Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated through ROS-Triggered Apoptosis
Abstract
:1. Introduction
2. Results and Discussion
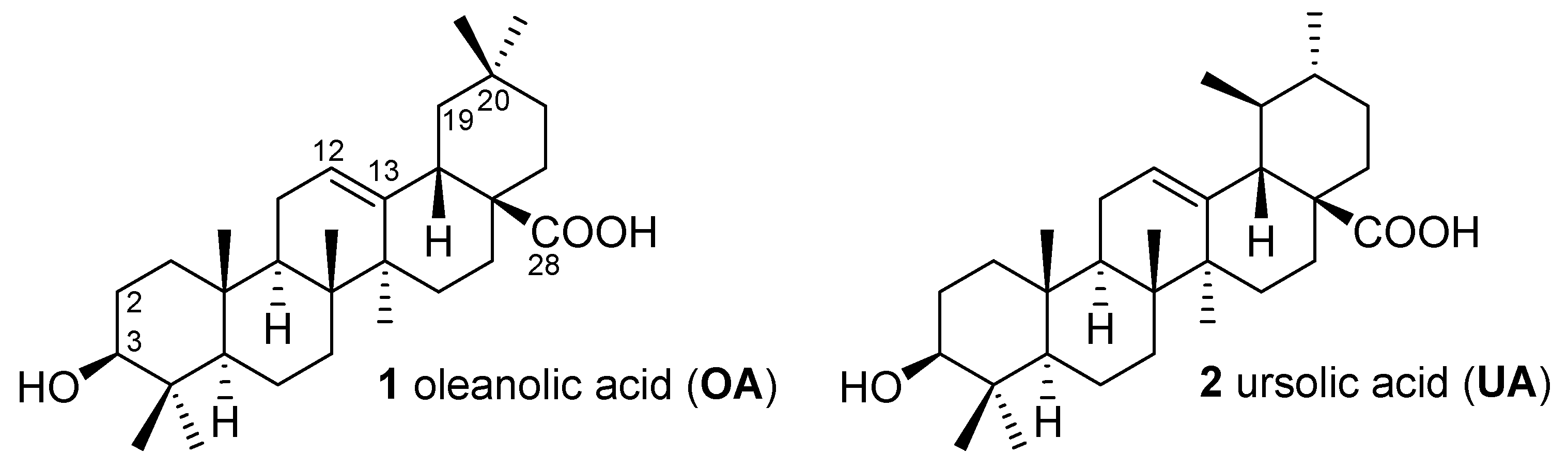
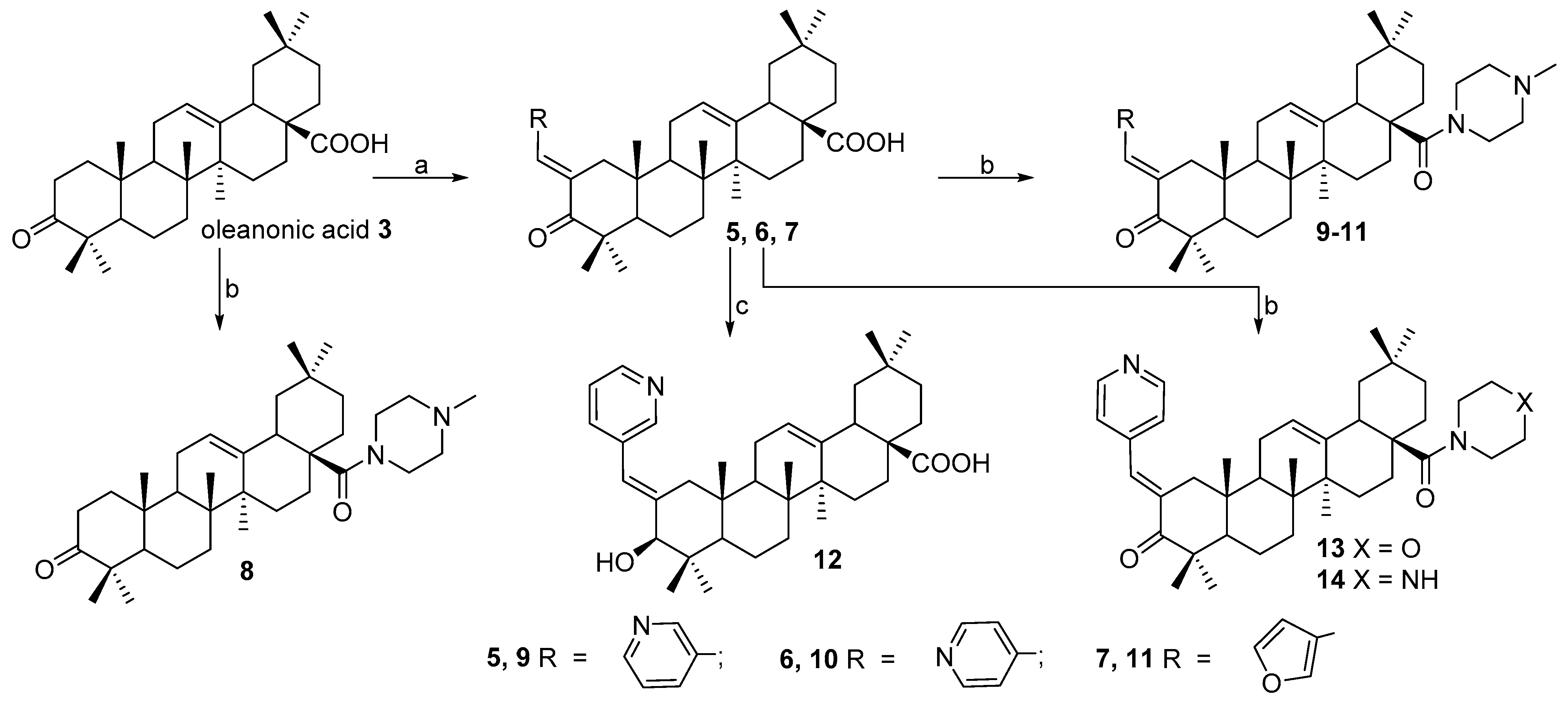
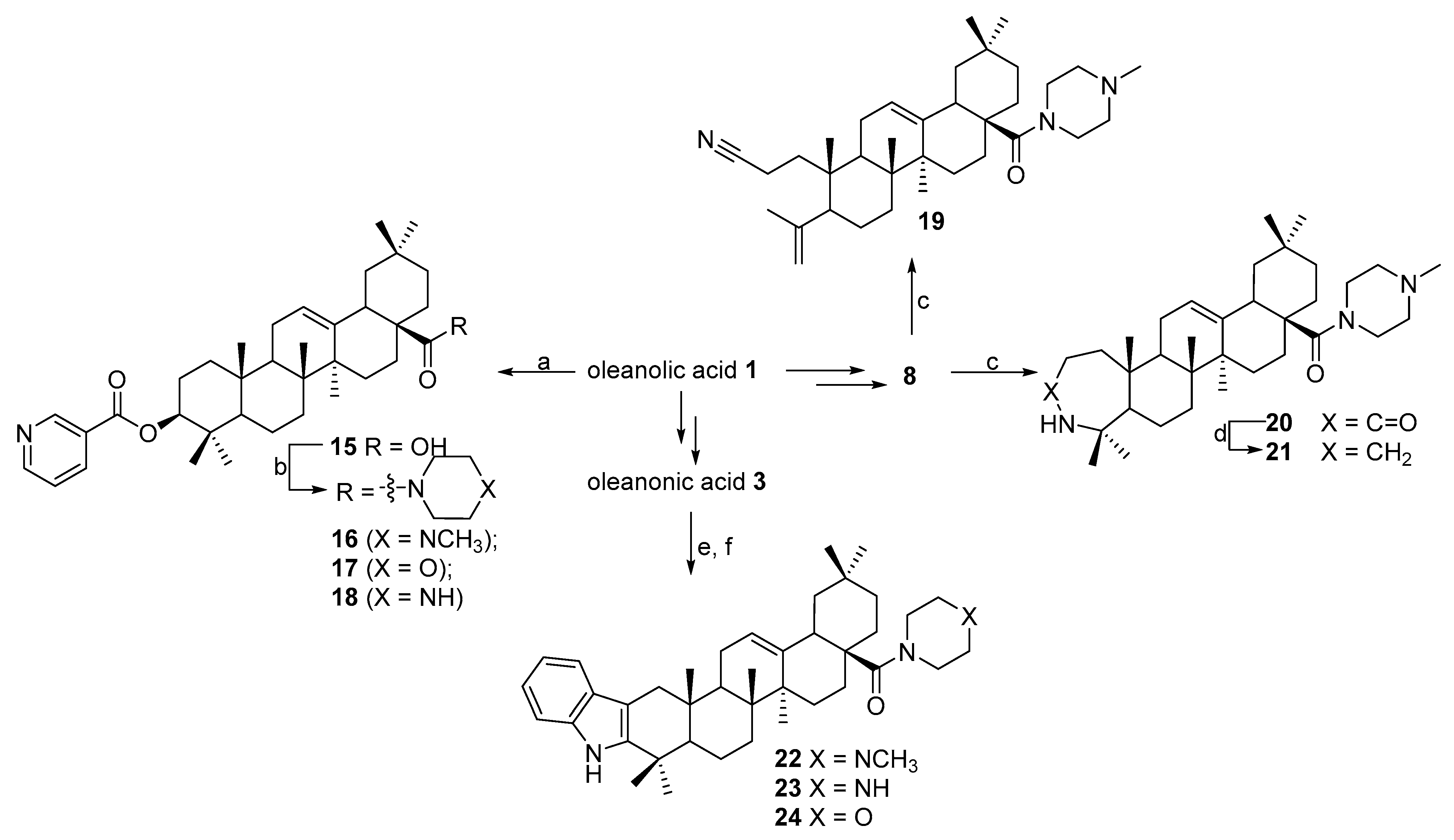
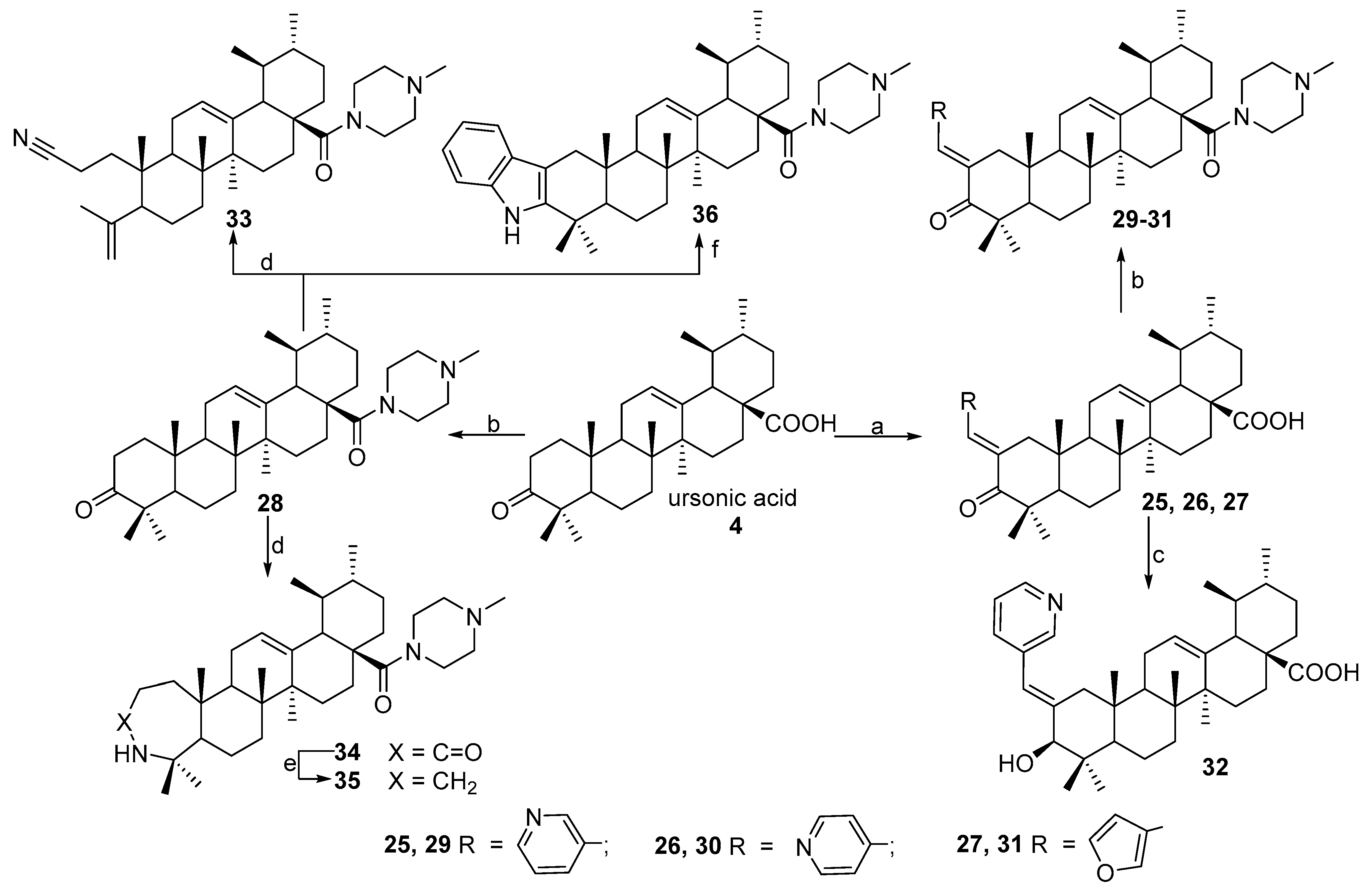
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. NCI-60 Anticancer Drug Screening
2.2.2. Mechanisms In Vitro Studies
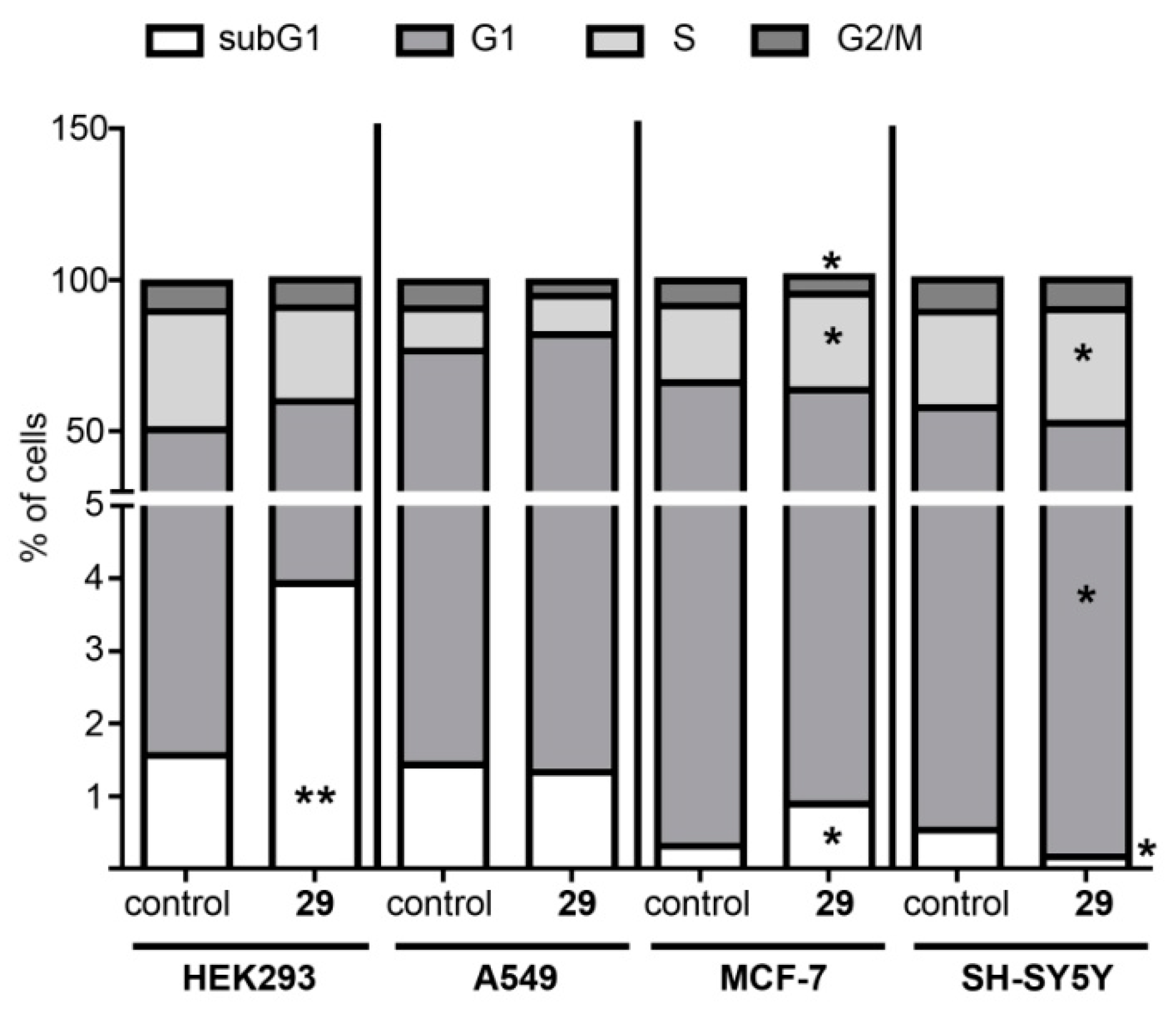
Cell Cycle Analysis
The Cell Apoptosis Assay for Compound 29
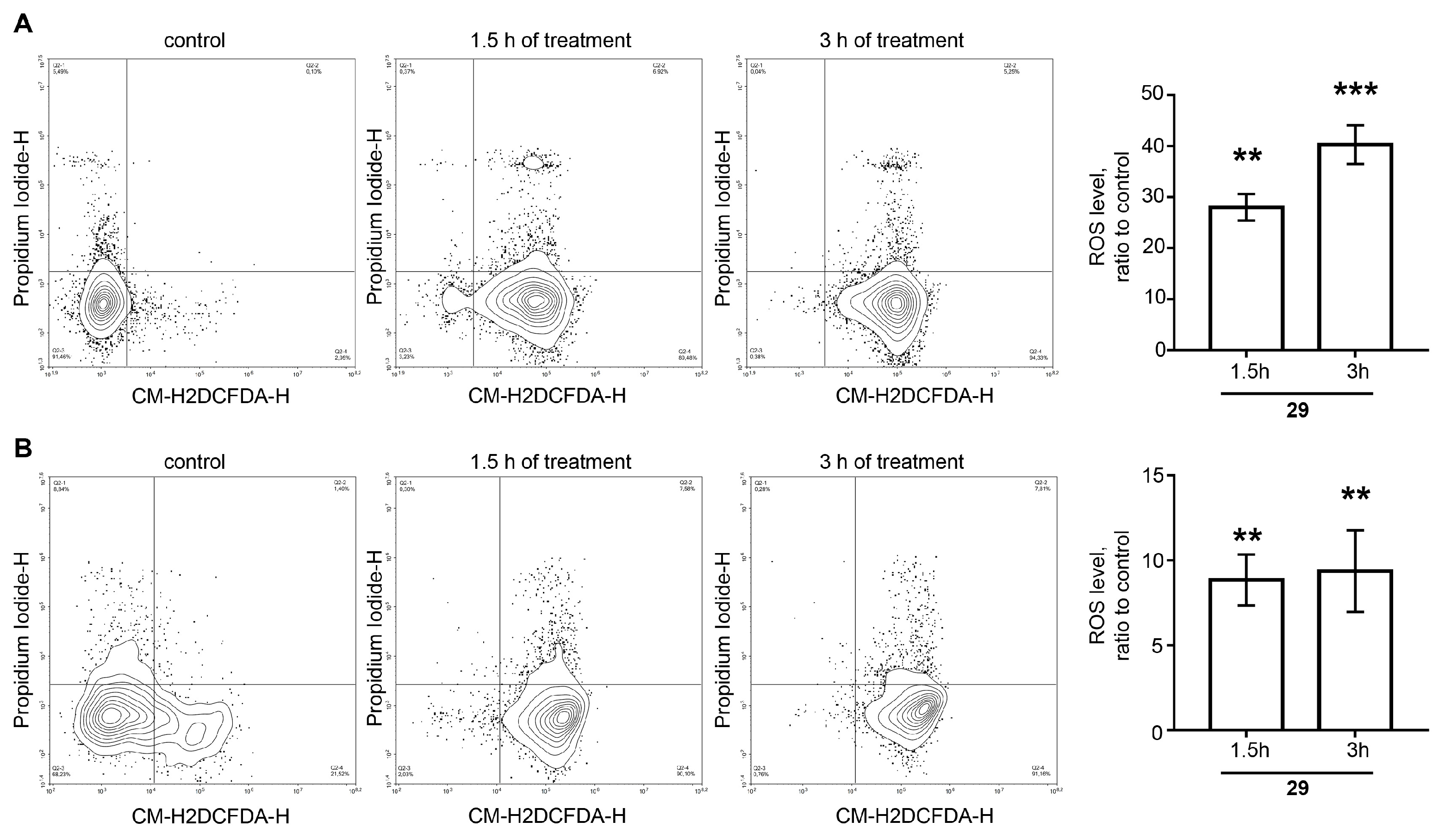
Measurement of Intracellular Reactive Oxygen Species Level for Compound 29
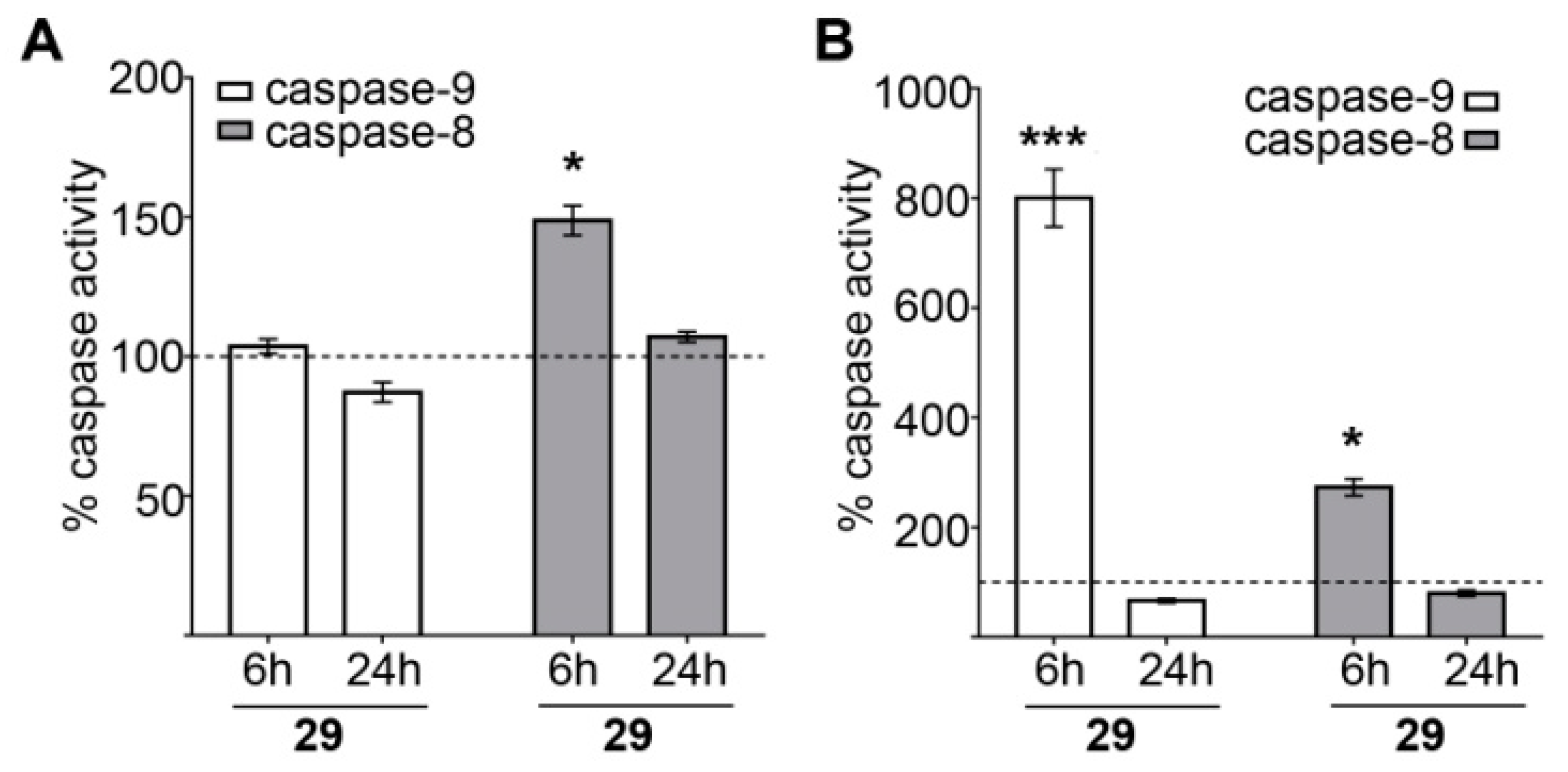
Caspase 8, 9 Activity Assay for Compound 29
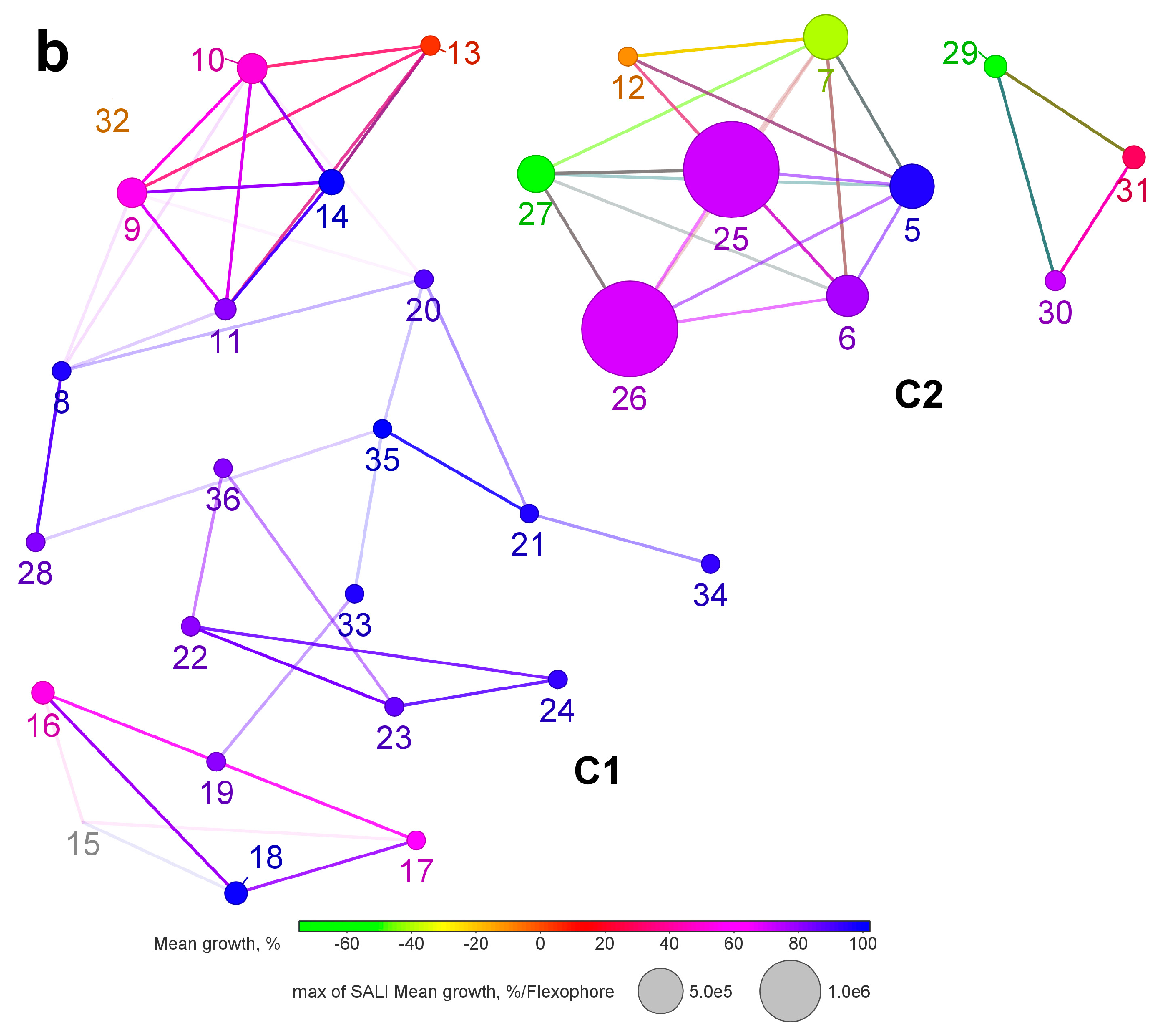
2.3. Structure-Activity Analysis with Network-like Similarity Graphs
2.4. CellMiner and Gene Enrichment Analysis
2.5. Computational ADMET Profiling of Compound 29
3. Materials and Methods
3.1. Experimental Part
3.1.1. General
3.1.2. Synthesis of Compounds 7 and 27
3.1.3. Synthesis of Compounds 9–11, 13, 14, 16–18, 22–24, 29–31, 36
3.1.4. Synthesis of Compounds 12 and 32
3.1.5. Synthesis of Compounds 19, 20, 33 and 34
3.1.6. Synthesis of Compounds 21 and 35
3.2. NCI-60 Cytotoxicity Drug Screen
3.3. Cell Cycle Analysis
3.4. Cell Apoptosis Assay
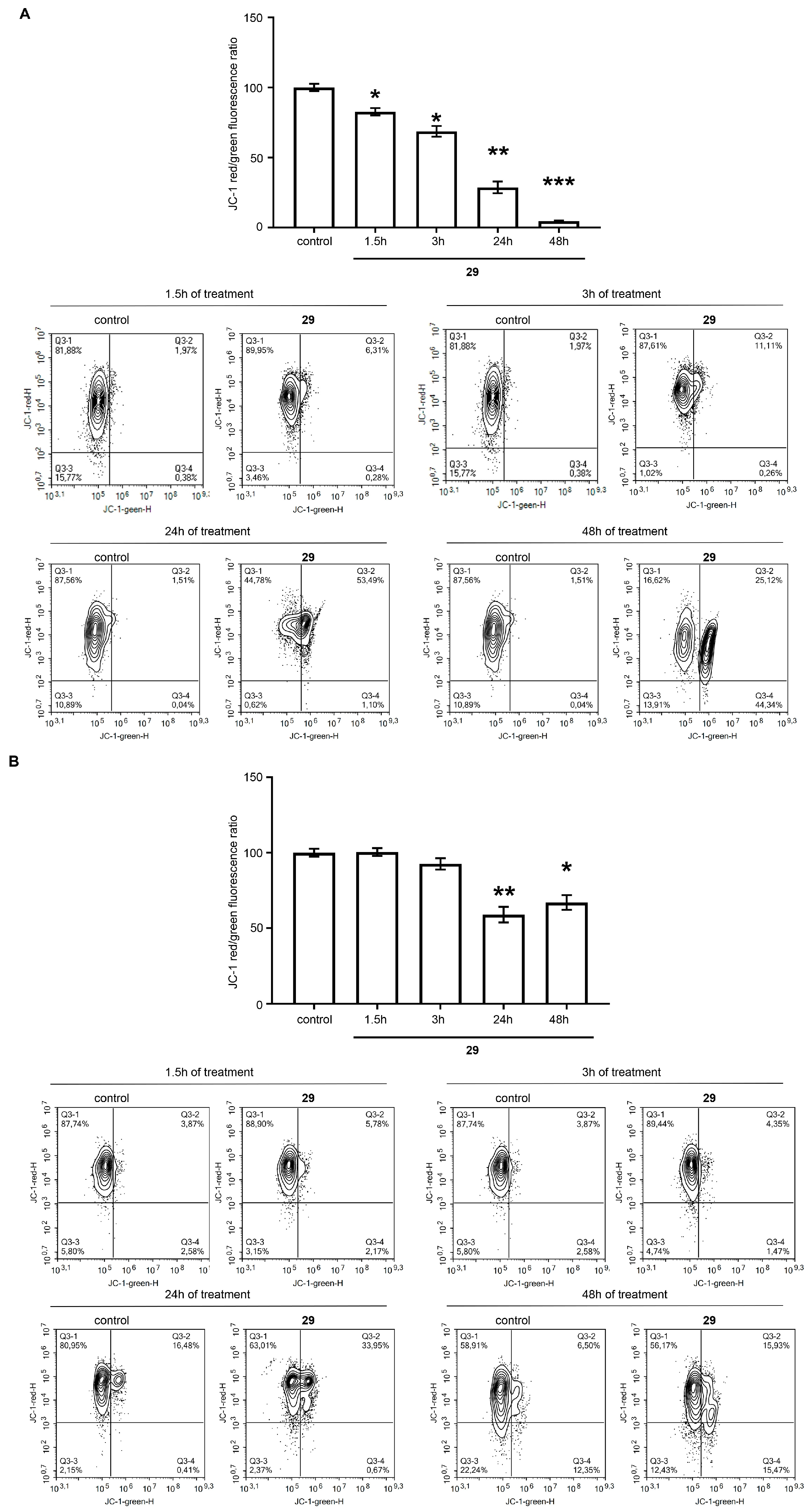
3.5. Mitochondrial Membrane Potential Assay
3.6. Caspase 8, 9 Activity Assay
3.7. Measurement of Intracellular Reactive Oxygen Species Level
3.8. Statistical Analysis
3.9. Network-like Similarity Graphs Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023; WHO Press: Geneva, Switzerland, 2013. [Google Scholar]
- Che, C.-T.; Zhang, H. Plant Natural Products for Human Health. Int. J. Mol. Sci. 2019, 20, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Kinghorn, A.D. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Med. 2019, 85, 802. [Google Scholar] [CrossRef] [Green Version]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a gamma-cyclodextrin derivative decreases proliferation and in vivo tumor development of non-metastatic and metastatic B164A5 cells. Int. J. Mol. Sci. 2014, 15, 8235. [Google Scholar] [CrossRef] [Green Version]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Marzaro, G.; Dall Acqua, S. Known Triterpenes and their Derivatives as Scaffolds for the Development of New Therapeutic Agents for Cancer. Curr. Med. Chem. 2018, 25, 1259. [Google Scholar] [CrossRef] [PubMed]
