The Adaptive Immune Landscape of the Colorectal Adenoma–Carcinoma Sequence
Abstract
:1. Introduction
2. Results
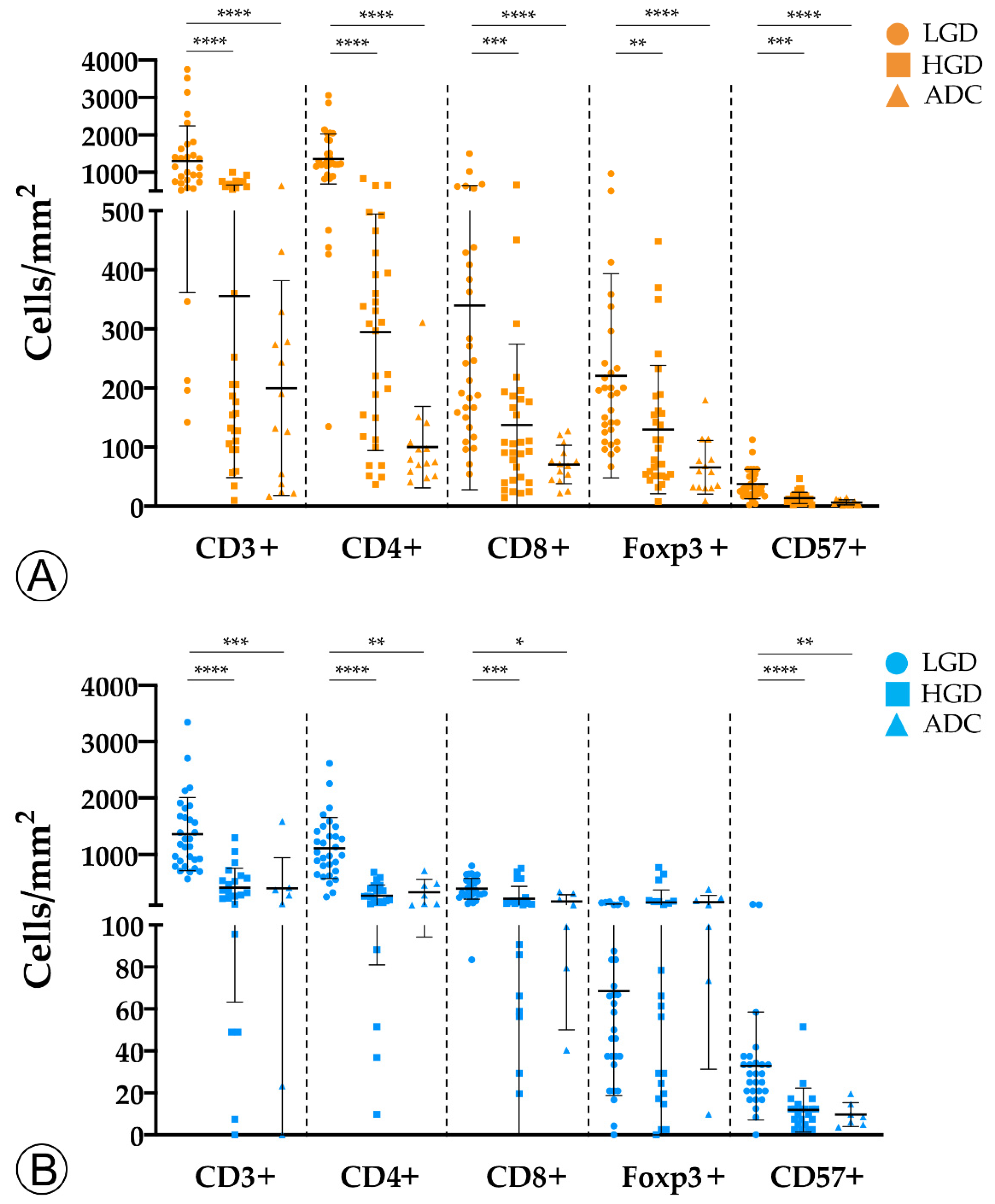
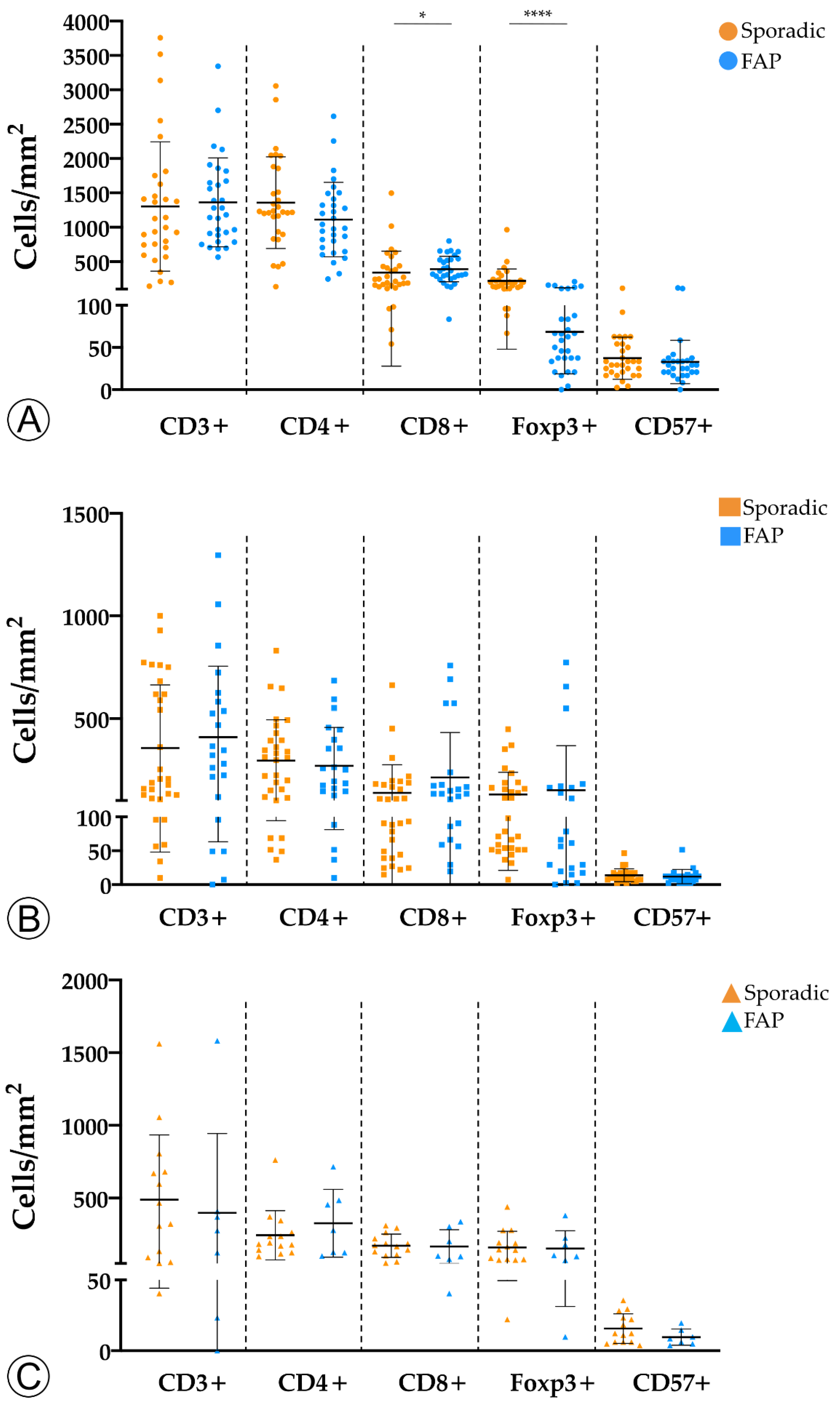
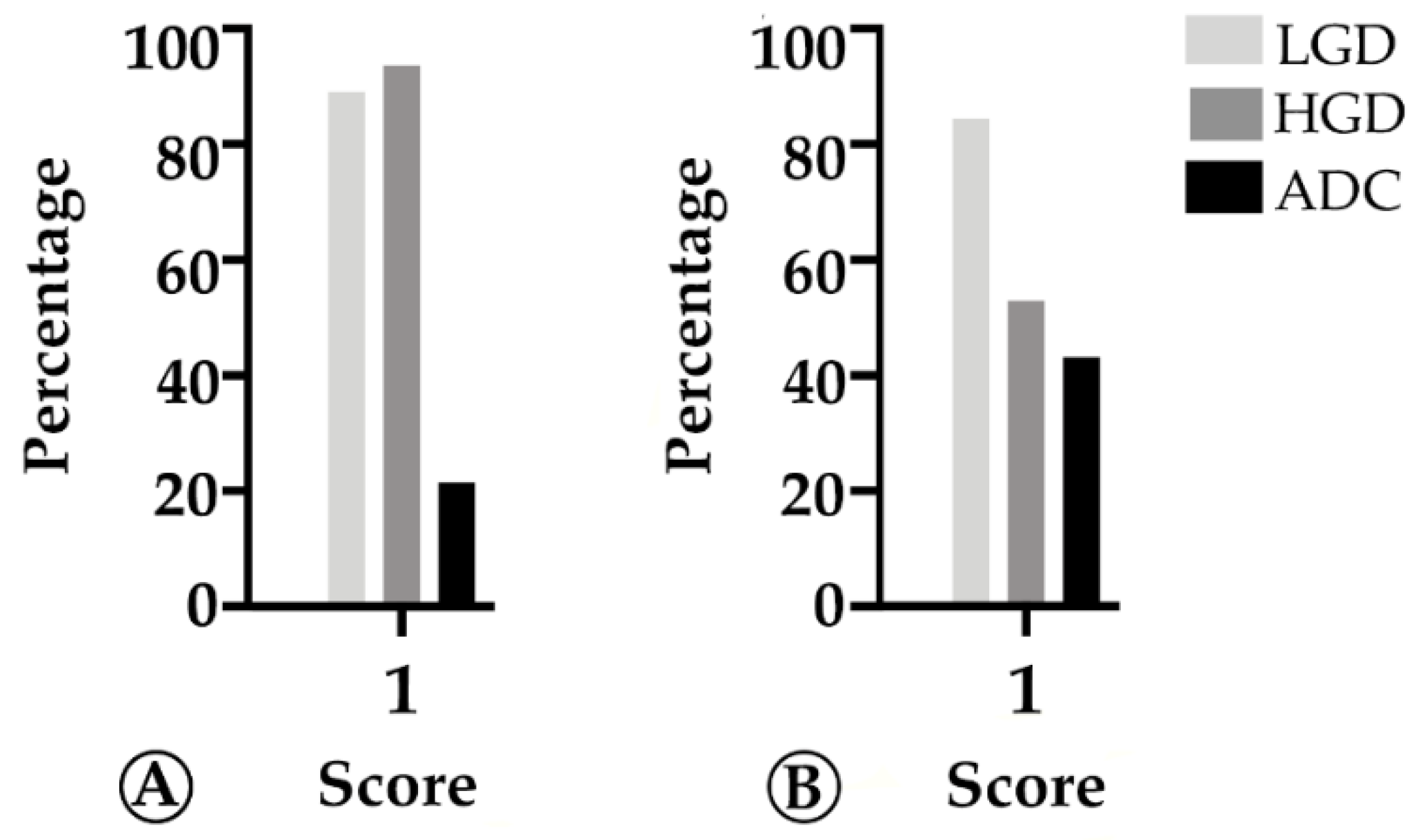
2.1. Tumor Infiltration with Immune Cells Decreases throughout the Colorectal ACS
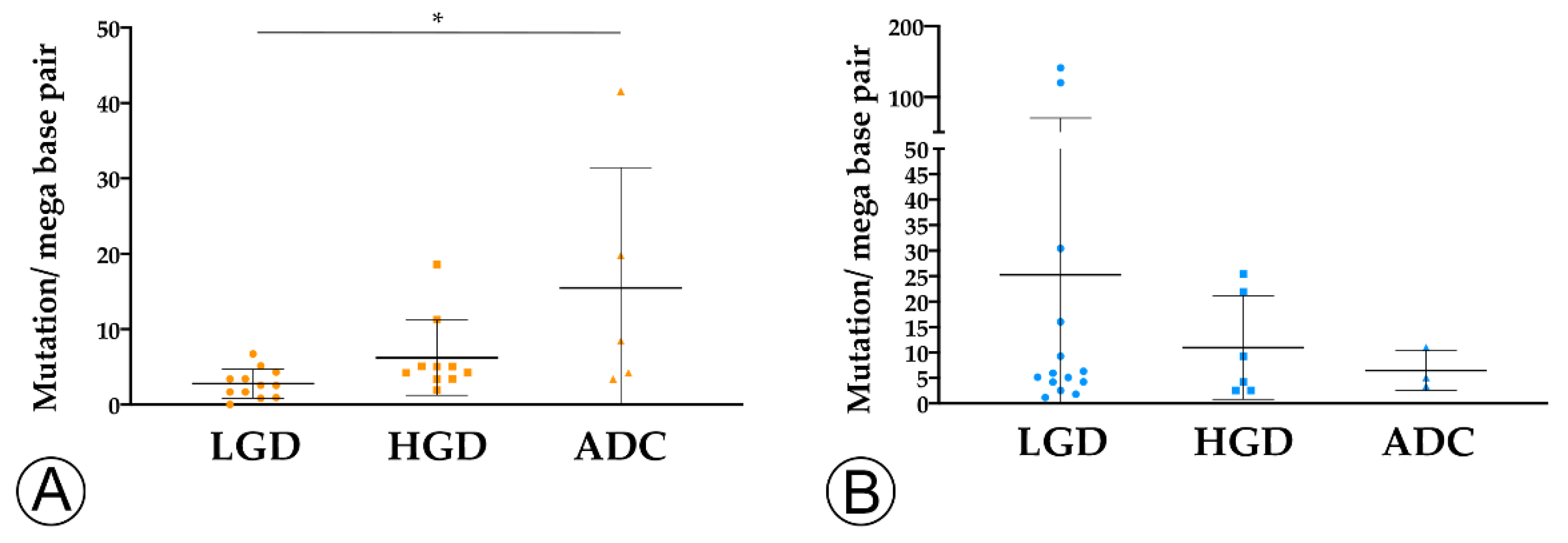
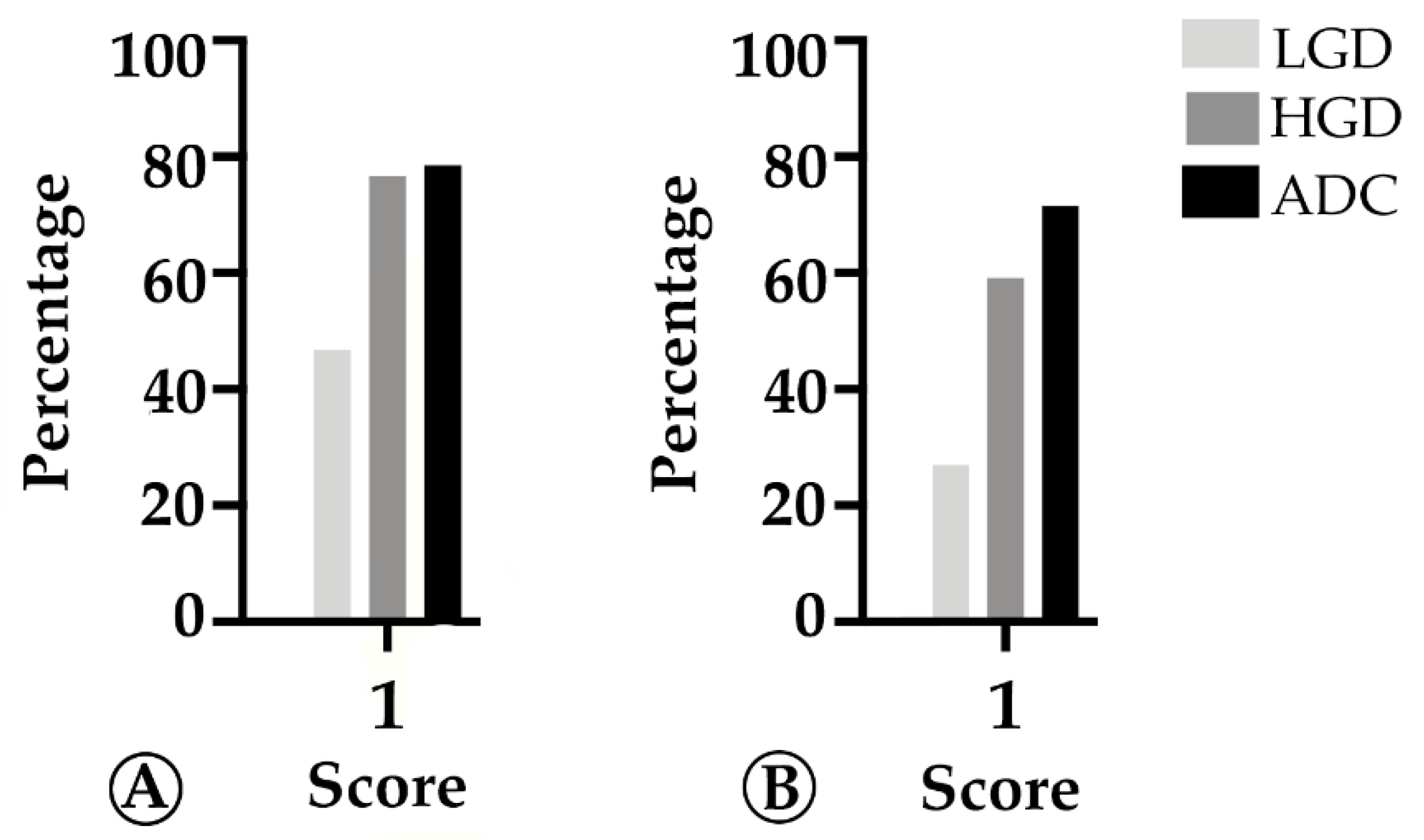
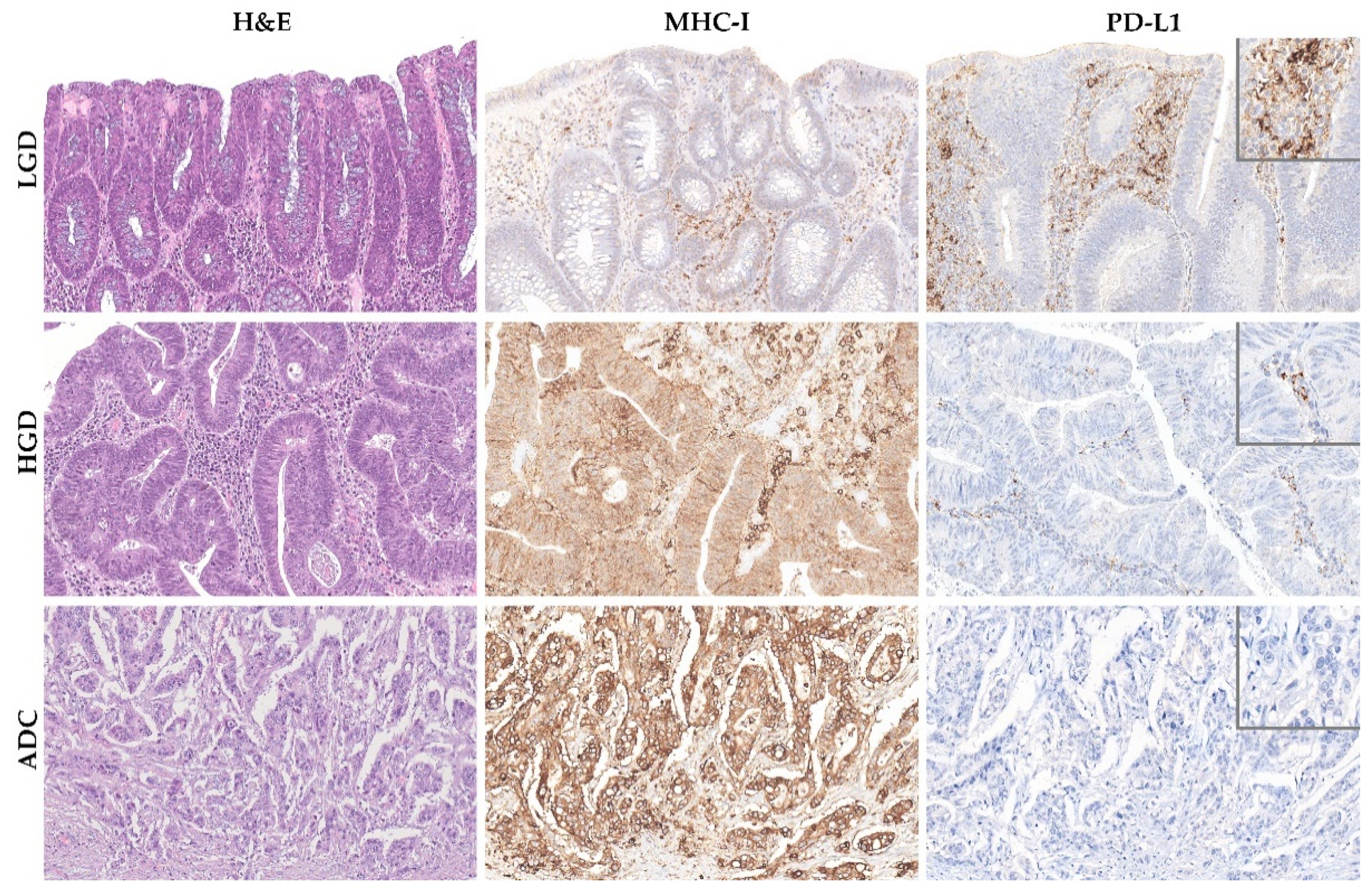
2.2. TMB and MHC-I Expression Increase along the Colorectal ACS
2.3. PD-L1 Expression Decreases throughout the Colorectal Adenoma Carcinoma Sequence
3. Discussion
4. Materials and Methods
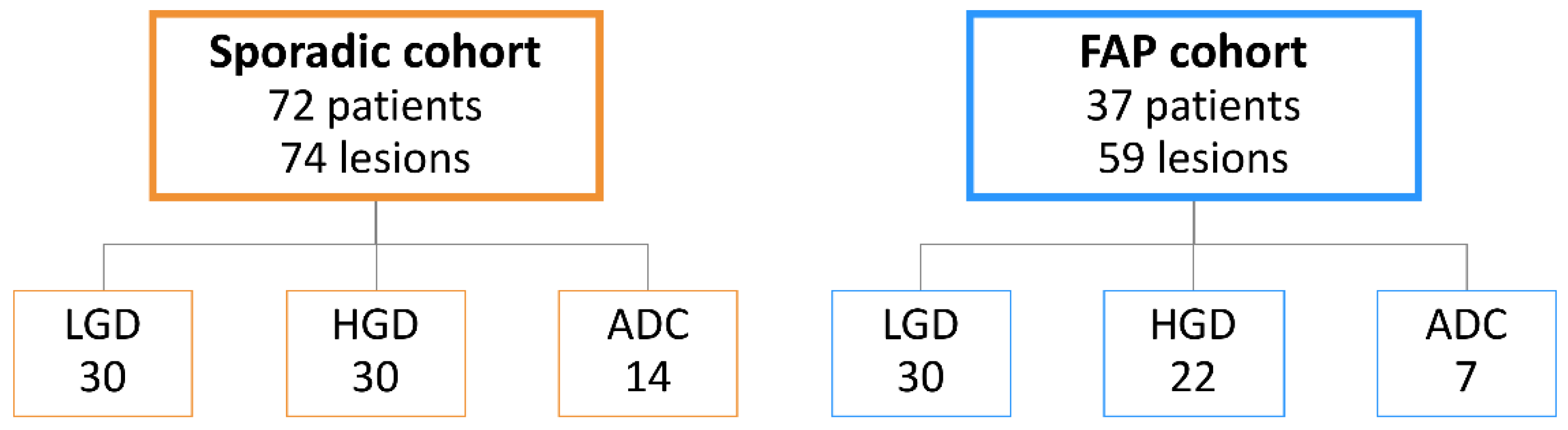
4.1. Patient Series
4.2. Immunohistochemistry and Scoring
4.3. MSI Testing
4.4. Tumor Mutation Burden
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Powell, S.M.; Zilz, N.; Beazer-Barclay, Y.; Bryan, T.M.; Hamilton, S.R.; Thibodeau, S.N.; Vogelstein, B.; Kinzler, K.W. APC mutations occur early during colorectal tumorigenesis. Nature 1992, 359, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Shi, Y.; Cui, J.; Tang, F.; Florholmen, J. Immune