Identification and Characterization of Short Crown Root 8, a Temperature-Sensitive Mutant Associated with Crown Root Development in Rice
Abstract
:1. Introduction
2. Results
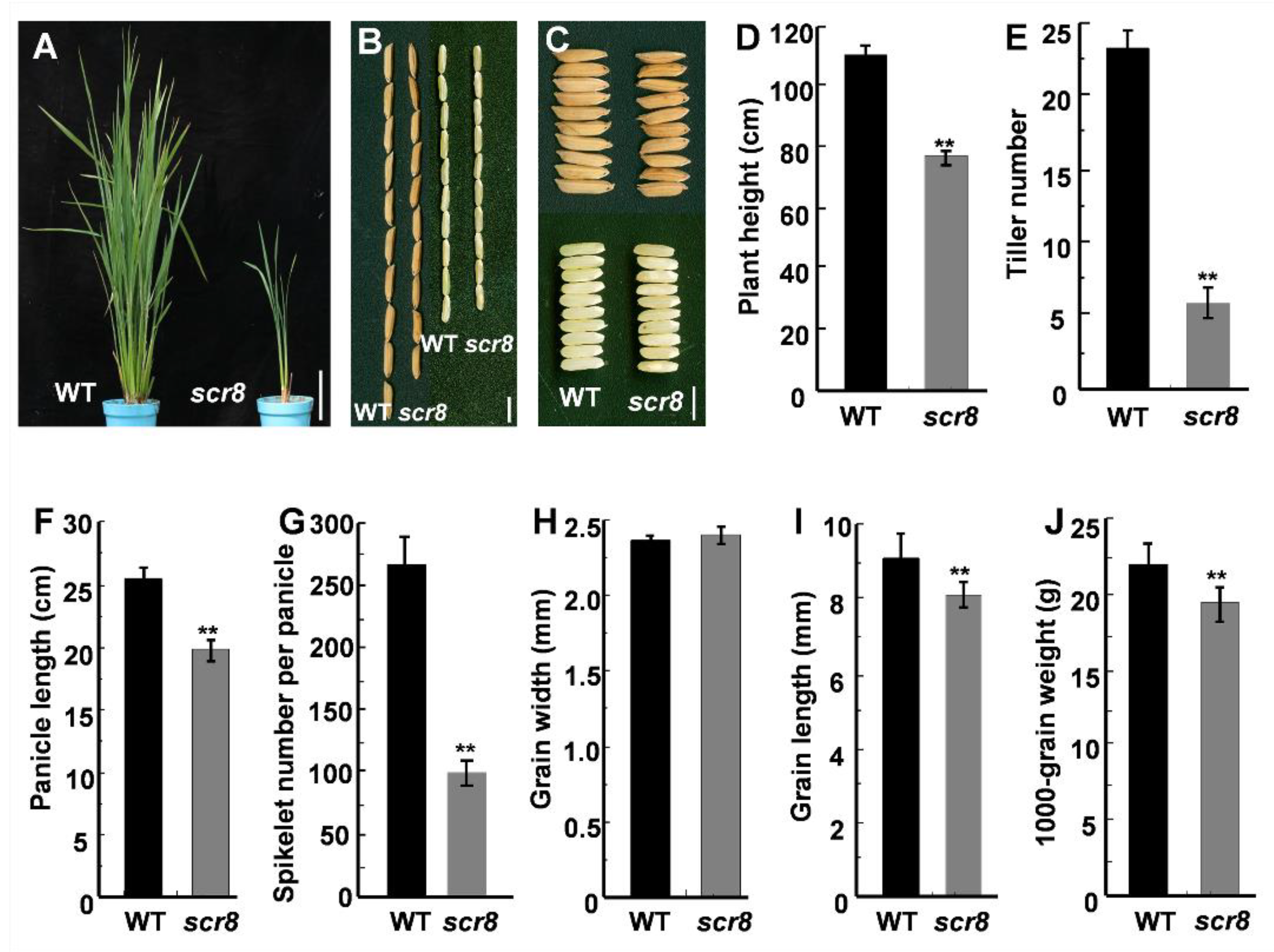
2.1. Identification of Scr8 Mutant
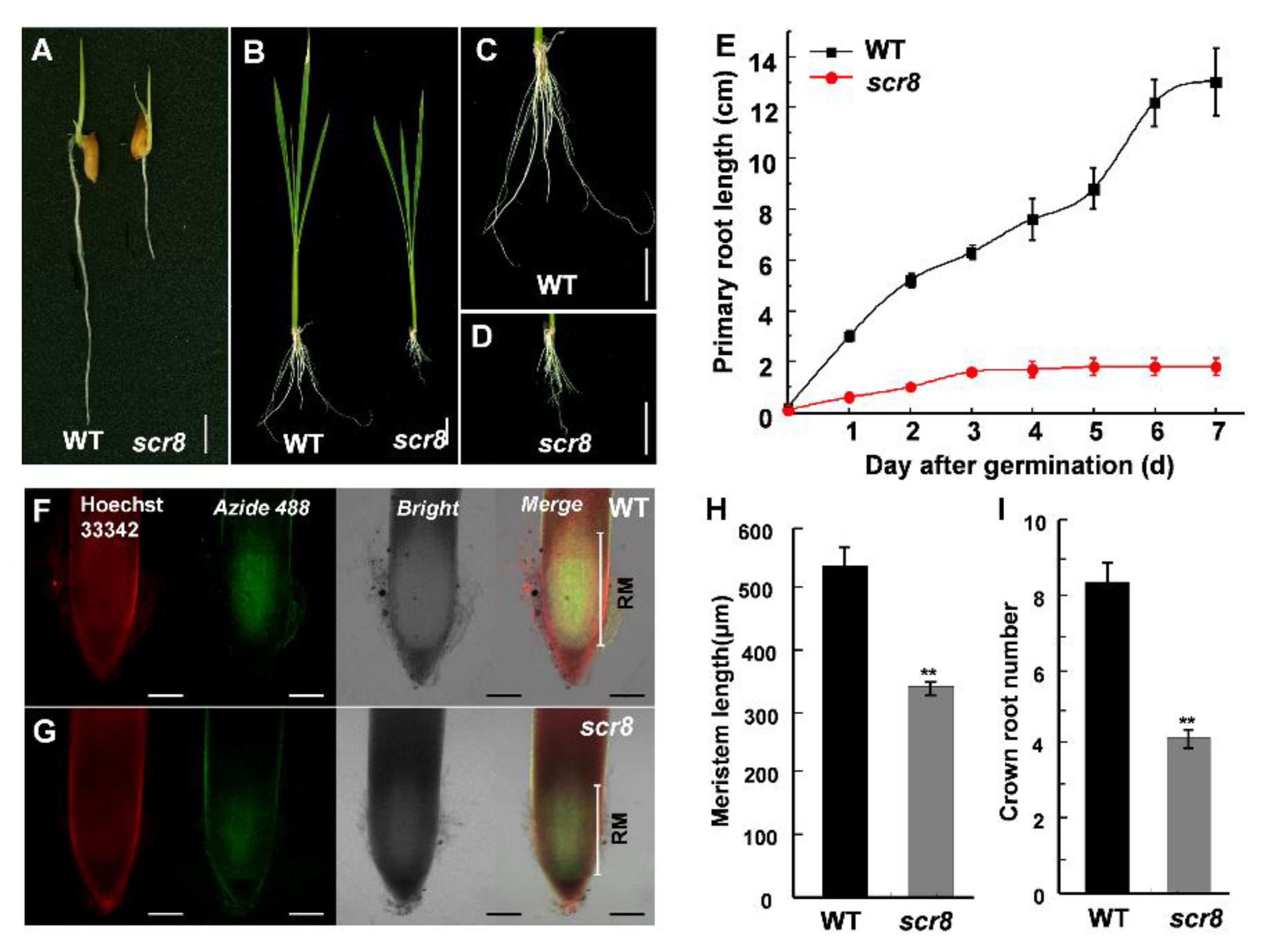
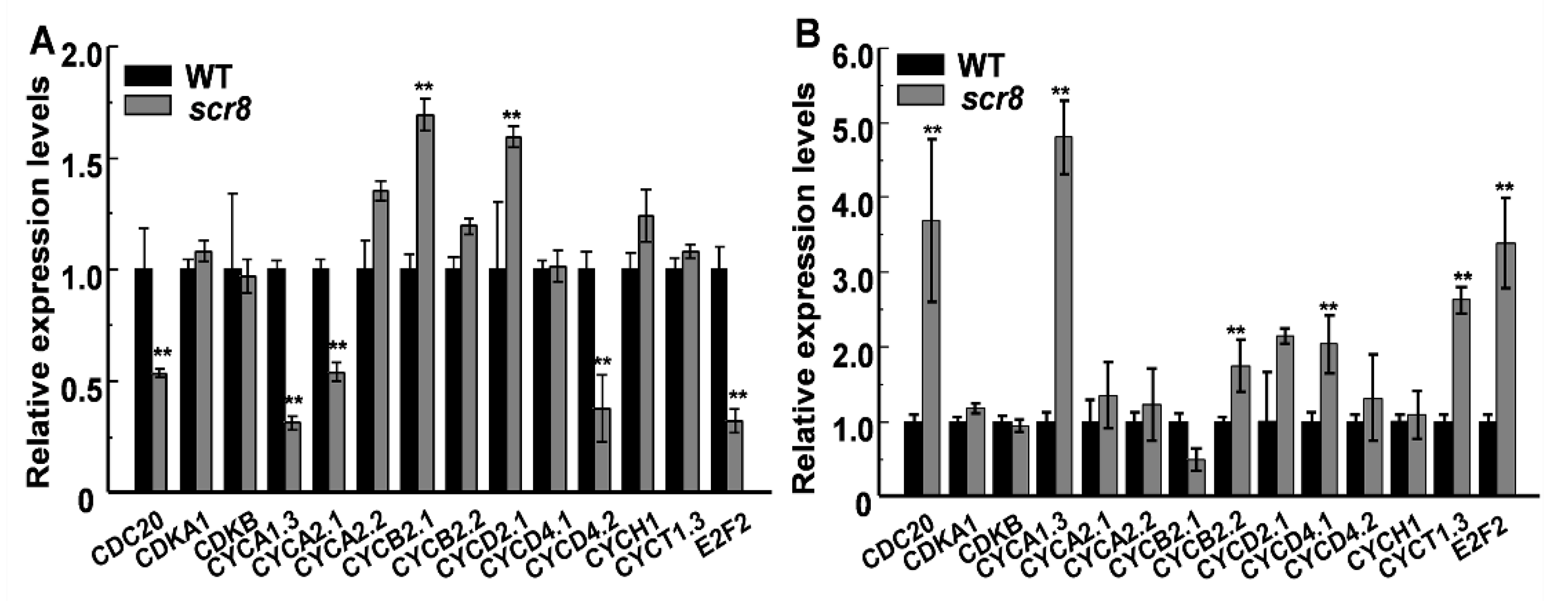
2.2. Reduced Root Meristem Activity of Scr8
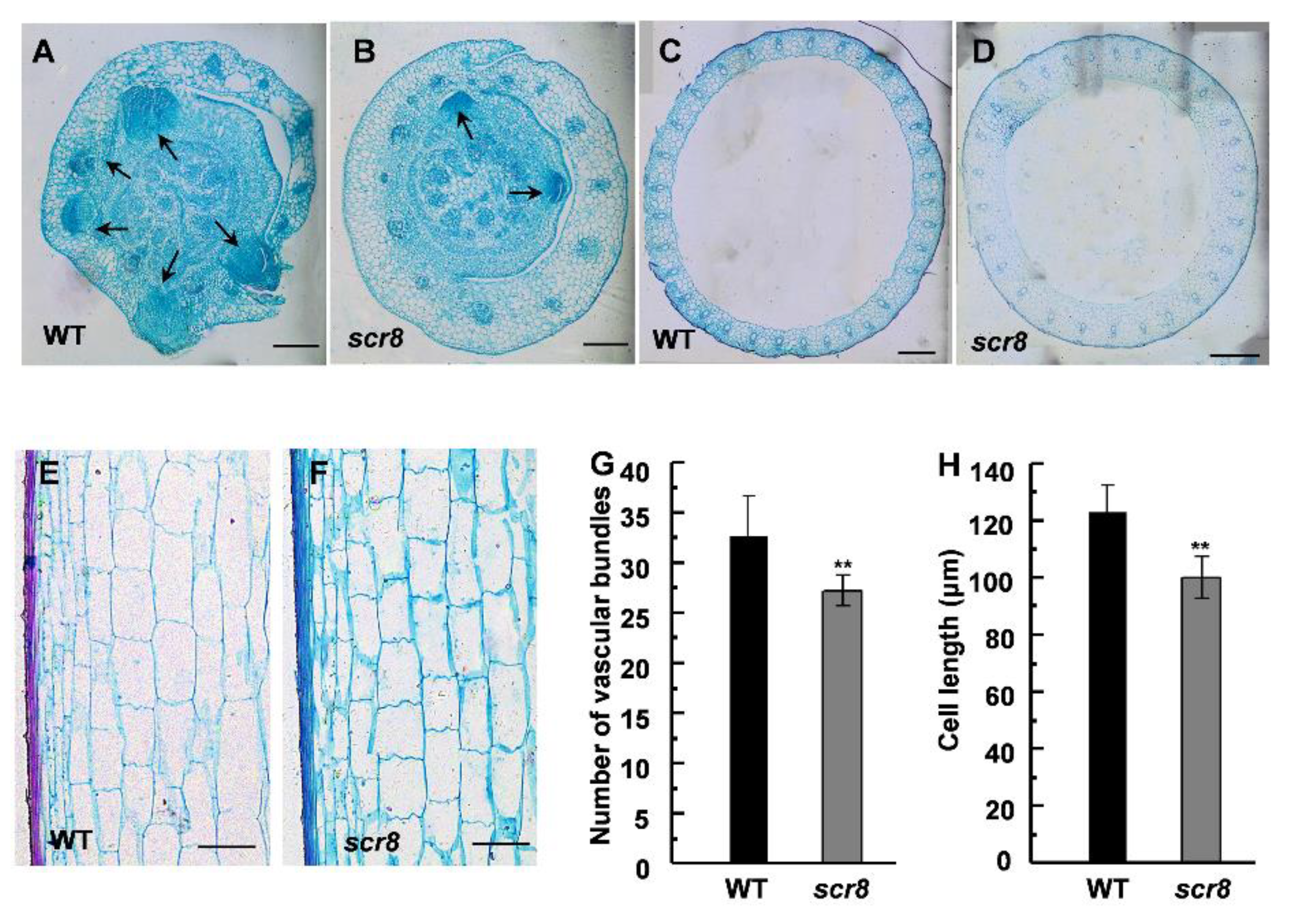
2.3. Anatomic Analysis of Scr8
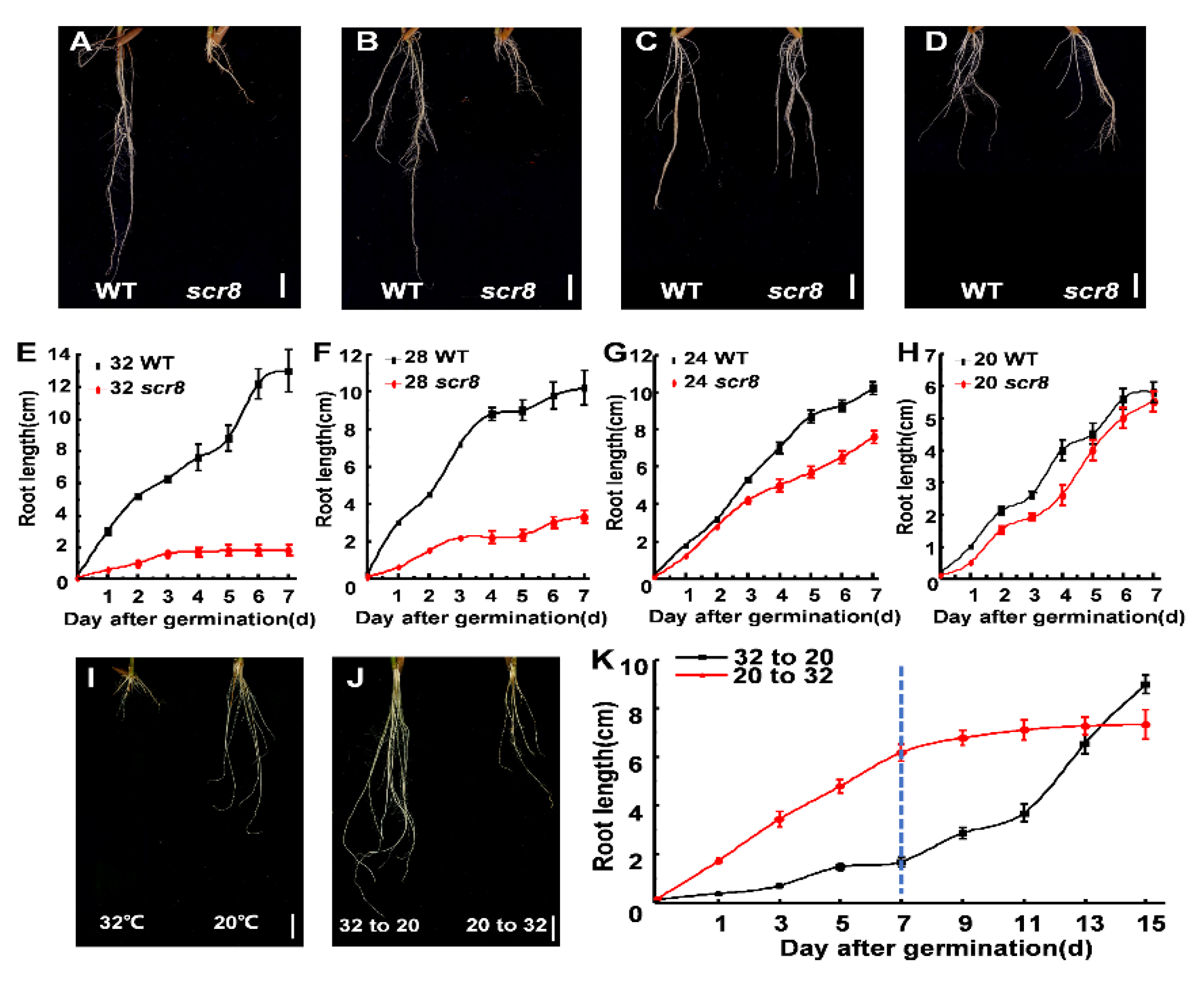
2.4. Temperature Affected Scr8 Root Growth
2.5. Scr8 Showed Increased SA and JA Content
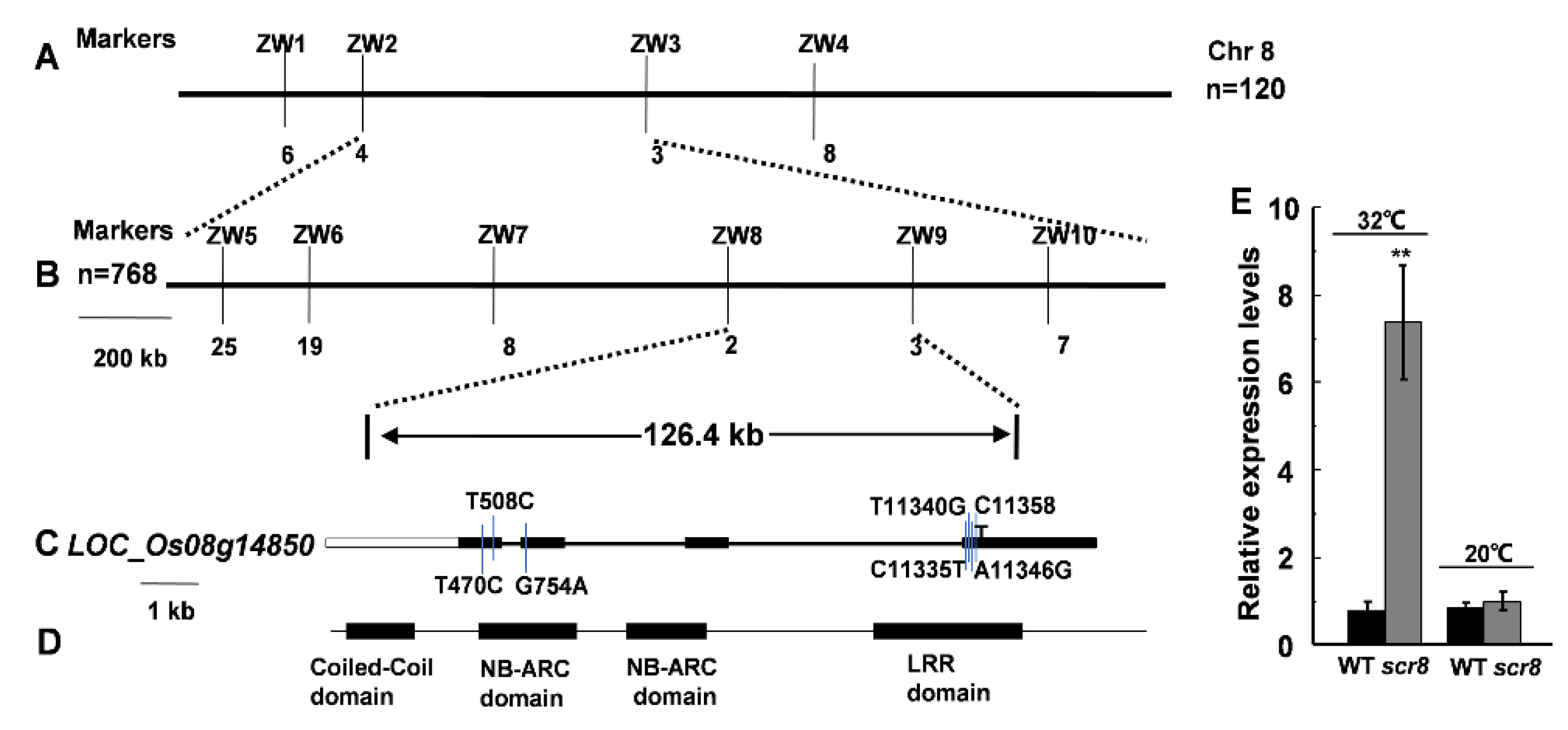
2.6. Fine Mapping of Scr8 and Candidate Gene Analysis
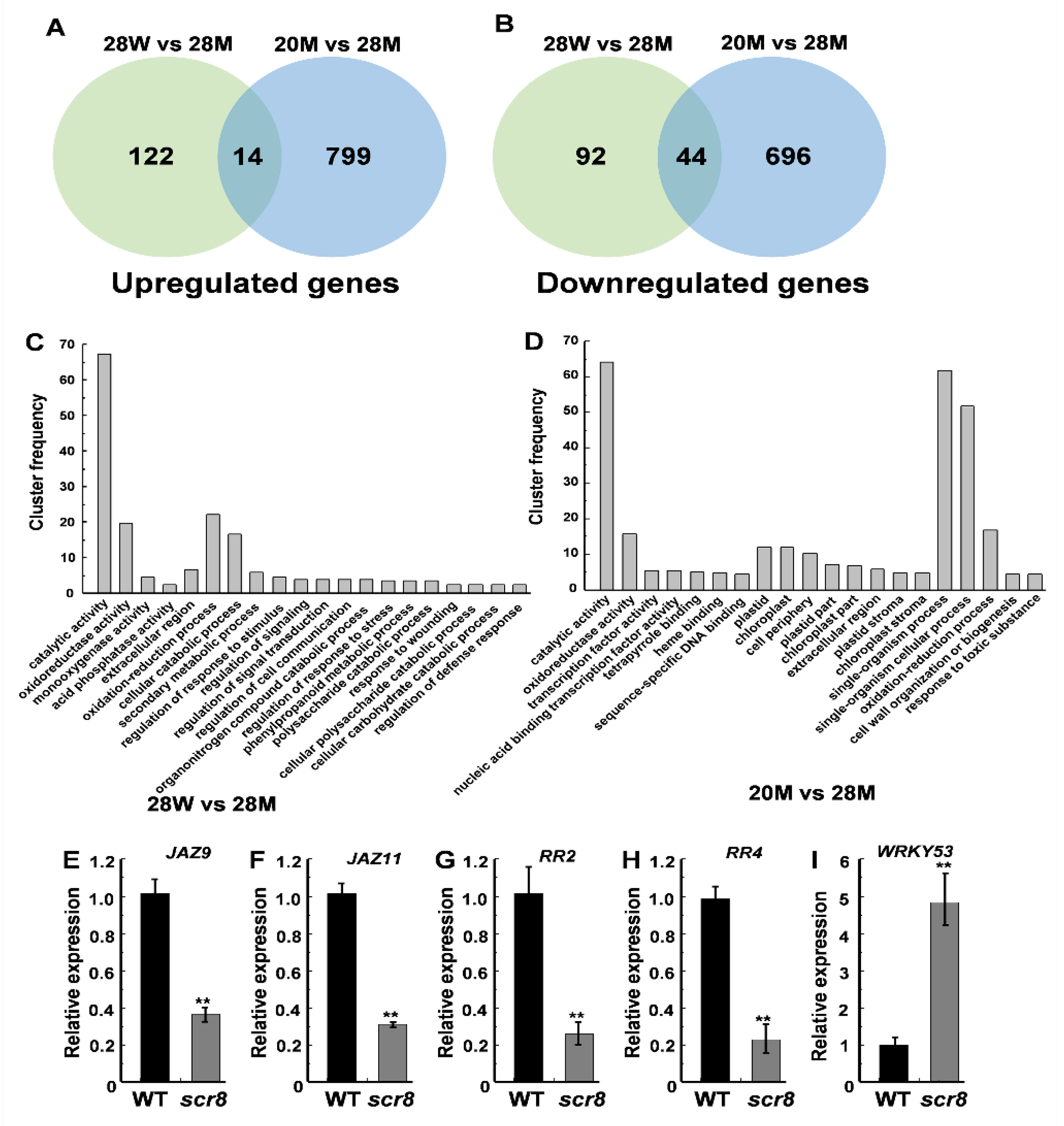
2.7. Transcriptome Analysis of the Wildtype and Scr8 Mutant
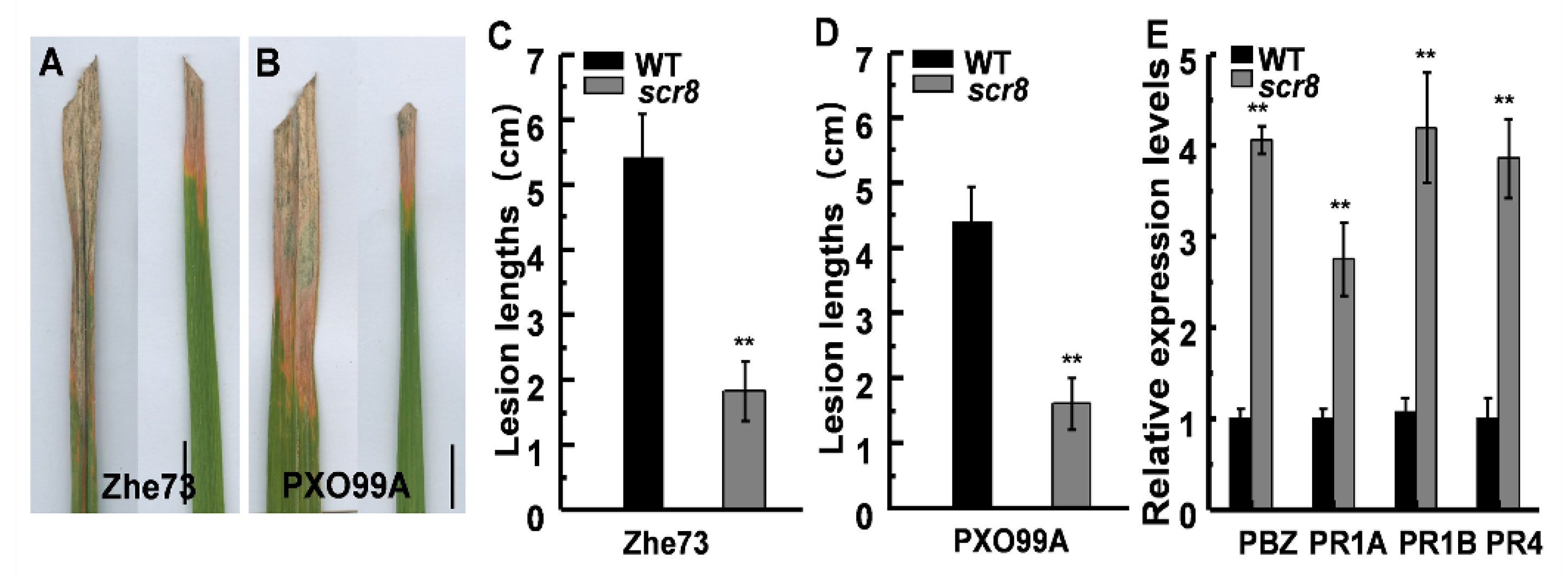
2.8. Scr8 Showed Enhanced Resistance to Xoo
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Phenotypical Characterization and Histological Analysis
4.3. EdU Staining
4.4. Pathological Analysis
4.5. Determination of Free SA and JA by HPLC
4.6. Map-Based Cloning
4.7. RNA Extraction and qRT-PCR Analysis
4.8. RNA-Seq and Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, S.; Song, Y.; Huang, Y.; Zhou, S.; Liu, X.; Zhou, D.X. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell 2015, 27, 2469–2483. [Google Scholar] [CrossRef]
- Wang, X.F.; He, F.F.; Ma, X.X.; Mao, C.Z.; Hodgman, C.; Lu, C.G.; Wu, P. OsCAND1 is required for crown root emergence in rice. Mol. Plant 2011, 4, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Perilli, S.; Di-Mambro, R.; Sabatini, S. Growth and development of the root apical meristem. Curr. Opin. Plant Biol. 2012, 15, 17–23. [Google Scholar] [CrossRef]
