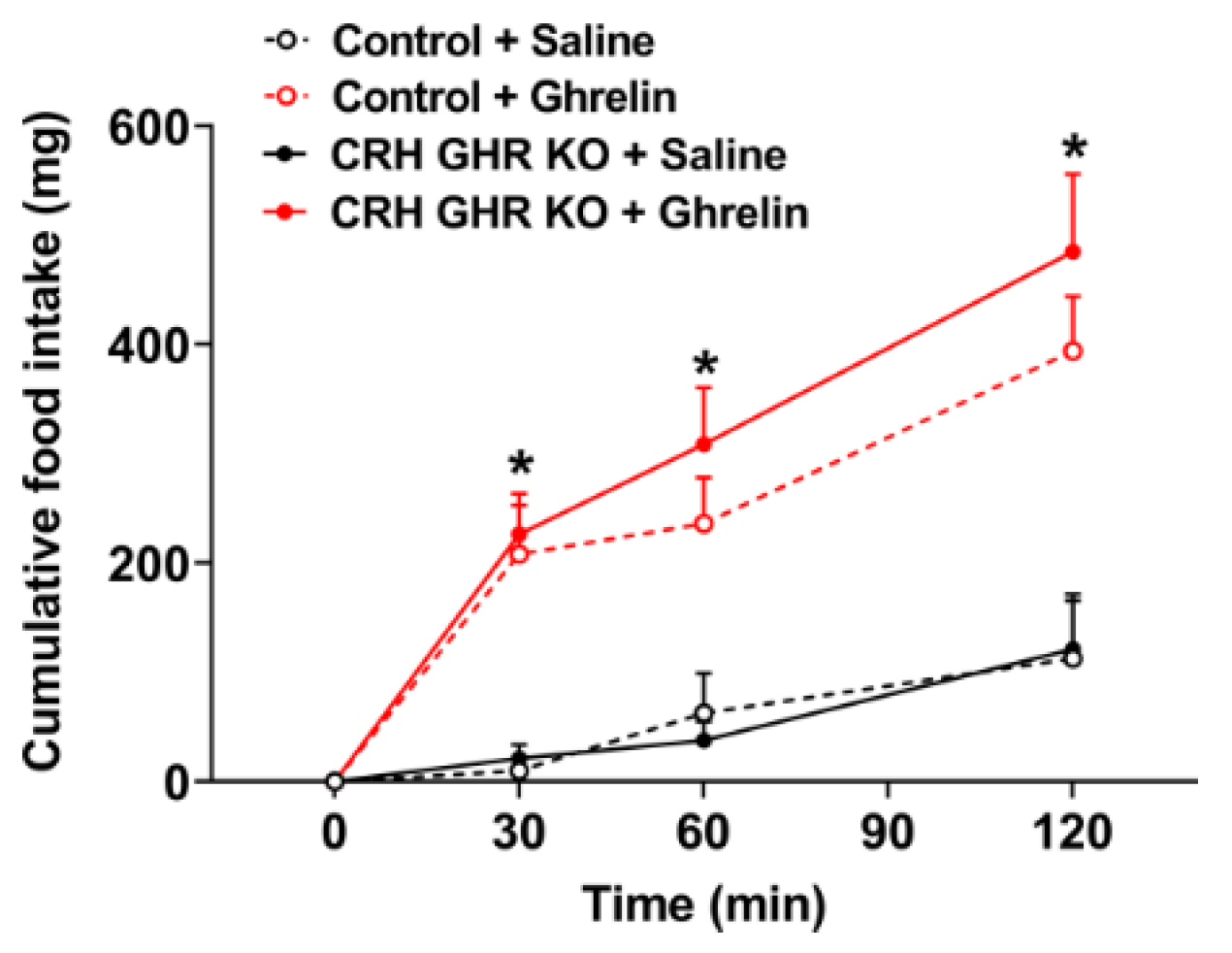
2.4. Ghrelin-Induced Food Intake Is Normal in CRH GHR KO Mice
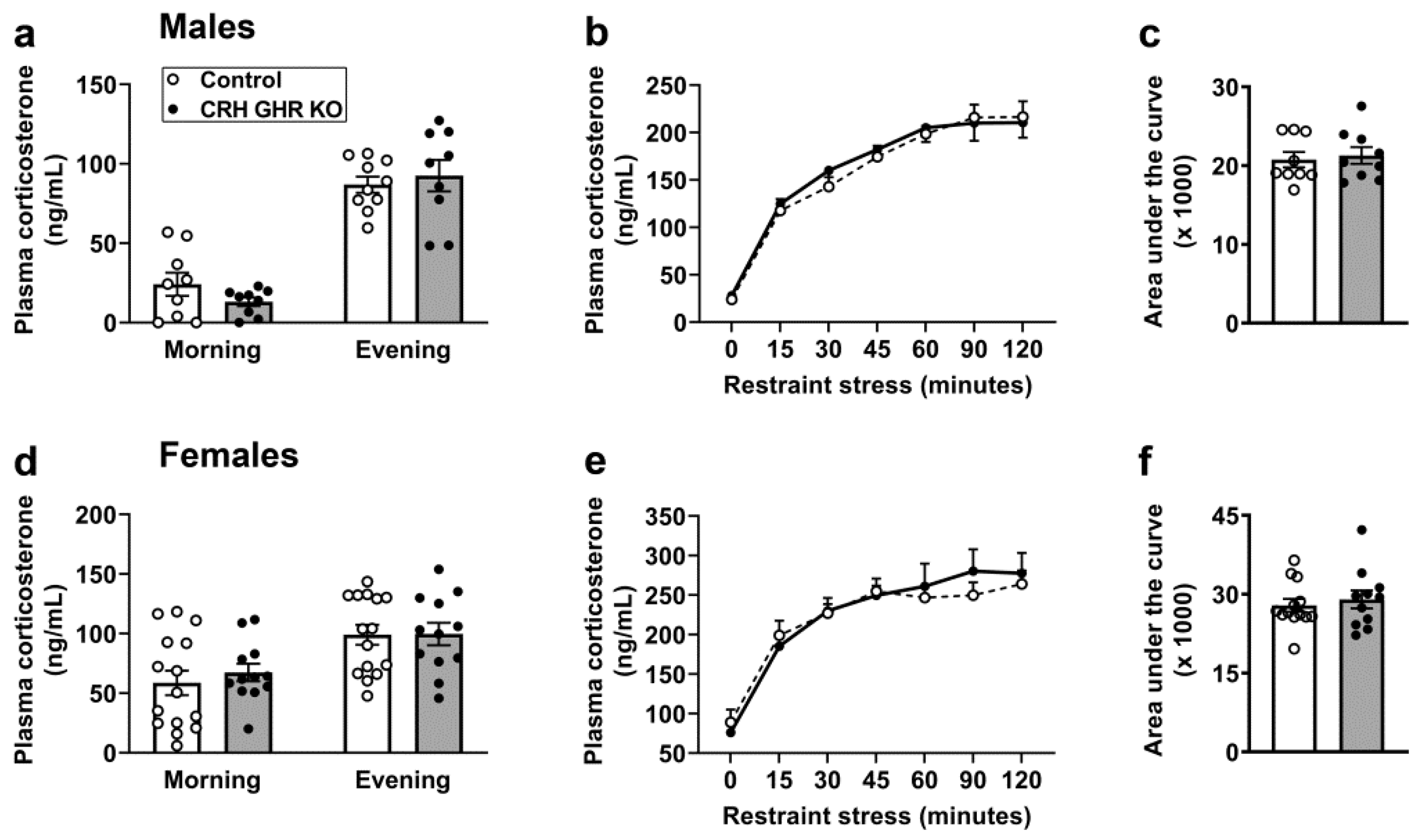
2.5. GHR Ablation in CRH Cells Does Not Affect Circadian or Restraint Stress-Induced Corticosterone Secretion
2.6. CRH GHR KO Mice Exhibit Normal Activation of PVH Neurons after Restraint Stress
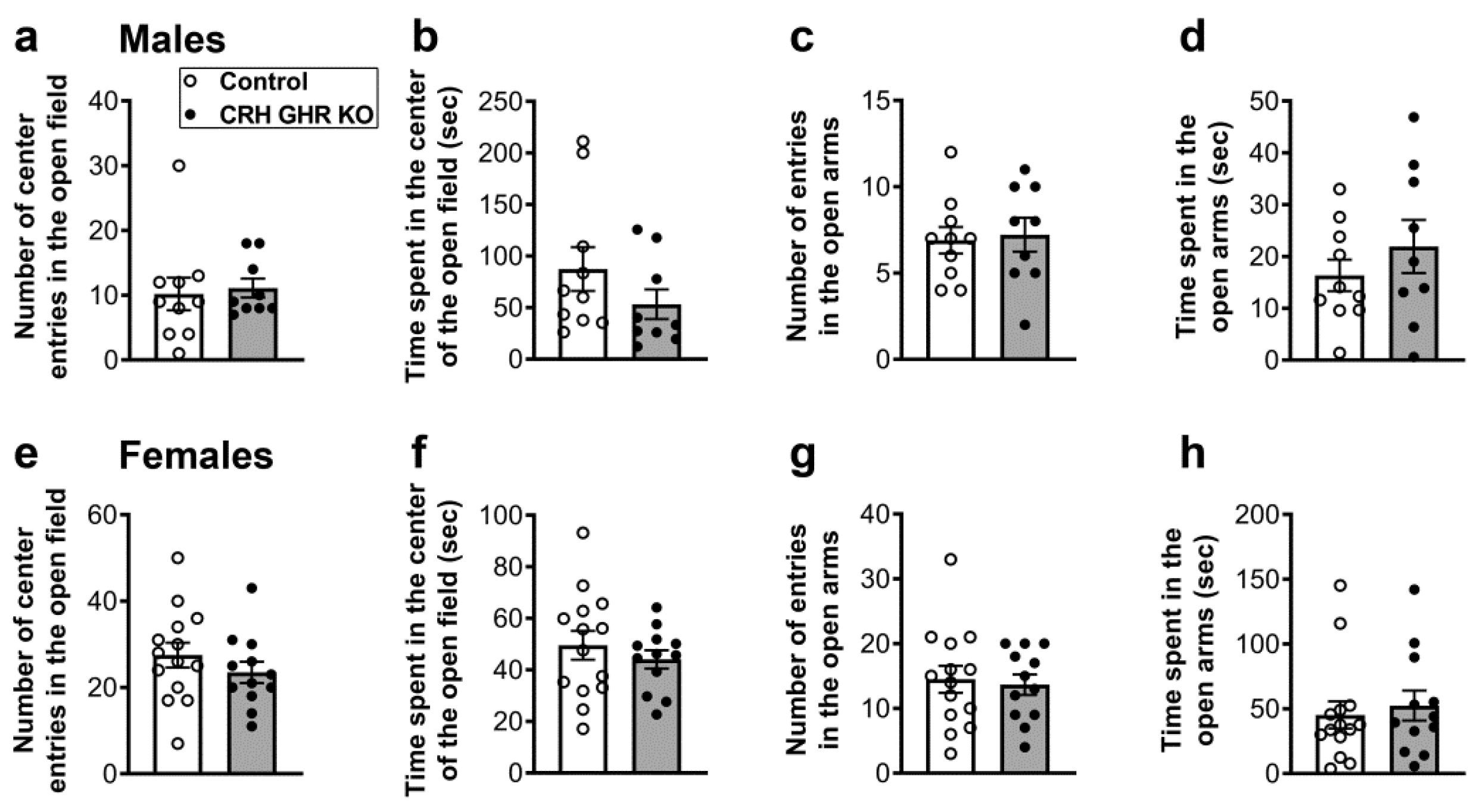
2.7. CRH GHR KO Mice Exhibit Normal Anxiety
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Detection of GH Responsive neurons
4.3. Metabolic Measurements
4.4. Evaluation of Ghrelin-Induced Food Intake
4.5. Evaluation of Circadian and Restraint-Stress-Induced Corticosterone Secretion
4.6. Analysis of Stress-Induced Fos Expression
4.7. Open Field and Elevated Plus Maze Tests
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehkhoda, F.; Lee, C.M.M.; Medina, J.; Brooks, A.J. The growth hormone receptor: Mechanism of receptor activation, cell signaling, and physiological aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Moller, N.; Jorgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Devesa, J.; Almenglo, C.; Devesa, P. Multiple effects of growth hormone in the body: Is it really the hormone for growth? Clin. Med. Insights Endocrinol. Diabetes 2016, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Glick, S.M.; Yalow, R.S.; Berson, S.A. Hypoglycemia: A potent stimulus to secretion of growth hormone. Science 1963, 140, 987–988. [Google Scholar] [CrossRef]
- Furigo, I.C.; de Souza, G.O.; Teixeira, P.D.S.; Guadagnini, D.; Frazao, R.; List, E.O.; Kopchick, J.J.; Prada, P.O.; Donato, J., Jr. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J. 2019, 33, 11909–11924. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.B.; Dos Santos, L.B.P.; Furigo, I.C.; Spagnol, A.R.; Wasinski, F.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Deletion of growth hormone receptor in hypothalamic neurons affects the adaptation capacity to aerobic exercise. Peptides 2021, 135, 170426. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Donato, J., Jr.; Tirapegui, J.; Schneider, C.D. Growth hormone and physical exercise: Current considerations. Rev. Bras. Cienc. Farm. 2008, 44, 549–562. [Google Scholar] [CrossRef]
- Deemer, S.E.; Castleberry, T.J.; Irvine, C.; Newmire, D.E.; Oldham, M.; King, G.A.; Ben-Ezra, V.; Irving, B.A.; Biggerstaff, K.D. Pilot study: An acute bout of high intensity interval exercise increases 12.5 h Gh secretion. Physiol. Rep. 2018, 6, e13563. [Google Scholar] [CrossRef]
- Wideman, L.; Weltman, J.Y.; Hartman, M.L.; Veldhuis, J.D.; Weltman, A. Growth hormone release during acute and chronic aerobic and resistance exercise: Recent findings. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef]