microenvironmental shift along human colorectal adenoma-carcinoma sequence: Is it relevant to tumor development, biomarkers and biotherapeutic targets? Scand. J. Gastroenterol. 2012, 47, 367–377. [Google Scholar] [CrossRef]
- Nosho, K.; Baba, Y.; Tanaka, N.; Shima, K.; Hayashi, M.; Meyerhardt, J.A.; Giovannucci, E.; Dranoff, G.; Fuchs, C.S.; Ogino, S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J. Pathol. 2010, 222, 350–366. [Google Scholar] [CrossRef] [Green Version]
- Jakubowska, K.; Kisielewski, W.; Kanczuga-Koda, L.; Koda, M.; Famulski, W. Diagnostic value of inflammatory cell infiltrates, tumor stroma percentage and disease-free survival in patients with colorectal cancer. Oncol. Lett. 2017, 14, 3869–3877. [Google Scholar] [CrossRef] [Green Version]
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 7527. [Google Scholar] [CrossRef]
- Agarwal, P.; Le, D.T.; Boland, P.M. Immunotherapy in colorectal cancer. Adv. Cancer Res. 2021, 151, 137–196. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Banner, B.F.; Savas, L.; Baker, S.; Woda, B.A. Characterization of the inflammatory cell populations in normal colon and colonic carcinomas. Virchows Arch. B Cell Pathol. 1993, 64, 213–220. [Google Scholar] [CrossRef]
