One-Step Surface Immobilization of Protein A on Hydrogel Nanofibers by Core-Shell Electrospinning for Capturing Antibodies
Abstract
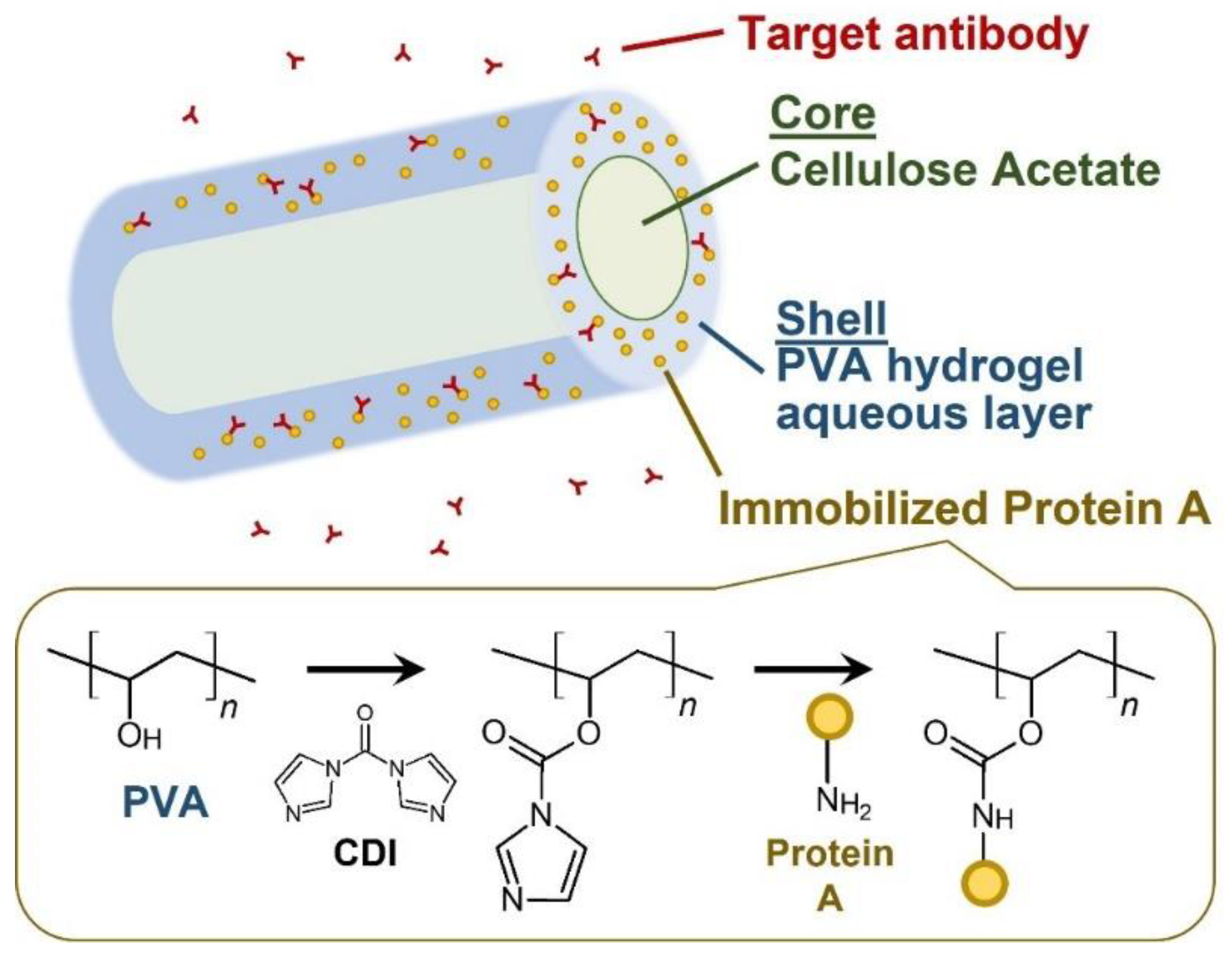
:1. Introduction
2. Experimental Methods
2.1. Fabrication of the NF Sheet
2.2. Morphological Observations
2.3. Attenuated Total Reflection Fourier-Transform Infrared Spectrometry (ATR-FTIR)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Quantification of the Antibody Absorbed
3. Results
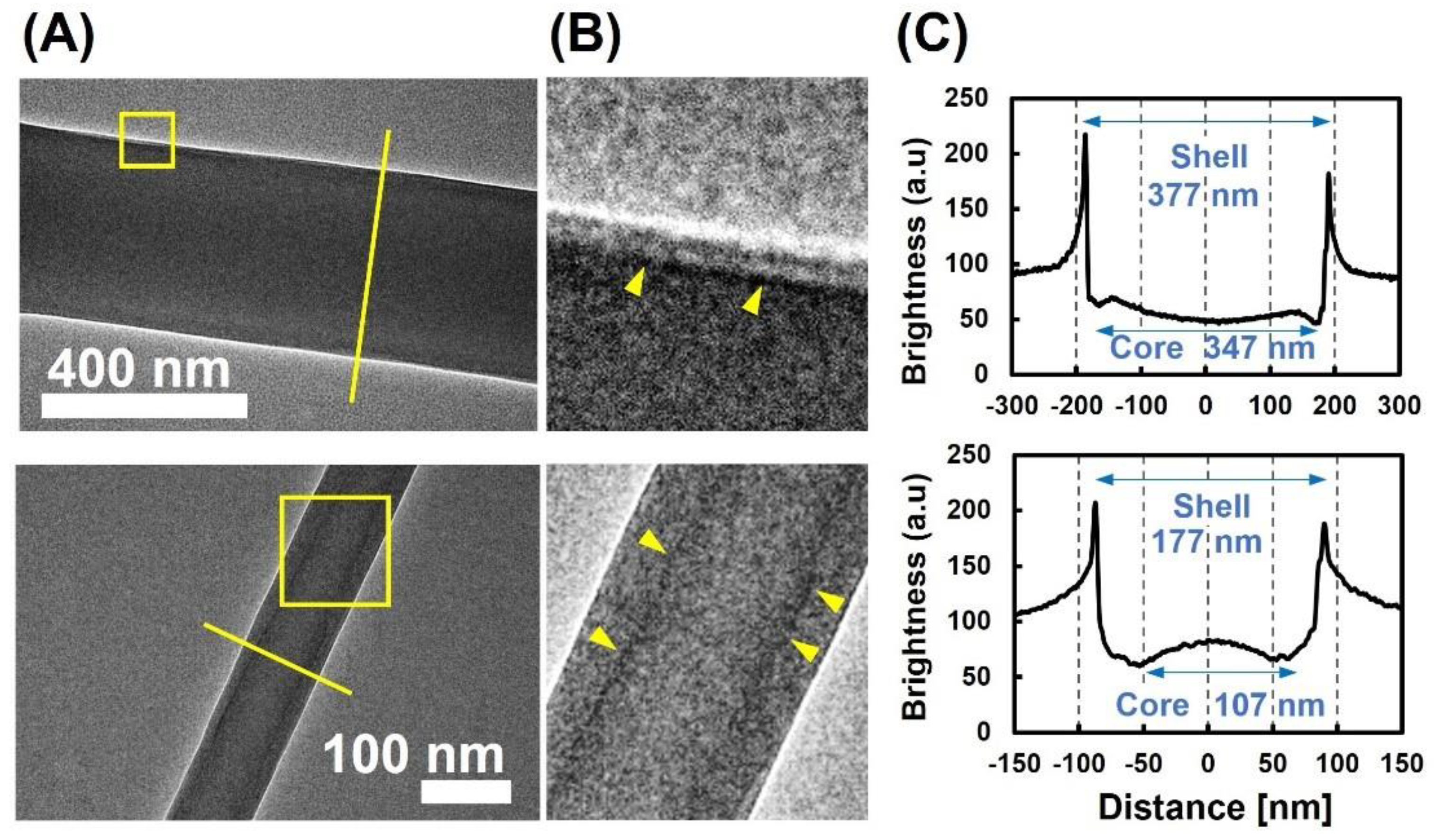
3.1. Morphological Observation of Protein-Immobilized Core-Shell NFs
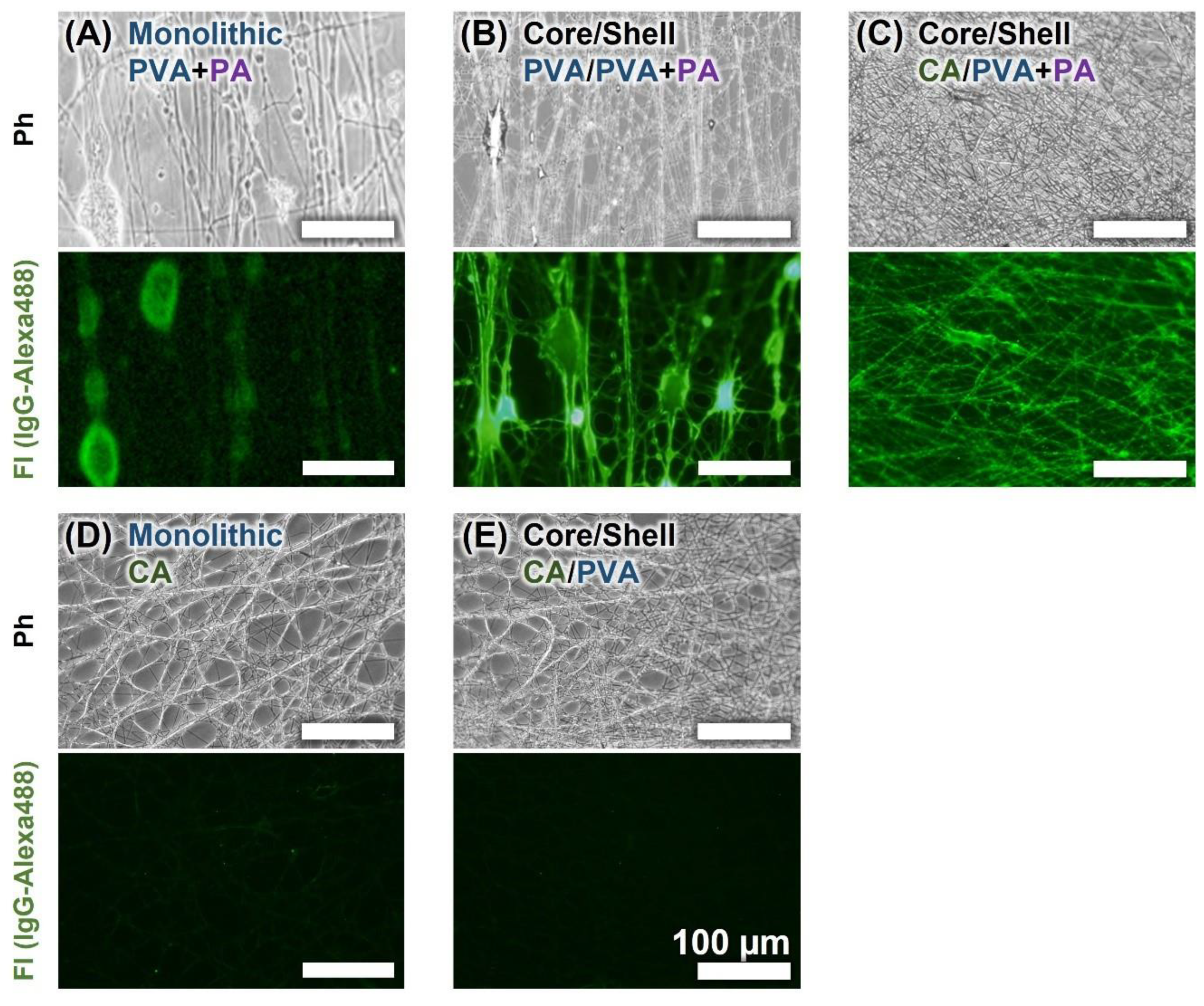
3.2. Fluorescent Observation of Protein-Immobilized Core-Shell NFs
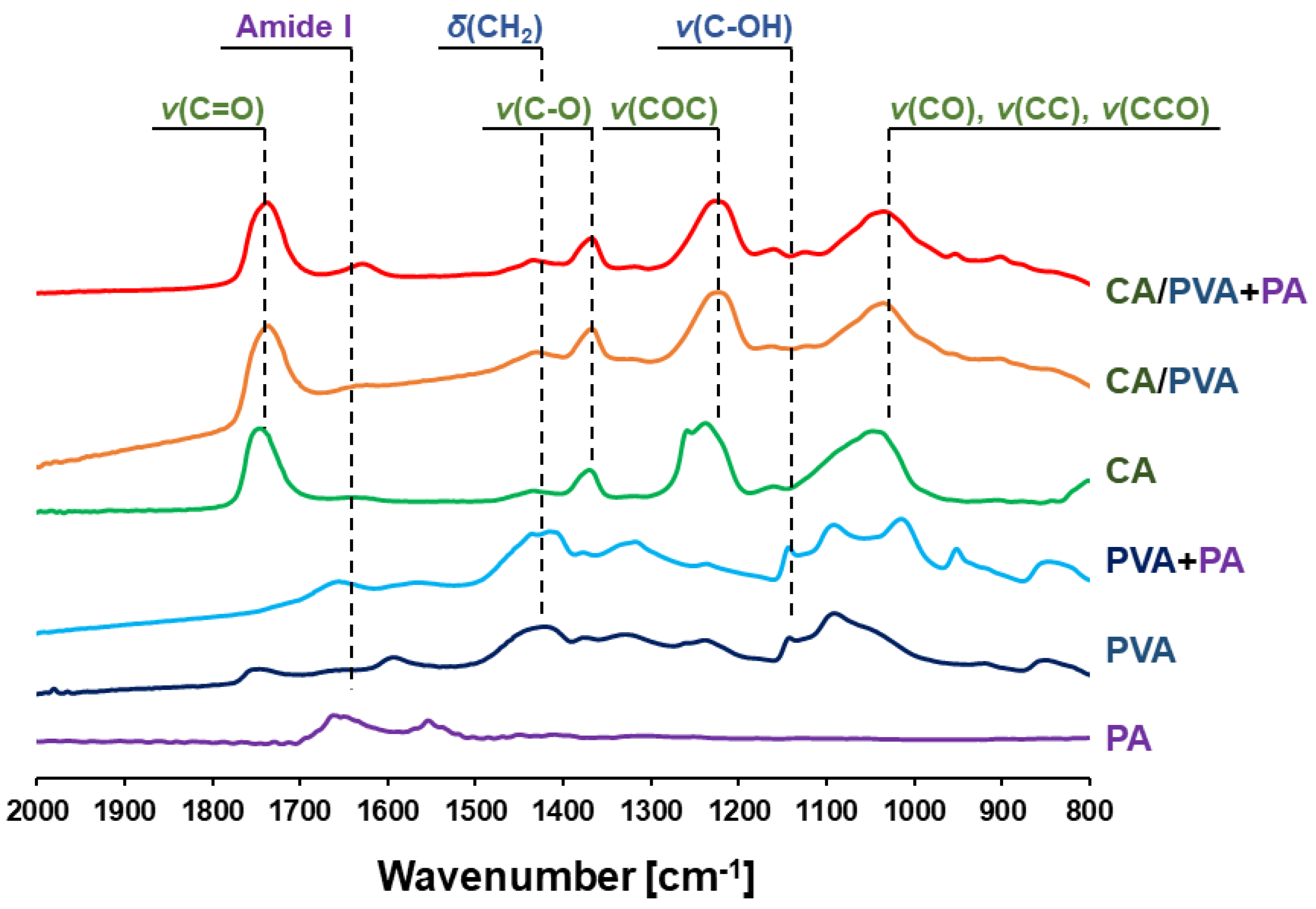
3.3. ATR-FTIR
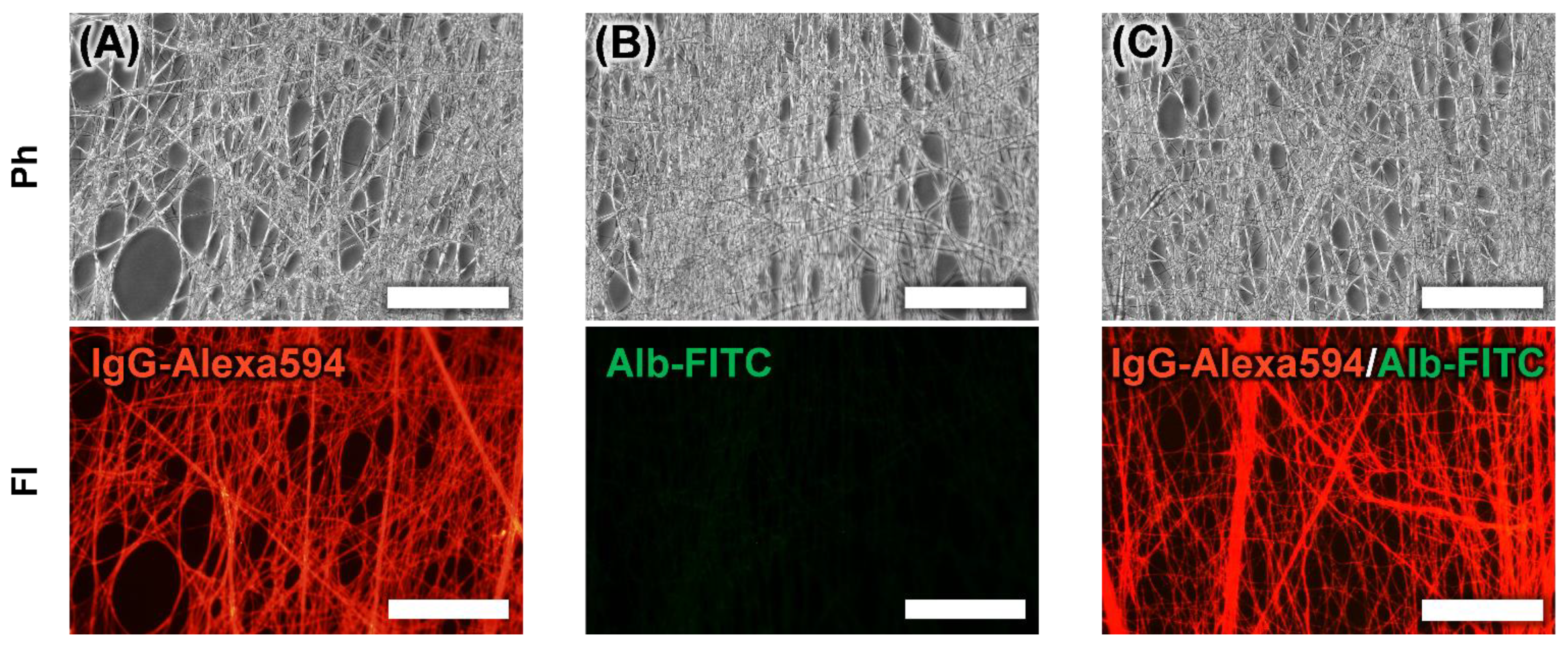
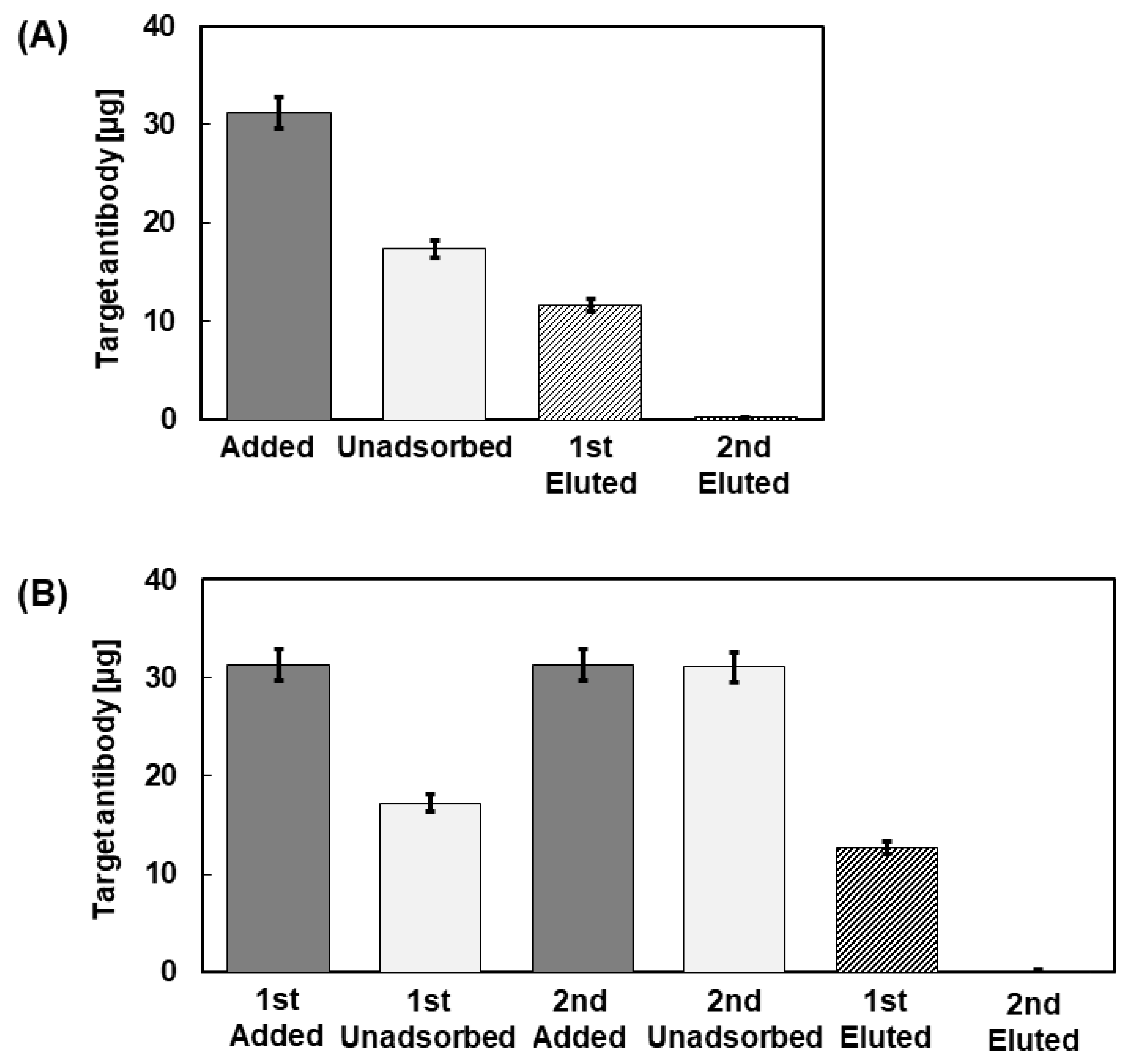
3.4. Adsorption of IgG to PA-Immobilized Core-Shell Fiber
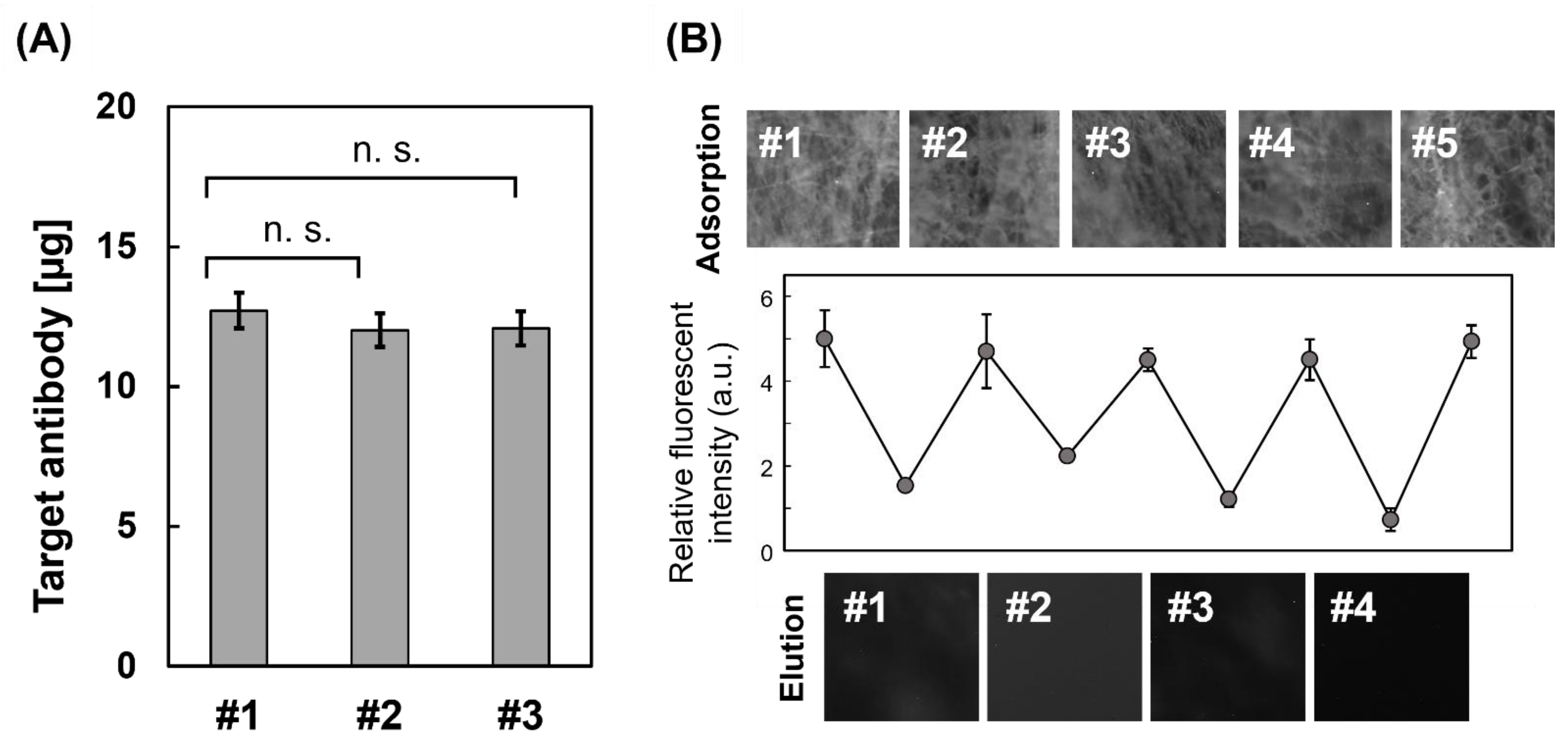
3.5. Evaluation of the Maximum Adsorption on the CA/PVA + PA Core-Shell Fiber
3.6. Reusability as an Affinity Carrier
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | protein A |
| CA | cellulose acetate |
| CDI | 1,1’-carbonyldiimidazole |
| PVA | polyvinyl alcohol |
| NF | nanofiber |
| SEM | scanning electron microscopy |
| ATR-FTIR | attenuated total reflection Fourier-transform infrared spectrometry |
References