- Zhao, T.J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin o-acyltransferase (goat) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef]
- Furigo, I.C.; Teixeira, P.D.S.; de Souza, G.O.; Couto, G.C.L.; Romero, G.G.; Perello, M.; Frazao, R.; Elias, L.L.; Metzger, M.; List, E.O.; et al. Growth hormone regulates neuroendocrine responses to weight loss via agrp neurons. Nat. Commun. 2019, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Sherbet, D.P.; Elsbernd, B.L.; Goldstein, J.L.; Brown, M.S.; Zhao, T.J. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J. Biol. Chem. 2012, 287, 17942–17950. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr.; Wasinski, F.; Furigo, I.C.; Metzger, M.; Frazao, R. Central regulation of metabolism by growth hormone. Cells 2021, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Hindmarsh, P.; Aynsley-Green, A. Spontaneous hypoglycemia in childhood is accompanied by paradoxically low serum growth hormone and appropriate cortisol counterregulatory hormonal responses. J. Clin. Endocrinol. Metab. 2003, 88, 3715–3723. [Google Scholar] [CrossRef]
- Hinrichs, A.; Renner, S.; Bidlingmaier, M.; Kopchick, J.J.; Wolf, E. Mechanisms in endocrinology: Transient juvenile hypoglycemia in growth hormone receptor deficiency—mechanistic insights from laron syndrome and tailored animal models. Eur. J. Endocrinol. 2021, 185, R35–R47. [Google Scholar] [CrossRef]
- Gatford, K.L.; Muhlhausler, B.S.; Huang, L.; Sim, P.S.; Roberts, C.T.; Velhuis, J.D.; Chen, C. Rising maternal circulating gh during murine pregnancy suggests placental regulation. Endocr. Connect. 2017, 6, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, T.M.; Zampieri, T.T.; Furigo, I.C.; Teixeira, P.D.; List, E.O.; Kopchick, J.; Donato, J., Jr.; Frazao, R. Central growth hormone signaling is not required for the timing of puberty. J. Endocrinol. 2019, 243, 161–173. [Google Scholar] [CrossRef]
- Teixeira, P.D.S.; Tavares, M.R.; Jose, D. Temporal characterization of the insulin resistance during puberty in mice. Endocr. Regul. 2021, 55, 1–4. [Google Scholar] [CrossRef]
- Amiel, S.A.; Sherwin, R.S.; Simonson, D.C.; Lauritano, A.A.; Tamborlane, W.V. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N. Engl. J. Med. 1986, 315, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Bloch, C.A.; Clemons, P.; Sperling, M.A. Puberty decreases insulin sensitivity. J. Pediatr. 1987, 110, 481–487. [Google Scholar] [CrossRef]
- Moran, A.; Jacobs, D.R., Jr.; Steinberger, J.; Cohen, P.; Hong, C.P.; Prineas, R.; Sinaiko, A.R. Association between the insulin resistance of puberty and the insulin-like growth factor-i/growth hormone axis. J. Clin. Endocrinol. Metab. 2002, 87, 4817–4820. [Google Scholar] [CrossRef]