- McLean, M.H.; Murray, G.I.; Stewart, K.N.; Norrie, G.; Mayer, C.; Hold, G.L.; Thomson, J.; Fyfe, N.; Hope, M.; Mowat, N.A.; et al. The inflammatory microenvironment in colorectal neoplasia. PLoS ONE 2011, 6, e15366. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Yuan, A.; Vonen, B.; Florholmen, J. Progressive cellular response in the lamina propria of the colorectal adenoma-carcinoma sequence. Histopathology 2009, 54, 550–560. [Google Scholar] [CrossRef]
- Cui, G.; Goll, R.; Olsen, T.; Steigen, S.E.; Husebekk, A.; Vonen, B.; Florholmen, J. Reduced expression of microenvironmental Th1 cytokines accompanies adenomas-carcinomas sequence of colorectum. Cancer Immunol. Immunother. 2007, 56, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Yuan, A.; Zheng, W.; Li, C.; Cui, J.; Pang, Z.; Zhang, L.; Li, Z.; Goll, R.; Cui, G. Accumulation of FoxP3+ T regulatory cells in the tumor microenvironment of human colorectal adenomas. Pathol. Res. Pract. 2016, 212, 106–112. [Google Scholar] [CrossRef]
- Cui, G. Immune battle at the premalignant stage of colorectal cancer: Focus on immune cell compositions, functions and cytokine products. Am. J. Cancer Res. 2020, 10, 1308–1320. [Google Scholar]
- Liu, F.; Hu, X.; Zimmerman, M.; Waller, J.L.; Wu, P.; Hayes-Jordan, A.; Lev, D.; Liu, K. TNFalpha cooperates with IFN-gamma to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS ONE 2011, 6, e16241. [Google Scholar] [CrossRef]
- Yang, J.; Wen, Z.; Li, W.; Sun, X.; Ma, J.; She, X.; Zhang, H.; Tu, C.; Wang, G.; Huang, D.; et al. Immune Microenvironment: New Insight for Familial Adenomatous Polyposis. Front. Oncol. 2021, 11, 570241. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.C.; Liu, R.Y.; Yan, J.P.; An, X.; Jiang, W.; Ling, Y.H.; Chen, J.W.; Bei, J.X.; Zuo, X.Y.; Cai, M.Y.; et al. The Heterogeneity Between Lynch-Associated and Sporadic MMR Deficiency in Colorectal Cancers. J. Natl. Cancer Inst. 2018, 110, 975–984. [Google Scholar] [CrossRef]
- Antonescu, C.R. WHO Classification of Tumours. Digestive System Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; WHO: Lyon, France, 2019. [Google Scholar]