- Ayyar, B.V.; Arora, S.; Murphy, C.; O’Kennedy, R. Affinity Chromatography as a tool for antibody purification. Methods 2012, 56, 116–129. [Google Scholar] [CrossRef]
- Avvakumova, S.; Colombo, M.; Tortora, P.; Prosperi, D. Biotechnological approaches toward nanoparticle biofunctionalization. Trends. Biotechnol. 2014, 32, 11–20. [Google Scholar] [CrossRef]
- Arora, S.; Saxena, V.; Ayyar, B.V. Affinitiy chromatography: A versatile technique for antibody purification. Methods 2017, 116, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sjöquist, J.; Meloun, B.; Hjelm, H. Protein A Isolated from Staphylococcus aureus after Digestion with Lysostaphin. Eur. J. Biochem. 1972, 29, 572–578. [Google Scholar] [CrossRef]
- Kim, D.; Herr, A.E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7, 041501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein immobilization strategies for protein biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Schoffelen, S. Recent advances in covalent, site-specific protein immobilization. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef]
- Sisson, T.H.; Castor, C.W. An improved method for immobilizing IgG antibodies on protein A-agarose. J. Immunol. Methods 1990, 127, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Chen, P. Chitosan/coarse filter paper composite membrane for fast purification of IgG from human serum. J. Membr. Sci. 2001, 205, 141–153. [Google Scholar] [CrossRef]