- Zhai, H.; Zhang, X.; You, Y.; Lin, L.; Zhou, W.; Li, C. SEUSS integrates transcriptional and epigenetic control of root stem cell organizer specification. EMBO J. 2020, 39, e105047. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, H.Y.; Ruan, W.Y.; Deng, M.J.; Wang, F.; Peng, J.R.; Luo, J.; Chen, Z.X.; Yi, K.K. ABNORMAL INFLORESCENCE MERISTEM1 functions in salicylic acid biosynthesis to maintain proper reactive oxygen species levels for root meristem activity in rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Jiang, W.; Long, F.; Cheng, S.; Yang, W.; Zhao, Y.; Zhou, D.X. Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell 2017, 29, 1088–1104. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.M.; Yu, X.X.; Xu, X.W.; Li, Y.; Yuan, W.X.; Xu, Y.; Mao, C.Z.; Zhang, S.Q.; Xu, J. The YDA-MKK4/MKK5-MPK3/MPK6 cascade functions downstream of the rgf1-rgi ligand-receptor pair in regulating mitotic activity in root apical meristem. Mol. Plant 2020, 13, 1608–1623. [Google Scholar] [CrossRef]
- Lu, X.T.; Shi, H.Y.; Ou, Y.; Cui, Y.W.; Chang, J.K.; Peng, L.; Gou, X.P.; He, K.; Li, J. RGF1-RGI1, a peptide-receptor complex, regulates Arabidopsis root meristem development via a MAPK signaling cascade. Mol. Plant 2020, 2, 1594–1607. [Google Scholar] [CrossRef]
- Shao, Y.L.; Zhou, H.Z.; Wu, Y.R.; Zhang, H.; Lin, J.; Jiang, X.Y.; He, Q.J.; Zhu, J.S.; Li, Y.; Yu, H.; et al. OsSPL3, an SBP-domain protein, regulates crown root development in rice. Plant Cell 2019, 31, 1257–1275. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.S.; Li, Y.; Lin, J.; Wu, Y.R.; Guo, H.X.; Shao, Y.L.; Wang, F.; Wang, X.F.; Mo, X.R.; Zheng, S.J.; et al. CRD1, an XPO1 domain protein, regulates mirna accumulation and crown root development in rice. Plant J. 2019, 100, 328–342. [Google Scholar] [CrossRef]
- Martins, S.; Montiel-Jorda, A.; Cayrel, A.; Huguet, S.; Roux, C.P.; Ljung, K.; Vert, G. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat. Commun. 2017, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Guo, T.; Li, M.X.; Zhang, Y.M.; Yang, Y.B.; Ye, W.W.; Dong, N.Q.; Shi, C.L.; Kan, Y.; Xiang, Y.H.; et al. Translational regulation of plant response to high temperature by a dual function trnahis guanylyltransferase in rice. Mol. Plant 2019, 12, 1123–1142. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Lin, Y.S.; Shen, J.B.; Shan, J.X.; Qi, P.; Shi, M.; Zhu, M.Z.; Huang, X.H.; Feng, Q.; et al. A two-locus interaction causes interspecific hybrid weakness in rice. Nat. Commun. 2014, 5, 3357. [Google Scholar] [CrossRef] [Green Version]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, L.; Mongelard, G.; Flokova, K.; Pacurar, D.I.; Novak, O.; Staswick, P.; Kowalczyk, M.; Pacurar, M.; Demailly, H.; Geiss, G.; et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of endogenous auxin levels in plant root development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xun, Q.; Wu, Y.; Li, H.; Chang, J.; Ou, Y.; He, K.; Gou, X.; Tax, F.E.; Li, J. Two receptor-like protein kinases, MUSTACHES and MUSTACHES-LIKE, regulate lateral root development in Arabidopsis thaliana. New Phytol. 2020, 227, 1157–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xun, Q.; Guo, Y.; Zhang, J.; Cheng, K.; Shi, T.; He, K.; Hou, S.; Gou, X.; Li, J. Genome-wide expression pattern analyses of the Arabidopsis leucine-rich repeat receptor-like kinases. Mol. Plant 2016, 9, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Goh, T.; Joi, S.; Mimura, T.; Fukaki, H. The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 2012, 139, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Tao, W.; Sun, R.; Wang, J.; Li, C.; Kong, X.; Tian, H.; Ding, Z. PRH1 mediates ARF7-LBD dependent auxin signaling to regulate lateral root development in Arabidopsis thaliana. PLoS Genet. 2020, 16, e1008044. [Google Scholar] [CrossRef]
- Coudert, Y.; Le, V.A.; Adam, H.; Bes, M.; Vignols, F.; Jouannic, S.; Guiderdoni, E.; Gantet, P. Identification of CROWN ROOTLESS1-regulated genes in rice reveals specific and conserved elements of postembryonic root formation. New Phytol. 2015, 206, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, S.; Yu, X.; Yu, J.; He, X.; Zhang, S.; Shou, H.; Wu, P. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005, 43, 47–56. [Google Scholar] [CrossRef]