- Rizza, R.A.; Mandarino, L.J.; Gerich, J.E. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 1982, 31, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.D.S.; Couto, G.C.; Furigo, I.C.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Central growth hormone action regulates metabolism during pregnancy. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E925–E940. [Google Scholar] [CrossRef]
- Wasinski, F.; Frazão, R.; Donato, J.J. Effects of growth hormone in the central nervous system. Arch. Endocrinol. Metab. 2019, 63, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Wasinski, F.; Klein, M.O.; Bittencourt, J.C.; Metzger, M.; Donato, J., Jr. Distribution of growth hormone-responsive cells in the brain of rats and mice. Brain Res. 2021, 1751, 147189. [Google Scholar] [CrossRef]
- Furigo, I.C.; Metzger, M.; Teixeira, P.D.; Soares, C.R.; Donato, J., Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct. Funct. 2017, 222, 341–363. [Google Scholar] [CrossRef]
- Furigo, I.C.; Teixeira, P.D.; Quaresma, P.G.F.; Mansano, N.S.; Frazao, R.; Donato, J. Stat5 ablation in agrp neurons increases female adiposity and blunts food restriction adaptations. J. Mol. Endocrinol. 2020, 64, 13–27. [Google Scholar] [CrossRef]
- Wasinski, F.; Furigo, I.C.; Teixeira, P.D.S.; Ramos-Lobo, A.M.; Peroni, C.N.; Bartolini, P.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Growth hormone receptor deletion reduces the density of axonal projections from hypothalamic arcuate nucleus neurons. Neuroscience 2020, 434, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Wasinski, F.; Barrile, F.; Pedroso, J.A.B.; Quaresma, P.G.F.; Dos Santos, W.O.; List, E.O.; Kopchick, J.J.; Perello, M.; Donato, J. Ghrelin-induced food intake, but not gh secretion, requires the expression of the gh receptor in the brain of male mice. Endocrinology 2021, 162, bqab097. [Google Scholar] [CrossRef]
- de Lima, J.B.M.; Debarba, L.K.; Rupp, A.C.; Qi, N.; Ubah, C.; Khan, M.; Didyuk, O.; Ayyar, I.; Koch, M.; Sandoval, D.A.; et al. Arcghr neurons regulate muscle glucose uptake. Cells 2021, 10, 1093. [Google Scholar] [CrossRef]
- Quaresma, P.G.F.; Teixeira, P.D.S.; Furigo, I.C.; Wasinski, F.; Couto, G.C.; Frazao, R.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Growth hormone/stat5 signaling in proopiomelanocortin neurons regulates glucoprivic hyperphagia. Mol. Cell. Endocrinol. 2019, 498, 110574. [Google Scholar] [CrossRef]