- Denizli, A.; Arica, Y. Protein A-immobilized microporous polyhydroxyethylmethacrylate affinity membranes for selective sorption of human-immunoglobulin-G from human plasma. J. Biomater. Sci. Polym. Ed. 2000, 11, 367–382. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Baillie, L.W.J.; Zheng, Y.; Lis, E.K.; Savina, I.N.; Howell, C.A.; Mikhalovsky, S.V.; Sandeman, S.R. Affinity binding of antibodies to supermacroporous cryogel adsorbents with immobilized protein A for removal of anthrax toxin protective antigen. Biomaterials 2015, 50, 140–153. [Google Scholar] [CrossRef] [Green Version]
- Castilho, L.; Anspach, F.; Deckwer, W. Comparison of affinity membranes for the purification of immunoglobulins. J. Membr. Sci. 2002, 207, 253–264. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhong, T.; Feng, X. Optimal Spacer Arm Microenvironment for the Immobilization of Recombinant Protein A on Heterofunctional Amino-Epoxy Agarose Supports. Process Biochem. 2019, 91, 90–98. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Ramakrishna, S. Electospun cellulose nanofiber as affinity membrane. J. Membr. Sci. 2005, 265, 115–123. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Fujita, S.; Shimizu, H.; Suye, S. Control of Differentiation of Human Mesenchymal Stem Cells by Altering the Geometry of Nanofibers. J. Nanotechnol. 2012, 2012, 429890. [Google Scholar] [CrossRef] [Green Version]