- Inukai, Y.; Sakamoto, T.; Ueguchi-Tanaka, M.; Yohko, S.; Kenji, G.; Iichiro, U.; Yasuko, H.; Motoyuki, A.; Hidemi, K.; Makoto, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an auxin response factor in auxin signaling. Plant Cell 2005, 17, 1387–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hu, Y.; Dai, M.; Huang, L.; Zhou, D.X. The wuschel-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 2009, 21, 736–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Fang, J.; Xu, F.; Wang, W.; Sun, X.; Chu, J.; Cai, B.; Feng, Y.; Chu, C. Cytokinin oxidase/dehydrogenase4 integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol. 2014, 165, 1035–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.; Zhang, Z.; Wu, F.; Feng, M.; Sun, Y.; Chen, W.W.; Cheng, Z.; Zhang, X.; Ren, Y.; Lei, C.; et al. The APC/CTE E3 ubiquitin ligase complex mediates the antagonistic regulation of root growth and tillering by aba and ga. Plant Cell 2020, 32, 1973–1987. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 2019, 177, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, J.; Zhai, Q.; Zhou, W.; Qi, L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.; Qi, J.; et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, J.; Wang, L.; Wang, X.; Xue, Y.; Wu, P.; Shou, H. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res. 2009, 19, 1110–1119. [Google Scholar] [CrossRef] [Green Version]
- Kitomi, Y.; Ito, H.; Hobo, T.; Aya, K.; Kitano, H.; Inukai, Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011, 67, 472–484. [Google Scholar] [CrossRef]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef] [Green Version]
- Pavelescu, I.; Vilarrasa-Blasi, J.; Planas-Riverola, A.; Gonzalez-Garcia, M.P.; Cano-Delgado, A.I.; Ibanes, M. A sizer model for cell differentiation in Arabidopsis thaliana root growth. Mol. Syst. Biol. 2018, 14, e7687. [Google Scholar] [CrossRef]