- Borella, R.; Forti, L.; Gibellini, L.; De Gaetano, A.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Synthesis and Anticancer Activity of CDDO and CDDO-Me, Two Derivatives of Natural Triterpenoids. Molecules 2019, 24, 4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, T.; Honda, Y.; Favaloro, F.G., Jr.; Gribble, G.W.; Suh, N.; Place, A.E.; Rendi, M.H.; Sporn, M.B. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg. Med. Chem. Lett. 2002, 12, 1027. [Google Scholar] [CrossRef]
- Liby, K.; Royce, D.B.; Williams, C.R.; Risingsong, R.; Yore, M.M.; Honda, T.; Gribble, G.W.; Dmitrovsky, E.; Sporn, T.A.; Sporn, M.B. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007, 67, 2414. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Waddington, J.C.; Tailor, A.; Lister, A.; Hamlett, J.; Berry, N.; Park, B.K.; Sporn, M.B. CDDO-imidazolide Targets Multiple Amino Acid Residues on the Nrf2 Adaptor, Keap1. J. Med. Chem. 2020, 63, 9965. [Google Scholar] [CrossRef]
- Song, D.; Gao, Y.; Wang, R.; Liu, D.; Zhao, L.; Jing, Y. Downregulation of c-FLIP, XIAP and Mcl-1 protein as well as depletion of reduced glutathione contribute to the apoptosis induction of glycyrrhetinic acid derivatives in leukemia cells. Cancer Biol. Ther. 2010, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subba, R.G.S.; Kondaiah, P.; Singh, S.K.; Ravanan, P.; Sporn, M.B. Chemical modifications of natural triterpenes-glycyrrhetinic and boswellic acids: Evaluation of their biological activity. Tetrahedron 2008, 64, 11541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadalapaka, G.; Jutooru, I.; McAlees, A.; Stefanac, T.; Safe, S. Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markov, A.V.; Odarenko, K.V.; Sen’kova, A.V.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A. Cyano Enone-Bearing Triterpenoid Soloxolone Methyl Inhibits Epithelial-Mesenchymal Transition of Human Lung Adenocarcinoma Cells In Vitro and Metastasis of Murine Melanoma In Vivo. Molecules 2020, 25, 5925. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lin, Q.X.; Liby, K.T.; Sporn, M.B.; Gribble, G.W. An efficient synthesis of methyl 2-cyano-3,12-dioxoursol-1,9-dien-28-oate (CDDU-methyl ester): Analogues, biological activities, and comparison with oleanolic acid derivatives. Org. Biomol. Chem. 2014, 12, 5192. [Google Scholar] [CrossRef] [PubMed]
- Bartona, D.H.R.; Head, J.; May, P.J. Long-range effects in alicyclic systems. Part II. The rates of condensation of some triterpenoid ketones with benzaldehyde. J. Chem. Soc. 1957, 935–944. [Google Scholar] [CrossRef]
- Wu, P.; Tu, B.; Liang, J.; Guo, S.; Cao, N.; Chen, S.; Luo, Z.; Li, J.; Zheng, W.; Tang, X.; et al. Synthesis and biological evaluation of pentacyclic triterpenoid derivatives as potential novel antibacterial agents. Bioorg. Chem. 2021, 109, 104692. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Zhang, T.; Wei, Z.; Wang, H.; Guo, F.; Zheng, C.; Piao, H. Synthesis and biological evaluation of ursolic acid derivatives containing an aminoguanidine moiety. Med. Chem. Res. 2019, 28, 959. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Gonçalves, C.; Ferreira, R.M.; Cardoso, S.M.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Functionalization of Betulinic Acid with Polyphenolic Fragments for the Development of New Amphiphilic Antioxidants. Antioxidants 2021, 10, 148. [Google Scholar] [CrossRef]
- Naglah, A.M.; El-Galil, A.B.D.E.A.M.R.; Al-Omar, M.A. Oleanolic Acid Methyl Ester Derivatives. U.S. Patent US-9969768-B1, 15 May 2018. [Google Scholar]
- Chu, T.; Linhui, Z.; Yu, C.; Rui, Q.; Zhi Nan, N.M.; Jing, X.; Guangzhong, Y. Synthesis and biologic evaluation of oleanolic acid derivative-chalcone conjugates as a-glucosidase inhibitors. RSC Adv. 2014, 4, 10862. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013, 62, 1791. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Li, J.H.; Zhang, Y.W.; Chen, X.; Wu, Y. Synthesis and alpha-glucosidase inhibitory activity of oleanolic acid derivatives. J. Asian Nat. Prod. Res. 2010, 12, 20. [Google Scholar] [CrossRef]