- Wakuda, Y.; Nishimoto, S.; Suye, S.; Fujita, S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Sci. Rep. 2018, 8, 6248. [Google Scholar] [CrossRef]
- Fujita, S.; Wakuda, Y.; Matsumura, M.; Suye, S. Geometrically customizable alginate hydrogel nanofibers for cell culture platforms. J. Mater. Chem. B 2019, 7, 6556–6563. [Google Scholar] [CrossRef]
- Huang, W.Y.; Hibino, T.; Suye, S.; Fujita, S. Electrospun collagen core/poly-l-lactic acid shell nanofibers for prolonged release of hydrophilic drug. RSC Adv. 2021, 11, 5703–5711. [Google Scholar] [CrossRef]
- Barrett, D.A.; Hartshorne, M.S.; Hussain, M.A.; Shaw, P.N.; Davies, M.C. Resistance to Nonspecific Protein Adsorption by Poly(vinyl alcohol) Thin Films Adsorbed to a Poly(styrene) Support Matrix Studied Using Surface Plasmon Resonance. Anal. Chem. 2001, 73, 5232–5239. [Google Scholar] [CrossRef]
- Castilho, L.R.; Deckwer, W.-D.; Anspach, F.B. Influence of matrix activation and polymer coating on the purification of human IgG with protein A affinity membranes. J. Membr. Sci. 2000, 172, 269–277. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Review of the research on anti-protein fouling coatings materials. Prog. Org. Coat. 2020, 147, 105860. [Google Scholar] [CrossRef]