- Amin, M.B.; Edge, S.; Greene, F. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Morrison, B.J.; Steel, J.C.; Morris, J.C. Reduction of MHC-I expression limits T-lymphocyte-mediated killing of Cancer-initiating cells. BMC Cancer 2018, 18, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Xie, X.; Wang, X.; Zhang, X.; Li, W.; Sun, T.; Cai, Y.; Wu, J.; Dang, C.; Zhang, H. Correlations Between Tumor Mutation Burden and Immunocyte Infiltration and Their Prognostic Value in Colon Cancer. Front. Genet. 2021, 12, 623424. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Robbins, P.F.; Lu, Y.C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobie, J.J.; Wu, R.S.; Kurt, R.A.; Lou, S.; Adelman, M.K.; Whitesell, L.J.; Ramanathapuram, L.V.; Arteaga, C.L.; Akporiaye, E.T. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003, 63, 1860–1864. [Google Scholar]
- Munn, D.H.; Sharma, M.D.; Baban, B.; Harding, H.P.; Zhang, Y.; Ron, D.; Mellor, A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005, 22, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Plowden, J.; Renshaw-Hoelscher, M.; Gangappa, S.; Engleman, C.; Katz, J.M.; Sambhara, S. Impaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol. 2004, 229, 86–92. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.N.; Xu, Z.; Qian, X.P.; Ding, Y.T.; Yu, L.X.; Liu, B.R. RNA-electroporated CD40-activated B cells induce functional T-cell responses against HepG2 cells. Eur. J. Cancer Care 2008, 17, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Shah, R.; Elias, J.; Mistry, Y.; Coral, K.; Shah, P.; Maurya, A.K.; Mittal, B.; D’Silva, J.K.; Murugan, S.; et al. A neoepitope derived from a novel human germline APC gene mutation in familial adenomatous polyposis shows selective immunogenicity. PLoS ONE 2018, 13, e0203845. [Google Scholar] [CrossRef] [PubMed]
- Sarode, V.R.; Robinson, L. Screening for Lynch Syndrome by Immunohistochemistry of Mismatch Repair Proteins: Significance of Indeterminate Result and Correlation With Mutational Studies. Arch. Pathol. Lab. Med. 2019, 143, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Mindiola-Romero, M.A.; Green, B.D.; Al-TurkmaniPh, D.M.; Godwin, B.K.; Mackay, B.A.; Tafe, M.L.; Ren, M.B.; TsongalisPh, D.G. Novel Biocartis Idylla cartridge-based assay for detection of microsatellite instability in colorectal cancer tissues. Exp. Mol. Pathol. 