- Gupta, N.; Rath, S.K.; Singh, J.; Qayum, A.; Singh, S.; Sangwan, P.L. Synthesis of novel benzylidene analogues of betulinic acid as potent cytotoxic agents. Eur. J. Med. Chem. 2017, 135, 517. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.; Galimova, Z.; Lobov, A.; Baikova, I.; Kazakova, O.; Thu, H.N.T.; Tuyen, N.V.; Gatilov, Y.; Csuk, R.; Serbian, I.; et al. Synthesis of messagenin and platanic acid chalcone derivatives and their biological potential. Nat. Prod. Res. 2021, 1–10. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Li, W.; Miao, D.; Li, Y.; Lu, J.; Zhao, Y. Synthesis and anti-cancer activity studies of dammarane-type triterpenoid derivatives. Eur. J. Med. Chem. 2020, 187, 111964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, B.; Yuan, H.; Feng, G.; Chen, F.; Wu, A.; Zhang, L.; Lin, H.; Zhuo, Z.; Wang, T. Synthesis and Antitumor Evaluation in Vitro of NO-Donating Ursolic Acid-Benzylidene Derivatives. Chem. Biodivers. 2019, 16, e1900111. [Google Scholar] [CrossRef] [PubMed]
- Tailor, N.K.; Boon, H.L.; Sharma, M. Synthesis and in vitro anticancer studies of novel C-2 arylidene congeners of lantadenes. Eur. J. Med. Chem. 2013, 64, 285. [Google Scholar] [CrossRef]
- Fan, H.; Geng, L.; Yang, F.; Dong, X.; He, D.; Zhang, Y. Ursolic acid derivative induces apoptosis in glioma cells through down-regulation of cAMP. Eur. J. Med. Chem. 2019, 176, 61. [Google Scholar] [CrossRef]
- He, B.; Zhu, Z.; Chen, F.; Zhang, R.; Chen, W.; Zhang, T.; Wang, T.; Lei, J. Synthesis and antitumor potential of new arylidene ursolic acid derivatives via caspase-8 activation. Arch. Pharm. 2021, 354, e2000448. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic triterpenoids with nitrogen-containing heterocyclic moiety, privileged hybrids in anticancer drug discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and cytotoxicity of 28-N-propargylaminoalkylated 2,3-indolotriterpenic acids. Nat. Prod. Commun. 2018, 13, 665. [Google Scholar] [CrossRef] [Green Version]