- de Jongh, H.H.; Goormaghtigh, E.; Ruysschaert, J.M. The different molar absorptivities of the secondary structure types in the amide I region: An attenuated total reflection infrared study on globular proteins. Anal. Biochem. 1996, 242, 95–103. [Google Scholar] [CrossRef]
- Beattie, J.W.; Rowland-Jones, R.C.; Farys, M.; Tran, R.; Kazarian, S.G.; Byrne, B. Insight into purification of monoclonal antibodies in industrial columns via studies of Protein A binding capacity by in situ ATR-FTIR spectroscopy. Analyst 2021. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J.P. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Matharu, Z.; Bandodkar, A.J.; Sumana, G.; Solanki, P.R.; Ekanayake, E.M.I.M.; Kaneto, K.; Gupta, V.; Malhotra, B.D. Low Density Lipoprotein Detection Based on Antibody Immobilized Self-Assembled Monolayer: Investigations of Kinetic and Thermodynamic Properties. J. Phys. Chem. B 2009, 113, 14405–14412. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Ozalp, V.C.; Arica, M.Y. Adsorption and separation of immunoglobulins by novel affinity core-shell beads decorated with Protein L and l-histidine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 936, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.W. Physical Chemistry of Surfaces, 5th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Nakanishi, K.; Tomita, M.; Masuda, Y.; Kato, K. Gold nanoparticle–mesoporous silica sheet composites with enhanced antibody adsorption capacity. New J. Chem. 2015, 39, 4070–4077. [Google Scholar] [CrossRef]
- Fahrner, R.; Iyer, H.; Blank, G. The optimal flow rate and column length for maximum production rate of protein A affinity chromatography. Bioprocess Eng. 1999, 21, 287–292. [Google Scholar] [CrossRef]
- Yang, L.; Biswas, M.E.; Chen, P. Study of binding between Protein A and Immunoglobulin G Using a Surface Tension Probe. Biophys. J. 2003, 84, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Jansson, B.; Uhlén, M.; Nygren, P.A. All individual domains of staphylococcal protein A show Fab binding. FEMS Immunol. Med. Microbiol. 1998, 20, 69–78. [Google Scholar] [CrossRef]
- Salva, M.L.; Rocca, M.; Niemeyer, C.M.; Delamarche, E. Methods for immobilizing receptors in microfluidic devices: A review. Micro Nano Eng. 2021, 11, 100085. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Zeng, X. Improved Performance of Recombinant Protein A Immobilized on Agarose Beads by Site-Specific Conjugation. ACS Omega 2017, 2, 1731–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaveh-Baghbaderani, Y.; Allgayer, R.; Schwaminger, S.P.; Fraga-García, P.; Berensmeier, S. Magnetic Separation of Antibodies with High Binding Capacity by Site-Directed Immobilization of Protein A-Domains to Bare Iron Oxide Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 4956–4963. [Google Scholar] [CrossRef]