- Yu, P.; Gutjahr, C.; Li, C.; Hochholdinger, F. Genetic control of lateral root formation in cereals. Trends Plant Sci. 2016, 21, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pasternak, T.; Eiblmeier, M.; Ditengou, F.; Kochersperger, P.; Sun, J.; Wang, H.; Rennenberg, H.; Teale, W.; Paponov, I.; et al. Plastid-localized Glutathione Reductase2—Regulated glutathione redox status is essential for Arabidopsis root apical meristem maintenance. Plant Cell 2013, 25, 4451–4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Zhang, X.; Tan, Y.; Huang, J.B.; Zheng, Z.; Tao, L.Z. Phosphoethanolamine N-methyltransferase 1 contributes to maintenance of root apical meristem by affecting ROS and auxin-regulated cell differentiation in Arabidopsis. New Phytol. 2019, 224, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, W.; Zhang, X.; Peng, T.; Zou, Y.; Zhang, T.; Wang, H.; Liu, X.; Tao, L. Cystathionine beta-lyase is crucial for embryo patterning and the maintenance of root stem cell niche in Arabidopsis. Plant J. 2019, 99, 536–555. [Google Scholar] [CrossRef]
- Tang, J.Y.; Zhu, X.D.; Wang, Y.Q.; Liu, L.C.; Xu, B.; Li, F.; Fang, J.; Chu, C.C. Semi-dominant mutations in the CC-NB-LRR-TYPE R gene, NLS1, lead to constitutive activation of defense responses in rice. Plant J. 2011, 66, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Dong, L.; Jiang, Z.; Yi, K.; Zhang, J.; Zhang, Z.; Zhu, Z.; Wu, Y.; Xu, M.; Ni, J. A semi-dominant mutation in a CC-NB-LRR-type protein leads to a short-root phenotype in rice. Rice 2018, 11, 54. [Google Scholar] [CrossRef]
- Takken, F.L.; Goverse, A. How to build a pathogen detector: Structural basis of NB-LRR function. Curr. Opin. Plant Biol. 2012, 15, 375–384. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, W.; Hua, J. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog. 2010, 6, e1000844. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.E.; Ning, Y.S.; Zhang, Y.X.; Yu, N.; Zhao, C.D.; Zhan, X.D.; Wu, W.X.; Chen, D.B.; Wei, X.J.; Wang, G.L.; et al. OsCUL3a negatively regulates cell death and immunity by degrading OsNPR1 in rice. Plant Cell 2017, 29, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Y.C.; Jiao, R.; Wang, S.; Wu, X.M.; Ye, H.F.; Pan, C.Y.; Li, S.F.; Xin, D.D.; Zhou, W.Y.; Dai, G.X.; et al. SPL36 encodes a receptor-like protein kinase that regulates programmed cell death and defense responses in rice. Rice 2021, 14, 34. [Google Scholar]
- Wigge, P.A. Ambient temperature signalling in plants. Curr. Opin. Plant Biol. 2013, 16, 661–666. [Google Scholar] [CrossRef]
- Jeuken, M.J.; Zhang, N.W.; McHale, L.K.; Pelgrom, K.; Boer, D.E.; Lindhout, P.; Michelmore, R.W.; Visser, R.G.; Niks, R.E. Rin4 causes hybrid necrosis and race-specific resistance in an interspecific lettuce hybrid. Plant Cell 2009, 21, 3368–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, K.A.; Lee, S.H.; Patel, D.; Kumar, S.V.; Spartz, A.K.; Gu, C.; Ye, S.; Yu, P.; Breen, G.; Cohen, J.D.; et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. USA 2011, 108, 20231–20235. [Google Scholar] [CrossRef] [Green Version]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [Green Version]
- Yoneya, Y.; Wakabayashi, T.; Kato, K. The temperature sensitive hybrid breakdown 1 induces low temperature-dependent intrasubspecific hybrid breakdown in rice. Breed. Sci. 2021, 71, 268–276. [Google Scholar] [CrossRef]
- Fu, D.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.; Sela, H.; Fahima, T.; Dubcovsky, J. A Kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Gao, X.Q.; Feng, B.M.; Sheen, J.; Shan, L.B.; He, P. Plant immune response to pathogens differs with changing temperatures. Nat. Commun. 2013, 4, 2530. [Google Scholar] [CrossRef] [Green Version]
- Webb, K.M.; Ona, I.; Bai, J.; Garrett, K.; Mew, T.; Vera-Cruz, C.M.; Leach, J.E. A benefit of high temperature: Increased effectiveness of a rice bacterial blight disease resistance gene. New Phytol. 