- Khusnutdinova, E.F.; Apryshko, G.N.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. The synthesis and selective cytotoxicity of new Mannich bases derivatives of 19- and 28-alkynyltriterpenoids. Russ. J. Bioorg. Chem. 2018, 1, 123. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Mustafin, A.G.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A. Evaluation of cytotoxicity and α-glucosidase inhibitory activity of amide and polyamino-derivatives of lupane triterpenoids. Molecules 2020, 25, 4833. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Smirnova, I.; Tret’yakova, E.; Csuk, R.; Hoenke, S.; Fischer, L. Cytotoxic Potential of a-Azepano- and 3-Amino-3,4-Seco-Triterpenoids. Int. J. Mol. Sci. 2021, 22, 1714. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Deigner, H.P. The potential of click reactions for the synthesis of bioactive triterpenes. Bioorg. Med. Chem. Lett. 2019, 29, 949. [Google Scholar] [CrossRef]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 182, 111653. [Google Scholar] [CrossRef] [PubMed]
- Valdeira, A.S.C.; Darvishi, E.; Woldemichael, G.M.; Beutler, J.A.; Gustafson, K.R.; Salvador, J.A.R. Madecassic Acid Derivatives as Potential Anticancer Agents: Synthesis and Cytotoxic Evaluation. J. Nat. Prod. 2019, 82, 2094. [Google Scholar] [CrossRef]
- Valdeira, A.S.C.; Ritt, D.A.; Morrison, D.K.; McMahon, J.B.; Gustafson, K.R.; Salvador, J.A.R. Synthesis and Biological Evaluation of New Madecassic Acid Derivatives Targeting ERK Cascade Signaling. Front. Chem. 2018, 6, 434. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91. [Google Scholar] [CrossRef]
- Hubbard, W.C.; Alley, M.C.; Gray, G.N.; Green, K.C.; McLemore, T.L.; Boyd, M.R. Evidence for prostanoid biosynthesis as a biochemical feature of certain subclasses of non-small cell carcinomas of the lung as determined in established cell lines derived from human lung tumors. Cancer Res. 1989, 49, 826. [Google Scholar] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Myers, T.G.; O’Connor, P.M.; Friend, S.H.; Fornace, A.J., Jr.; Kohn, K.W.; Fojo, T.; Bates, S.E.; Rubinstein, L.V.; Anderson, N.L.; et al. An information-intensive approach to the molecular pharmacology of cancer. Science 1997, 275, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622. [Google Scholar] [PubMed]
- Collins, J.M. Developmental Therapeutics Program NCI/NIH. Available online: http://dtp.cancer.gov/branches/btb/ivclsp.html (accessed on 23 September 2014).