- Putti, M.; de Jong, S.M.J.; Stassen, O.M.J.A.; Sahlgren, C.M.; Dankers, P.Y.W. A Supramolecular Platform for the Introduction of Fc-Fusion Bioactive Proteins on Biomaterial Surfaces. ACS Appl. Polym. Mater. 2019, 1, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Qafari, S.M.; Ahmadian, G.; Mohammadi, M. One-step purification and oriented attachment of protein A on silica and graphene oxide nanoparticles using sortase-mediated immobilization. RSC Adv. 2017, 7, 56006–56015. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Lan, Z.; Matsuura, T.; Ramakrishna, S. Electrospun polyethersulfone affinity membrane: Membrane preparation and performance evaluation. J. Chromatogr. B 2009, 29, 3686–3694. [Google Scholar] [CrossRef]
- Klein, E.; Yeager, D.; Seshadri, R.; Baurmeister, U. Affinity adsorption devices prepared from microporous poly(amide) hollow fibers and sheet membranes. J. Membr. Sci. 1997, 129, 31–46. [Google Scholar] [CrossRef]


| Carrier | Antibody Binding Capacity | Bed Volume | Max Flow Rate |
|---|---|---|---|
| Agarose gel beads 1 | 50 mg/mL(gel) = 100 mg/device | 2mL (gel) | 2 mL/min |