- Wasinski, F.; Chaves, F.M.; Pedroso, J.A.B.; Mansano, N.S.; Camporez, J.P.; Gusmao, D.O.; List, E.O.; Kopchick, J.J.; Frazao, R.; Szawka, R.E.; et al. Growth hormone receptor in dopaminergic neurones regulates stress-induced prolactin release in male mice. J. Neuroendocrinol. 2021, 33, e12957. [Google Scholar] [CrossRef] [PubMed]
- Wasinski, F.; Pedroso, J.A.B.; Dos Santos, W.O.; Furigo, I.C.; Garcia-Galiano, D.; Elias, C.F.; List, E.O.; Kopchick, J.J.; Szawka, R.E.; Donato, J., Jr. Tyrosine hydroxylase neurons regulate growth hormone secretion via short-loop negative feedback. J. Neurosci. 2020, 40, 4309–4322. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, P.G.F.; Teixeira, P.D.S.; Wasinski, F.; Campos, A.M.P.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Cholinergic neurons in the hypothalamus and dorsal motor nucleus of the vagus are directly responsive to growth hormone. Life Sci. 2020, 259, 118229. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Liu, Y. The molecular physiology of crh neurons. Front. Neuroendocrinol. 2012, 33, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Fuzesi, T.; Daviu, N.; Wamsteeker Cusulin, J.I.; Bonin, R.P.; Bains, J.S. Hypothalamic crh neurons orchestrate complex behaviours after stress. Nat. Commun. 2016, 7, 11937. [Google Scholar] [CrossRef]
- Quaresma, P.G.F.; Dos Santos, W.O.; Wasinski, F.; Metzger, M.; Donato, J., Jr. Neurochemical phenotype of growth hormone-responsive cells in the mouse paraventricular nucleus of the hypothalamus. J. Comp. Neurol. 2021, 529, 1228–1239. [Google Scholar] [CrossRef]
- Krahn, D.D.; Gosnell, B.A.; Majchrzak, M.J. The anorectic effects of crh and restraint stress decrease with repeated exposures. Biol. Psychiatry 1990, 27, 1094–1102. [Google Scholar] [CrossRef]
- Benoit, S.C.; Thiele, T.E.; Heinrichs, S.C.; Rushing, P.A.; Blake, K.A.; Steeley, R.J. Comparison of central administration of corticotropin-releasing hormone and urocortin on food intake, conditioned taste aversion, and c-fos expression. Peptides 2000, 21, 345–351. [Google Scholar] [CrossRef]
- George, S.A.; Khan, S.; Briggs, H.; Abelson, J.L. Crh-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 2010, 35, 607–612. [Google Scholar] [CrossRef]
- Bovetto, S.; Rouillard, C.; Richard, D. Role of crh in the effects of 5-ht-receptor agonists on food intake and metabolic rate. Am. J. Physiol. 1996, 271, R1231–R1238. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Cheng, Y.; Zhang, Q.; Xiao, F.; Liu, B.; Chen, S.; Guo, F. S6k1 in the central nervous system regulates energy expenditure via mc4r/crh pathways in response to deprivation of an essential amino acid. Diabetes 2012, 61, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Verberne, A.J.; Sabetghadam, A.; Korim, W.S. Neural pathways that control the glucose counterregulatory response. Front. Neurosci. 2014, 8, 38. [Google Scholar] [CrossRef]