2010, 185, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.X.; Liu, Y.; Liu, S.J.; Mao, C.Z.; Wu, Y.R.; Wu, P. A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol. Plant 2012, 5, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Behrens, I.V.; Komatsu, M.; Zhang, Y.X.; Berendzen, K.W.; Niu, X.M.; Sakai, H.; Taramino, G.; Hochholdinger, F. Rootless with undetectable meristem 1 encodes a monocot-specific AUX/IAA protein that controls embryonic seminal and post-embryonic lateral root initiation in maize. Plant J. 2011, 66, 341–353. [Google Scholar] [CrossRef]
- Zheng, H.; Li, S.; Ren, B.; Zhang, J.; Ichii, M.; Taketa, S.; Tao, Y.; Zuo, J.; Wang, H. LATERAL ROOTLESS2, a cyclophilin protein, regulates lateral root initiation and auxin signaling pathway in rice. Mol. Plant 2013, 6, 1719–1721. [Google Scholar] [CrossRef] [Green Version]
- Kusumi, K.; Yaeno, T.; Kojo, K.; Hirayama, M.; Hirokawa, D.; Yara, A.; Iba, K. The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiol Plant 2006, 128, 651–661. [Google Scholar] [CrossRef]
- Raya-Gonzalez, J.; Pelagio-Flores, R.; Lopez-Bucio, J. The jasmonate receptor COI1 plays a role in jasmonate-induced lateral root formation and lateral root positioning in Arabidopsis thaliana. J. Plant Physiol. 2012, 169, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhao, P.X.; Cai, X.T.; Mao, J.L.; Miao, Z.Q.; Xiang, C.B. Integration of jasmonic acid and ethylene into auxin signaling in root development. Front. Plant Sci. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.B.; Shang, G.D.; Pan, Y.; Xu, Z.G.; Zhou, C.M.; Mao, Y.B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.W. AP2/ERF transcription factors integrate age and wound signals for root regeneration. Plant Cell 2019, 32, 226–241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.X.; Qiu, Z.; Zhang, Y.; et al. Jasmonate-mediated wound signalling promotes plant regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Banos, PH, USA, 1971; p. 61. [Google Scholar]