| CA porous membrane 1 | 0.08 mg/cm2 = 6 mg/device | 2.1 mL (75 cm2) | 10 mL/min |
| CA/PVA + PA NF (this study) | 0.04 mg/cm2 = 3 mg/device | More than 2.1 mL (More than 75 cm2) | More than 10 mL/min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naganuma, C.; Moriyama, K.; Suye, S.-i.; Fujita, S. One-Step Surface Immobilization of Protein A on Hydrogel Nanofibers by Core-Shell Electrospinning for Capturing Antibodies. Int. J. Mol. Sci. 2021, 22, 9857. https://doi.org/10.3390/ijms22189857
Naganuma C, Moriyama K, Suye S-i, Fujita S. One-Step Surface Immobilization of Protein A on Hydrogel Nanofibers by Core-Shell Electrospinning for Capturing Antibodies. International Journal of Molecular Sciences. 2021; 22(18):9857. https://doi.org/10.3390/ijms22189857
Chicago/Turabian StyleNaganuma, Chihiro, Kosuke Moriyama, Shin-ichiro Suye, and Satoshi Fujita. 2021. "One-Step Surface Immobilization of Protein A on Hydrogel Nanofibers by Core-Shell Electrospinning for Capturing Antibodies" International Journal of Molecular Sciences 22, no. 18: 9857. https://doi.org/10.3390/ijms22189857
APA StyleNaganuma, C., Moriyama, K., Suye, S.-i., & Fujita, S. (2021). One-Step Surface Immobilization of Protein A on Hydrogel Nanofibers by Core-Shell Electrospinning for Capturing Antibodies. International Journal of Molecular Sciences, 22(18), 9857. https://doi.org/10.3390/ijms22189857