- Jacobson, L.; Ansari, T.; Potts, J.; McGuinness, O.P. Glucocorticoid-deficient corticotropin-releasing hormone knockout mice maintain glucose requirements but not autonomic responses during repeated hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E15–E22. [Google Scholar] [CrossRef][Green Version]
- Kageyama, K.; Kumata, Y.; Akimoto, K.; Takayasu, S.; Tamasawa, N.; Suda, T. Ghrelin stimulates corticotropin-releasing factor and vasopressin gene expression in rat hypothalamic 4b cells. Stress 2011, 14, 520–529. [Google Scholar] [CrossRef]
- Cabral, A.; Suescun, O.; Zigman, J.M.; Perello, M. Ghrelin indirectly activates hypophysiotropic crf neurons in rodents. PLoS ONE 2012, 7, e31462. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos, R.C.; Grover, H.M.; Reis, L.C.; Ferguson, A.V.; Mecawi, A.S. Electrophysiological effects of ghrelin in the hypothalamic paraventricular nucleus neurons. Front. Cell Neurosci. 2018, 12, 275. [Google Scholar] [CrossRef]
- Cabral, A.; Portiansky, E.; Sanchez-Jaramillo, E.; Zigman, J.M.; Perello, M. Ghrelin activates hypophysiotropic corticotropin-releasing factor neurons independently of the arcuate nucleus. Psychoneuroendocrinology 2016, 67, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Kordon, C.; Epelbaum, J. Anatomy of the hypophysiotropic somatostatinergic and growth hormone-releasing hormone system minireview. Neurochem. Res. 2006, 31, 137–143. [Google Scholar] [CrossRef]
- Steyn, F.J.; Tolle, V.; Chen, C.; Epelbaum, J. Neuroendocrine regulation of growth hormone secretion. Compr. Physiol. 2016, 6, 687–735. [Google Scholar]
- Murray, P.G.; Higham, C.E.; Clayton, P.E. 60 years of neuroendocrinology: The hypothalamo-gh axis: The past 60 years. J. Endocrinol. 2015, 226, T123–T140. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C.; Cornish, L.C.; Lawrence, A.J.; Campbell, E.J. The effect of acute or repeated stress on the corticotropin releasing factor system in the crh-ires-cre mouse: A validation study. Neuropharmacology 2019, 154, 96–106. [Google Scholar] [CrossRef]
- Zhang, R.; Asai, M.; Mahoney, C.E.; Joachim, M.; Shen, Y.; Gunner, G.; Majzoub, J.A. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry 2017, 22, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Rajamanickam, S.; Justice, N.J. Crf signaling between neurons in the paraventricular nucleus of the hypothalamus (pvn) coordinates stress responses. Neurobiol. Stress 2019, 11, 100192. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.L.; Picetti, R.; Contarino, A.; Koob, G.F.; Vale, W.W.; Lee, K.F. Mice deficient for both corticotropin-releasing factor receptor 1 (crfr1) and crfr2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci. 2002, 22, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F.; Ising, M. Central crh system in depression and anxiety—evidence from clinical studies with crh1 receptor antagonists. Eur. J. Pharmacol. 2008, 583, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.G.; Sanchez-Watts, G.; Kelly, A.B. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J. Neurosci. 1999, 19, 6111–6121. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.G.; Sanchez-Watts, G. Rapid and preferential activation of fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J. Comp. Neurol. 2007, 502, 768–782. [Google Scholar] [CrossRef]
- Watts, A.G. Osmotic stimulation differentially affects cellular levels of corticotropin-releasing hormone and neurotensin/neuromedin n mrnas in the lateral hypothalamic area and central nucleus of the amygdala. Brain Res. 1992, 581, 208–216. [Google Scholar] [CrossRef]
- Peng, J.; Long, B.; Yuan, J.; Peng, X.; Ni, H.; Li, X.; Gong, H.; Luo, Q.; Li, A. A quantitative analysis of the distribution of crh neurons in whole mouse brain. Front. Neuroanat. 2017, 11, 63. [Google Scholar] [CrossRef]
- Magableh, A.; Lundy, R. Somatostatin and corticotrophin releasing hormone cell types are a major source of descending input from the forebrain to the parabrachial nucleus in mice. Chem. Senses 2014, 39, 673–682. [Google Scholar] [CrossRef]
- Lundy, R. Comparison of gaba, somatostatin, and corticotrophin-releasing hormone expression in axon terminals that target the parabrachial nucleus. Chem. Senses 2020, 45, 275–282. [Google Scholar] [CrossRef]
- Raver, C.; Uddin, O.; Ji, Y.; Li, Y.; Cramer, N.; Jenne, C.; Morales, M.; Masri, R.; Keller, A. An amygdalo-parabrachial pathway regulates pain perception and chronic pain. J. Neurosci. 2020, 40, 3424–3442. [Google Scholar] [CrossRef]
- Meyer, R.M.; Burgos-Robles, A.; Liu, E.; Correia, S.S.; Goosens, K.A. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatry 2014, 19, 1284–1294. [Google Scholar] [CrossRef]
- Gisabella, B.; Farah, S.; Peng, X.; Burgos-Robles, A.; Lim, S.H.; Goosens, K.A. Growth hormone biases amygdala network activation after fear learning. Transl. Psychiatry 2016, 6, e960. [Google Scholar] [CrossRef]
- Yousufzai, M.; Harmatz, E.S.; Shah, M.; Malik, M.O.; Goosens, K.A. Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans. Transl. Psychiatry 2018, 8, 74. [Google Scholar] [CrossRef]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.J.; Ford, C.P.; Bruchas, M.R. Crh engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef]