- Montoya, A.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J.; Insuasty, B. Synthesis and in vitro antitumor activity of a novel series of 2-pyrazoline derivatives bearing the 4-aryloxy-7-chloroquinoline fragment. Molecules 2014, 19, 18656–18675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, A.F.; Stefan, C.; Nobel, I.; van den Dobbelsteen, D.J.; Orrenius, S. Signalling mechanisms and oxidative stress in apoptosis. Toxicol. Lett. 1995, 82, 149. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Júnior, H.V.; Seleghim, M.H.; Berlinck, R.G.; Cunha, G.M.; Moraes, M.O.; Pessoa, C. Cytotoxic and genotoxic effects of tambjamine D, an alkaloid isolated from the nudibranch Tambja eliora, on Chinese hamster lung fibroblasts. Chem. Biol. Interact. 2008, 174, 155. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Lv, C.; Shi, Z.R.; Zeng, R.T.; Dong, X.Y.; Zhang, W.D.; Liu, R.H.; Shan, L.; Shen, Y.H. Abieslactone induces cell cycle arrest and apoptosis in human hepatocellular carcinomas through the mitochondrial pathway and the generation of reactive oxygen species. PLoS ONE 2014, 9, e115151. [Google Scholar] [CrossRef]
- Gupta, S. Molecular signaling in death receptor and mitochondrial pathways of apoptosis (Review). Int. J. Oncol. 2003, 22, 15. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375. [Google Scholar]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011, 89, 713. [Google Scholar] [CrossRef]
- Wawer, M.; Peltason, L.; Weskamp, N.; Teckentrup, A.; Bajorath, J. Structure-activity relationship anatomy by network-like similarity graphs and local structure-activity relationship indices. J. Med. Chem. 2008, 51, 6075. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; von Korff, M.; Rufenerm, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, W.C.; Sunshine, M.; Liu, H.; Varma, S.; Kohn, K.W.; Morris, J.; Doroshow, J.; Pommier, Y. CellMiner: A web-based suite of genomic and pharmacologic tools to explore transcript and drug patterns in the NCI-60 cell line set. Cancer Res. 2012, 72, 3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Kunos, C.A.; Andrews, S.J.; Moore, K.N.; Chon, H.S.; Ivy, S.P. Randomized Phase II Trial of Triapine-Cisplatin-Radiotherapy for Locally Advanced Stage Uterine Cervix or Vaginal Cancers. Front. Oncol. 2019, 9, 1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slichenmyer, W.J.; Rowinsky, E.K.; Donehower, R.C.; Kaufmann, S.H. The current status of camptothecin analogues as antitumor agents. J. Natl. Cancer Inst. 1993, 85, 271. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimada, M.; Harimoto, N.; Rikimaru, T.; Shirabe, K.; Tanaka, S.; Sugimach, K. Histone deacetylase inhibitor trichostatin A induces cell-cycle arrest/apoptosis and hepatocyte differentiation in human hepatoma cells. Int. J. Cancer 2003, 103, 572. [Google Scholar] [CrossRef]
- Veith, H.; Southall, N.; Huang, R.; James, T.; Fayne, D.; Artemenko, N.; Shen, M.; Inglese, J.; Austin, C.P.; Lloyd, D.G.; et al. Comprehensive characterization of cytochrome P450 isozyme selectivity across chemical libraries. Nat. Biotechnol. 2009, 27, 1050. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, C.; Moir, E.M.; Rankovic, Z.; Wishart, G. Medicinal chemistry of hERG optimizations: Highlights and hang-ups. J. Med. Chem. 2006, 49, 5029. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.N.; Yao, Z.J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.P.; Cao, D.S. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazakova, O.; Medvedeva, N.; Samoilova, I.; Baikova, I.; Tolstikov, G.; Kataev, V.; Mironov, V. Conjugates of several lupane, oleanane, and ursane triterpenoids with the antituberculosis drug isoniazid and pyridinecarboxaldehydes. Chem. Nat. Compd. 2011, 47, 752. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniiatullina, G.V.; Tolstikov, G.A.; Medvedeva, N.I.; Utkina, T.M.; Kartashova, O.L. Synthesis, modifications, and antimicrobial activity of the methylpiperazinyl amides of triterpenic acids. Russ. J. Bioorg. Chem. 2010, 36, 416. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Smirnova, I.E.; Kazakova, O.B.; Petrova, A.V.; Thu, N.T.H.; Viet, D.Q. Synthesis and evaluation of 2,3-indolotriterpenoids as new α-glucosidase inhibitors. Med. Chem. Res. 2017, 26, 2737. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Baikova, I.P.; Tolstikov, G.A.; Lopatina, T.V.; Yunusov, M.S.; Zaprutko, L. Synthesis of triterpenoid acylates: Effective reproduction inhibitors of influenza A (H1N1) and papilloma viruses. Russ. J. Bioorg. Chem. 2010, 36, 771. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Collins, J.M.; Doroshow, J.H. Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol. Cancer Ther. 2010, 9, 1451. [Google Scholar] [CrossRef] [Green Version]
- Monga, M.; Sausville, E.A. Developmental therapeutics program at the NCI: Molecular target and drug discovery process. Leukemia 2002, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H.; Juhasz, A.; Ge, Y.; Holbeck, S.; Lu, J.; Antony, S.; Wu, Y.; Jiang, G.; Roy, K. Antiproliferative mechanisms of action of the flavin dehydrogenase inhibitors diphenylene iodonium and di-2-thienyliodonium based on molecular profiling of the NCI-60 human tumor cell panel. Biochem. Pharmacol. 2012, 83, 1195. [Google Scholar] [CrossRef] [Green Version]


| Compound | NCS Number 1 | Range of Growth, % 2 | One-Dose Mean Growth (%) 3 | Five-Dose Mean GI50 (μM) 4 | Most Sensitive Cancer Cell Line |
|---|---|---|---|---|---|
| 7 | 804711 | −95.08 to 21.69 | −40.62 | 3.39 | LOXIMVI, melanoma |