2020, 116, 104519. [Google Scholar] [CrossRef]
| Feature | Sporadic ADCs n = 14 | FAP ADCs n = 7 |
|---|---|---|
| Median value (range) | Median value (range) | |
| Age | 62 (35–82) | 51 (45–63) |
| Gender | n (%) | n (%) |
| Male | 8 (57.1) | 3 (42.9) |
| Female | 6 (42.9) | 4 (57.1) |
| WHO classification (2019) [21] | ||
| ADC NOS | 14 (100) | 7 (100) |
| pT stage (AJCC 8th Ed) [22] | ||
| pT1 | 5 (35.7) | 2 (28.6) |
| pT2 | 4 (28.6) | 1 (14.3) |
| pT3 | 4 (28.6) | 3 (42.8) |
| pT4 | 1 (7.1) | 1 (14.3) |
| pN stage (AJCC 8th Ed) [22] | ||
| pN0 | 10 (71.4) | 4 (57.1) |
| pN1a | 2 (14.3) | 1 (14.3) |
| pN1b | 2 (14.3) | 2 (28.6) |
| Grading | ||
| Low-grade | 13 (92.9) | 7 (100) |
| High-grade | 1 (7.1) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, J.A.; Gullo, I.; Garcia, D.; Miranda, S.; Spaans, L.; Pinho, L.; Reis, J.; Sousa, F.; Baptista, M.; Resende, C.; et al. The Adaptive Immune Landscape of the Colorectal Adenoma–Carcinoma Sequence. Int. J. Mol. Sci. 2021, 22, 9791. https://doi.org/10.3390/ijms22189791