- Donner, N.C.; Davies, S.M.; Fitz, S.D.; Kienzle, D.M.; Shekhar, A.; Lowry, C.A. Crh receptor priming in the bed nucleus of the stria terminalis (bnst) induces tph2 gene expression in the dorsomedial dorsal raphe nucleus and chronic anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109730. [Google Scholar] [CrossRef]
- Hammack, S.E.; Roman, C.W.; Lezak, K.R.; Kocho-Shellenberg, M.; Grimmig, B.; Falls, W.A.; Braas, K.; May, V. Roles for pituitary adenylate cyclase-activating peptide (pacap) expression and signaling in the bed nucleus of the stria terminalis (bnst) in mediating the behavioral consequences of chronic stress. J. Mol. Neurosci. 2010, 42, 327–340. [Google Scholar] [CrossRef]
- Pomrenze, M.B.; Tovar-Diaz, J.; Blasio, A.; Maiya, R.; Giovanetti, S.M.; Lei, K.; Morikawa, H.; Hopf, F.W.; Messing, R.O. A corticotropin releasing factor network in the extended amygdala for anxiety. J. Neurosci. 2019, 39, 1030–1043. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Bohlooly, Y.M.; Olsson, B.; Bruder, C.E.; Linden, D.; Sjogren, K.; Bjursell, M.; Egecioglu, E.; Svensson, L.; Brodin, P.; Waterton, J.C.; et al. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes 2005, 54, 51–62. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Jiang, Z.; Tian, J.B.; Cassidy, R.M.; Cai, Z.L.; Shu, G.; Xu, Y.; Xue, M.; Arenkiel, B.R.; et al. Disrupted hypothalamic crh neuron responsiveness contributes to diet-induced obesity. EMBO Rep. 2020, 21, e49210. [Google Scholar] [CrossRef]
- Garcia-Caceres, C.; Lagunas, N.; Calmarza-Font, I.; Azcoitia, I.; Diz-Chaves, Y.; Garcia-Segura, L.M.; Baquedano, E.; Frago, L.M.; Argente, J.; Chowen, J.A. Gender differences in the long-term effects of chronic prenatal stress on the hpa axis and hypothalamic structure in rats. Psychoneuroendocrinology 2010, 35, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Manti, M.; Fornes, R.; Qi, X.; Folmerz, E.; Linden Hirschberg, A.; de Castro Barbosa, T.; Maliqueo, M.; Benrick, A.; Stener-Victorin, E. Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring. FASEB J. 2018, 32, 4158–4171. [Google Scholar] [CrossRef] [PubMed]
- Senst, L.; Baimoukhametova, D.; Sterley, T.L.; Bains, J.S. Sexually dimorphic neuronal responses to social isolation. eLife 2016, 5, e18726. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.J.; Suzuki, S.; Miller, L.K.; Handa, R.; Uht, R.M. Estrogen receptor (er)beta isoforms rather than eralpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J. Neurosci. 2004, 24, 10628–10635. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liu, J.; Yasrebi, A.; Gotthardt, J.D.; Bello, N.T.; Pang, Z.P.; Roepke, T.A. Gq protein-coupled membrane-initiated estrogen signaling rapidly excites corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus in female mice. Endocrinology 2016, 157, 3604–3620. [Google Scholar] [CrossRef]