| 9 | 797794 | −32.37 to100.15 | 55.08 | NT 5 | HL-60(TB), leukemia |
| 10 | 797795 | −41.72 to 86.87 | 50.20 | NT | HL-60(TB), leukemia |
| 12 | 804688 | −79.45 to 37.32 | −10.67 | 8.13 | HT29, Colon Cancer |
| 13 | 806889 | −84.00 to 48.59 | 4.46 | 1.55 | LOX IMVI, Melanoma |
| 17 | 806887 | 18.33 to 89.94 | 61.35 | NT | NCI-H460, non-small cell lung cancer |
| 23 | 806876 | 25.52 to 110.87 | 87.33 | NT | SR, leukemia |
| 27 | 804712 | −99.47 to 31.21 | −58.89 | 4.57 | UO-31, renal cancer |
| 29 | 801984 | −97.65 to −9.56 | −74.90 | 0.75 | SK-MEL-5, melanoma |
| 31 | 804510 | −97.52 to 63.50 | 29.45 | NT | UO-31, renal cancer |
| 32 | 804692 | −80.36 to 25.43 | −12.29 | 15.14 | COLO-205, colon cancer |
| Compound | Log GI50 | Log TGI50 | Log LC50 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 | Median | ∆ | Range | Median | ∆ | Range | Median | ∆ | Range |
| 13 | −5.47 | 0.51 | 0.77 | −4.67 | 0.74 | 1.41 | −4.13 | 0.99 | 1.12 |
| 27 | −5.81 | 1.65 | 2.3 | −4.60 | 1.10 | 1.70 | −4.13 | 1.10 | 1.23 |
| 29 | −6.12 | 0.65 | 0.98 | −5.67 | 0.60 | 1.04 | −5.21 | 0.22 | 1.43 |
| Subpanel/Cell Lines (μM) | Compound 13 | Compound 29 | Doxorubicine * NSC 123127 | |||||
|---|---|---|---|---|---|---|---|---|
| GI50 | TGI | LC50 | GI50 | TGI | LC50 | GI50 | LC50 | |
| Leukemia | ||||||||
| CCRF-CEM | 0.871 | >100 | >100 | 0.360 | 1.59 | — | 0.08 | 100.00 |
| HL-60(TB) | 1.16 | 3.96 | >100 | 0.200 | 0.981 | 7.66 | 0.12 | 89.33 |
| K-562 | 0.637 | >100 | >100 | 0.266 | >100 | 0.19 | 100.00 | |
| MOLT-4 | 0.787 | >100 | >100 | 0.309 | 1.38 | — | 0.03 | 100.00 |
| RPMI-8226 | 0.476 | 3.36 | >100 | 0.171 | 0.541 | — | 0.08 | 100.00 |
| SR | 0.365 | >100 | >100 | — | — | 0.03 | 100.00 | |
| Non-Small Cell Lung Cancer | ||||||||
| A549/ATCC | 1.22 | >100 | >100 | 1.06 | 2.32 | 5.06 | 0.06 | 100.00 |
| EKVX | 2.87 | >100 | >100 | 1.21 | 2.60 | — | 0.41 | 47.97 |
| HOP-62 | 1.76 | 6.73 | >100 | 1.41 | 2.80 | 5.54 | 0.07 | 67.61 |
| HOP-92 | 0.0347 | 11.60 | >100 | 0.177 | 1.33 | 3.87 | 0.10 | 42.27 |
| NCI-H226 | 2.09 | 9.22 | >100 | 1.63 | 3.62 | — | 0.05 | 6.40 |
| NCI-H23 | 2.41 | >100 | >100 | 1.35 | 2.89 | 6.18 | 0.15 | 13.15 |
| NCI-H322M | 4.32 | >100 | >100 | 1.27 | 2.55 | 5.10 | — | — |
| NCI-H460 | 1.32 | 4.17 | >100 | 0.535 | 1.96 | 5.78 | 0.02 | 51.29 |
| NCI-H522 | 1.30 | 6.72 | >100 | 0.328 | 1.30 | 3.88 | 0.03 | 2.80 |
| Colon Cancer | ||||||||
| COLO 205 | 1.38 | — | >100 | 0.751 | 2.17 | — | 0.18 | 4.33 |
| HCC-2998 | 1.66 | 7.82 | >100 | 1.09 | 2.41 | 5.29 | 0.26 | 21.68 |
| HCT-116 | 0.319 | 2.00 | 6.95 | 0.332 | 1.24 | 3.69 | 0.08 | 54.58 |
| HCT-15 | 0.939 | 11.00 | >100 | 0.654 | 2.02 | 4.99 | 6.46 | 100.00 |
| HT29 | 0.912 | 54.5 | >100 | 0.515 | 1.73 | 4.23 | 0.12 | 67.45 |
| KM-12 | 2.70 | >100 | >100 | 0.651 | 2.07 | 5.23 | 0.27 | 92.68 |
| SW-620 | 2.29 | >100 | >100 | 0.924 | 1.51 | 6.51 | 0.09 | 58.61 |
| CNS Cancer | ||||||||
| SF-268 | 6.33 | >100 | >100 | 1.19 | 2.90 | — | 0.10 | 30.48 |
| SF-295 | 2.03 | 34.8 | >100 | 1.53 | 2.89 | 5.49 | 0.10 | 69.98 |
| SF-539 | 1.73 | 3.70 | 7.94 | 1.28 | 2.58 | 5.19 | 0.12 | 27.23 |
| SNB-19 | 6.93 | 27.7 | 85.3 | 0.956 | 2.23 | 5.05 | 0.04 | 49.77 |
| SNB-75 | 3.21 | >100 | >100 | 1.00 | 2.24 | 4.98 | 0.07 | 3.30 |
| U251 | 1.89 | 10.3 | >100 | 0.547 | 1.87 | 4.53 | 0.04 | 30.62 |
| Melanoma | ||||||||
| LOX IMVI | 1.24 | 2.72 | 5.95 | 0.800 | 2.13 | — | 0.07 | 50.35 |
| MALME-3M | 1.57 | 4.59 | 38.4 | 1.08 | 2.45 | 5.58 | 0.12 | 3.97 |
| M14 | 1.41 | 5.23 | >100 | 0.432 | 1.73 | 4.73 | 0.18 | 4.05 |
| MDA-MB-435 | 1.82 | — | >100 | 0.844 | 2.13 | 4.81 | 0.25 | 9.57 |
| SK-MEL-2 | 2.33 | 7.26 | >100 | 1.21 | 2.56 | 5.41 | 0.17 | 1.06 |
| SK-MEL-28 | 1.84 | 5.41 | 31.2 | 1.15 | 2.40 | 5.00 | 0.21 | 15.92 |
| SK-MEL-5 | 1.48 | 3.01 | 6.15 | 0.760 | 2.05 | 4.64 | 0.08 | 0.49 |
| UACC-257 | 3.68 | >100 | >100 | 1.24 | 2.59 | 5.41 | 0.14 | 8.15 |
| UACC-62 | 1.36 | 40.9 | >100 | 0.870 | 2.13 | 4.74 | 0.12 | 0.74 |
| Ovarian Cancer | ||||||||
| IGROV1 | 3.09 | >100 | >100 | 1.52 | 3.31 | 7.21 | 0.17 | 100.00 |
| OVCAR-3 | 2.06 | >100 | >100 | 0.651 | 1.95 | 100 | 0.39 | 84.33 |
| OVCAR-4 | 2.36 | >100 | >100 | 0.777 | 2.17 | 5.30 | 0.37 | 74.30 |
| OVCAR-5 | 2.27 | 9.45 | 49.5 | 1.52 | 2.84 | 5.33 | 0.41 | 100.00 |
| OVCAR-8 | 3.52 | >100 | >100 | 1.03 | 2.50 | — | 0.10 | 43.25 |
| NCI/ADR-RES | 2.49 | 46.4 | >100 | 0.911 | 2.53 | — | 7.16 | 100.00 |
| SK-OV-3 | 3.58 | >100 | >100 | 1.44 | 2.76 | 5.29 | 0.22 | 100.00 |
| Renal Cancer | ||||||||
| 786–0 | 1.66 | 12.4 | >100 | 0.670 | 2.00 | 4.58 | 0.13 | 51.64 |
| A498 | 1.83 | 4.96 | 83.0 | 1.39 | 2.76 | 5.48 | 0.10 | 1.90 |
| ACHN | 2.20 | 21.7 | >100 | 1.03 | 2.20 | 4.69 | 0.08 | 100.00 |
| CAKI-1 | 1.07 | >100 | >100 | 1.22 | 2.47 | 5.00 | 0.95 | 100.00 |
| RXF 393 | 1.22 | 3.03 | 7.53 | 1.00 | 2.22 | 4.91 | 0.10 | 4.69 |
| SN12C | 2.37 | 20.6 | >100 | 0.804 | 2.16 | 5.05 | 0.07 | 72.44 |
| TK-10 | 6.46 | >100 | >100 | 1.34 | 2.64 | 5.20 | — | — |
| UO-31 | 0.893 | 14.2 | 45.9 | 0.497 | 1.87 | 4.47 | 0.49 | 26.18 |
| Prostate Cancer | ||||||||
| PC-3 | 0.357 | 46.1 | >100 | 0.275 | 1.33 | 3.82 | 0.32 | 87.10 |
| DU-145 | 2.77 | 36.4 | >100 | 0.989 | 2.22 | 4.95 | 0.11 | 100.00 |
| Breast Cancer | ||||||||
| MCF7 | 0.509 | 15.3 | >100 | 0.484 | 2.05 | 5 | 0.03 | 51.29 |
| MDA-MB-231/ATCC | 3.06 | 41.1 | >100 | 0.943 | 2.25 | 5.16 | 0.51 | 34.75 |
| HS 578T | 2.56 | >100 | >100 | 1.22 | 5.85 | >100 | 0.33 | 85.70 |
| BT-549 | 1.39 | 10.0 | >100 | 1.04 | 2.31 | — | 0.23 | 21.33 |