Freitas JA, Gullo I, Garcia D, Miranda S, Spaans L, Pinho L, Reis J, Sousa F, Baptista M, Resende C, et al. The Adaptive Immune Landscape of the Colorectal Adenoma–Carcinoma Sequence. International Journal of Molecular Sciences. 2021; 22(18):9791. https://doi.org/10.3390/ijms22189791
Chicago/Turabian StyleFreitas, João Augusto, Irene Gullo, Diogo Garcia, Sara Miranda, Louisa Spaans, Lídia Pinho, Joana Reis, Fabiana Sousa, Manuela Baptista, Carlos Resende, and et al. 2021. "The Adaptive Immune Landscape of the Colorectal Adenoma–Carcinoma Sequence" International Journal of Molecular Sciences 22, no. 18: 9791. https://doi.org/10.3390/ijms22189791
APA StyleFreitas, J. A., Gullo, I., Garcia, D., Miranda, S., Spaans, L., Pinho, L., Reis, J., Sousa, F., Baptista, M., Resende, C., Leitão, D., Durães, C., Costa, J. L., Carneiro, F., & Machado, J. C. (2021). The Adaptive Immune Landscape of the Colorectal Adenoma–Carcinoma Sequence. International Journal of Molecular Sciences, 22(18), 9791. https://doi.org/10.3390/ijms22189791