- Ren, D.Y.; Rao, Y.C.; Huang, L.C.; Leng, Y.J.; Hu, J.; Lu, M.; Zhang, G.H.; Zhu, L.; Gao, Z.Y.; Dong, G.J.; et al. Fine mapping identifies a new QTL for brown rice rate in rice (oryza sativa l.). Rice 2016, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, B.P.; Hua, Z.H.; Zhao, J.; Zhang, B.; Ren, D.Y.; Liu, C.L.; Yang, S.L.; Zhang, A.P.; Jiang, H.Z.; Yu, H.P.; et al. OsACL-A2 negatively regulates cell death and disease resistance in rice. Plant Biotechnol. J. 2019, 17, 1344–1356. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhou, M.; Yoo, H.; Pruneda-Paz, J.L.; Spivey, N.W.; Kay, S.A.; Dong, X. N Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 9166–9173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Wen, Y.; Wang, Y.; Wu, H.; Wang, J.; Wu, K.; Chai, B.; Zhu, L.; Zhang, G.; Gao, Z.; et al. Identification and Characterization of Short Crown Root 8, a Temperature-Sensitive Mutant Associated with Crown Root Development in Rice. Int. J. Mol. Sci. 2021, 22, 9868. https://doi.org/10.3390/ijms22189868
Hu P, Wen Y, Wang Y, Wu H, Wang J, Wu K, Chai B, Zhu L, Zhang G, Gao Z, et al. Identification and Characterization of Short Crown Root 8, a Temperature-Sensitive Mutant Associated with Crown Root Development in Rice. International Journal of Molecular Sciences. 2021; 22(18):9868. https://doi.org/10.3390/ijms22189868
Chicago/Turabian StyleHu, Peng, Yi Wen, Yueying Wang, Hao Wu, Junge Wang, Kaixiong Wu, Bingze Chai, Lixin Zhu, Guangheng Zhang, Zhenyu Gao, and et al. 2021. "Identification and Characterization of Short Crown Root 8, a Temperature-Sensitive Mutant Associated with Crown Root Development in Rice" International Journal of Molecular Sciences 22, no. 18: 9868. https://doi.org/10.3390/ijms22189868
APA StyleHu, P., Wen, Y., Wang, Y., Wu, H., Wang, J., Wu, K., Chai, B., Zhu, L., Zhang, G., Gao, Z., Ren, D., Zhu, L., Guo, L., Zeng, D., Xu, J., Yan, S., Qian, Q., Rao, Y., & Hu, J. (2021). Identification and Characterization of Short Crown Root 8, a Temperature-Sensitive Mutant Associated with Crown Root Development in Rice. International Journal of Molecular Sciences, 22(18), 9868. https://doi.org/10.3390/ijms22189868