| T -47D | 0.781 | >100 | >100 | 0.511 | 2.16 | 6.74 | 0.06 | 85.70 |
| MDA-MB-468 | 0.712 | >100 | >100 | 0.352 | 1.46 | 5.11 | 0.05 | 2.52 |
| Apoptosis, % of Cells | ||
|---|---|---|
| Early | Late | |
| HEK293; 24 h | ||
| control | 0.45 ± 0.02 | 1.07 ± 0.04 |
| 29 | 4.96 ± 1.3 ** | 7.62 ± 1.04 ** |
| HEK293; 48 h | ||
| control | 2.16 ± 0.98 | 1.84 ± 0.78 |
| 29 | 12.11 ± 1.7 ** | 65.39 ± 3.5 *** |
| MCF-7; 24 h | ||
| control | 4.88 ± 1.23 | 1.64 ± 0.095 |
| 29 | 5.38 ± 1.2 | 17.36 ± 3.5 ** |
| MCF-7; 48 h | ||
| control | 2.12 ± 1.03 | 0.85 ± 0.01 |
| 29 | 10.23 ± 2.4 * | 41.76 ± 3.6 *** |
| Compound | Pearson’s Correlation 2 | p Value | NSC # | Name | Target or Mechanism of Action | FDA Status |
|---|---|---|---|---|---|---|
| 7 | – | – | – | – | – | – |
| 12 | 0.511 | 0.000174 | 730001 | N-(2-Aminophenyl)-4-(3-(3,4-dihydro-1H-pyrido [3,4-b]indol-2(9H)-yl)prop-1-en-2-yl)benzamide | HDAC | – |
| 13 | 0.694 | 0 | 734945 | N-(4-Aminophenyl)-4-(3-(3,4-dihydroisoquinolin-2(1H)-yl)prop-1-en-2-yl)benzamide | HDAC | – |
| 0.594 | 0.000001 | 88536 | Calusterone | Hormone | FDA approved | |
| 0.554 | 0.000005 | 753686 | Olaparib | PARP1 | FDA approved | |
| 0.563 | 0.000017 | 77213 | Procarbazine | Alkylating at N7 position of guanine | FDA approved | |
| 0.594 | 0.000021 | 172112 | Spirohydantoin mustard | Alkylating at N7 position of guanine | – | |
| 0.521 | 0.000023 | 73754 | Fluorodopan | Alkylating at N7 position of guanine | – | |
| 0.531 | 0.000025 | 755809 | Vismodegib | SMO and tyrosine kinase | FDA approved | |
| 0.518 | 0.000031 | 777193 | LDK-378 | ALK | FDA approved | |
| 0.528 | 0.000067 | 23759 | Testolactone | Hormone | FDA approved | |
| 27 | 0.514 | 0.000031 | 663249 | Triapine | Ribonucleotide reductase | Clinical trial |
| 0.500 | 0.000054 | 681634 | Camptothecin derivative | Topoisomerase 1 | – | |
| 29 | 0.528 | 0.000021 | 735408 | N-(2-Aminophenyl)-4-(3-(6-oxophenanthridin-5(6H)-yl)prop-1-en-2-yl)benzamide | HDAC | – |
| 0.564 | 0.000024 | 730001 | N-(2-Aminophenyl)-4-(3-(3,4-dihydro-1H-pyrido [3,4-b]indol-2(9H)-yl)prop-1-en-2-yl)benzamide | HDAC | – | |
| 0.518 | 0.000044 | 734945 | N-(4-Aminophenyl)-4-(3-(3,4-dihydroisoquinolin-2(1H)-yl)prop-1-en-2-yl)benzamide | HDAC | – | |
| 32 | 0.588 | 0.000001 | 761191 | AP-26113 | ALK, EGFR | Clinical trial |
| 0.571 | 0.000002 | 764040 | Alectinib | PIK3, ALK, mTOR | FDA approved | |
| 0.567 | 0.000008 | 730003 | N-(2-Aminophenyl)-4-(3-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)prop-1-en-2-yl)benzamide | HDAC | – | |
| 0.549 | 0.000008 | 776422 | LDK-378 | ALK | FDA approved | |
| 0.507 | 0.000042 | 89201 | Estramustine | Alkylating at N7 position of guanine, tubulin | FDA approved | |
| 0.505 | 0.000053 | 777193 | LDK-378 | ALK | FDA approved | |
| 0.524 | 0.000079 | 702294 | Estramustine | Alkylating at N7 position of guanine, tubulin | FDA approved |
| Category | Property | Predicted Value | Mean Value 1 | ||
|---|---|---|---|---|---|
| pkCSM [61] | SwissADME [63] | ADMETlab [64] | |||
| Physicochemical | Water solubility (lg mol/L) | −5.402 | −8.51 | −6.08 | −6.96 |
| LogP | 8.08 | 6.53 | 8.08 | 7.56 | |
| Absorption | Intestinal absorption (human, %) | 100 | Low | ++ (0.714) | Yes |
| P-glycoprotein substrate | Yes | No | −−− (0.24) | No | |
| P-glycoprotein I inhibitor | Yes | +++ (0.908) | |||
| Distribution | VDss (human, L/kg) | 0.209 | 0.433 | 0.32 | |
| BBB permeability (lg BB) | −0.106 | No | + (0.68) | ||
| Metabolism | CYP2D6 substrate | No | − (0.39) | No | |
| CYP3A4 substrate | Yes | + (0.591) | Yes | ||
| CYP1A2 inhibitior | No | No | −−− (0.064) | No | |
| CYP2C19 inhibitior | No | No | − (0.408) | No | |
| CYP2C9 inhibitior | No | No | − (0.452) | No | |
| CYP2D6 inhibitior | No | No | − (0.374) | No | |
| CYP3A4 inhibitior | No | No | + (0.664) | No | |
| Excretion | Total clearance (mL/min/kg) | 0.244 | 1.331 | 0.79 | |
| Renal OCT2 substrate | No | ||||
| T1/2 (h) | 2.01 | ||||
| Toxicity | AMES toxicity | No | −−− (0.262) | No | |
| hERG I inhibitor | No | ||||
| hERG II inhibitor | Yes | + (0.573) | Yes | ||
| Oral rat acute LD50 (mg/kg) | 164,3 | 191.2 | 177.75 | ||
| Hepatotoxicity | Yes | − (0.308) | |||
| Skin Sensitisation | No | − (0.416) | No | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khusnutdinova, E.; Petrova, A.; Zileeva, Z.; Kuzmina, U.; Zainullina, L.; Vakhitova, Y.; Babkov, D.; Kazakova, O. Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated through ROS-Triggered Apoptosis. Int. J. Mol. Sci. 2021, 22, 9796. https://doi.org/10.3390/ijms22189796
Khusnutdinova E, Petrova A, Zileeva Z, Kuzmina U, Zainullina L, Vakhitova Y, Babkov D, Kazakova O. Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated through ROS-Triggered Apoptosis. International Journal of Molecular Sciences. 2021; 22(18):9796. https://doi.org/10.3390/ijms22189796
Chicago/Turabian StyleKhusnutdinova, Elmira, Anastasiya Petrova, Zulfia Zileeva, Ulyana Kuzmina, Liana Zainullina, Yulia Vakhitova, Denis Babkov, and Oxana Kazakova. 2021. "Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated through ROS-Triggered Apoptosis" International Journal of Molecular Sciences 22, no. 18: 9796. https://doi.org/10.3390/ijms22189796
APA StyleKhusnutdinova, E., Petrova, A., Zileeva, Z., Kuzmina, U., Zainullina, L., Vakhitova, Y., Babkov, D., & Kazakova, O. (2021). Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated through ROS-Triggered Apoptosis. International Journal of Molecular Sciences, 22(18), 9796. https://doi.org/10.3390/ijms22189796