- Egecioglu, E.; Bjursell, M.; Ljungberg, A.; Dickson, S.L.; Kopchick, J.J.; Bergstrom, G.; Svensson, L.; Oscarsson, J.; Tornell, J.; Bohlooly, Y.M. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E317–E325. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Uchida, A.; Chuang, J.C.; Walker, A.; Liu, T.; Osborne-Lawrence, S.; Mason, B.L.; Mosher, C.; Berglund, E.D.; et al. Arcuate agrp neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 2014, 3, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Bongmba, O.Y.N.; Yue, J.; Lee, J.H.; Lin, L.; Saito, K.; Pradhan, G.; Li, D.P.; Pan, H.L.; Xu, A.; et al. Suppression of ghs-r in agrp neurons mitigates diet-induced obesity by activating thermogenesis. Int. J. Mol. Sci. 2017, 18, 832. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Brown, L.M.; Zigman, J.M.; Kemp, C.J.; Strader, A.D.; Benoit, S.C.; Woods, S.C.; Mangiaracina, M.; Geary, N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 2007, 56, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- List, E.O.; Berryman, D.E.; Funk, K.; Gosney, E.S.; Jara, A.; Kelder, B.; Wang, X.; Kutz, L.; Troike, K.; Lozier, N.; et al. The role of gh in adipose tissue: Lessons from adipose-specific gh receptor gene-disrupted mice. Mol. Endocrinol. 2013, 27, 524–535. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, W.O.; Gusmao, D.O.; Wasinski, F.; List, E.O.; Kopchick, J.J.; Donato Jr., J. Effects of Growth Hormone Receptor Ablation in Corticotropin-Releasing Hormone Cells. Int. J. Mol. Sci. 2021, 22, 9908. https://doi.org/10.3390/ijms22189908
dos Santos WO, Gusmao DO, Wasinski F, List EO, Kopchick JJ, Donato Jr. J. Effects of Growth Hormone Receptor Ablation in Corticotropin-Releasing Hormone Cells. International Journal of Molecular Sciences. 2021; 22(18):9908. https://doi.org/10.3390/ijms22189908
Chicago/Turabian Styledos Santos, Willian O., Daniela O. Gusmao, Frederick Wasinski, Edward O. List, John J. Kopchick, and Jose Donato Jr. 2021. "Effects of Growth Hormone Receptor Ablation in Corticotropin-Releasing Hormone Cells" International Journal of Molecular Sciences 22, no. 18: 9908. https://doi.org/10.3390/ijms22189908
APA Styledos Santos, W. O., Gusmao, D. O., Wasinski, F., List, E. O., Kopchick, J. J., & Donato Jr., J. (2021). Effects of Growth Hormone Receptor Ablation in Corticotropin-Releasing Hormone Cells. International Journal of Molecular Sciences, 22(18), 9908. https://doi.org/10.3390/ijms22189908






