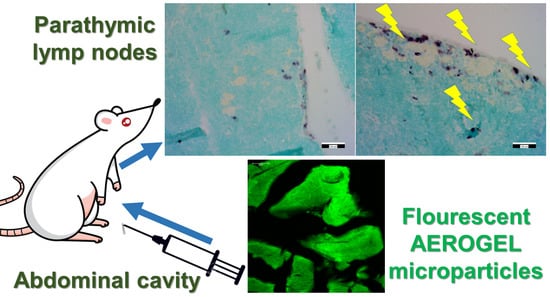
Mesoporous Aerogel Microparticles Injected into the Abdominal Cavity of Mice Accumulate in Parathymic Lymph Nodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
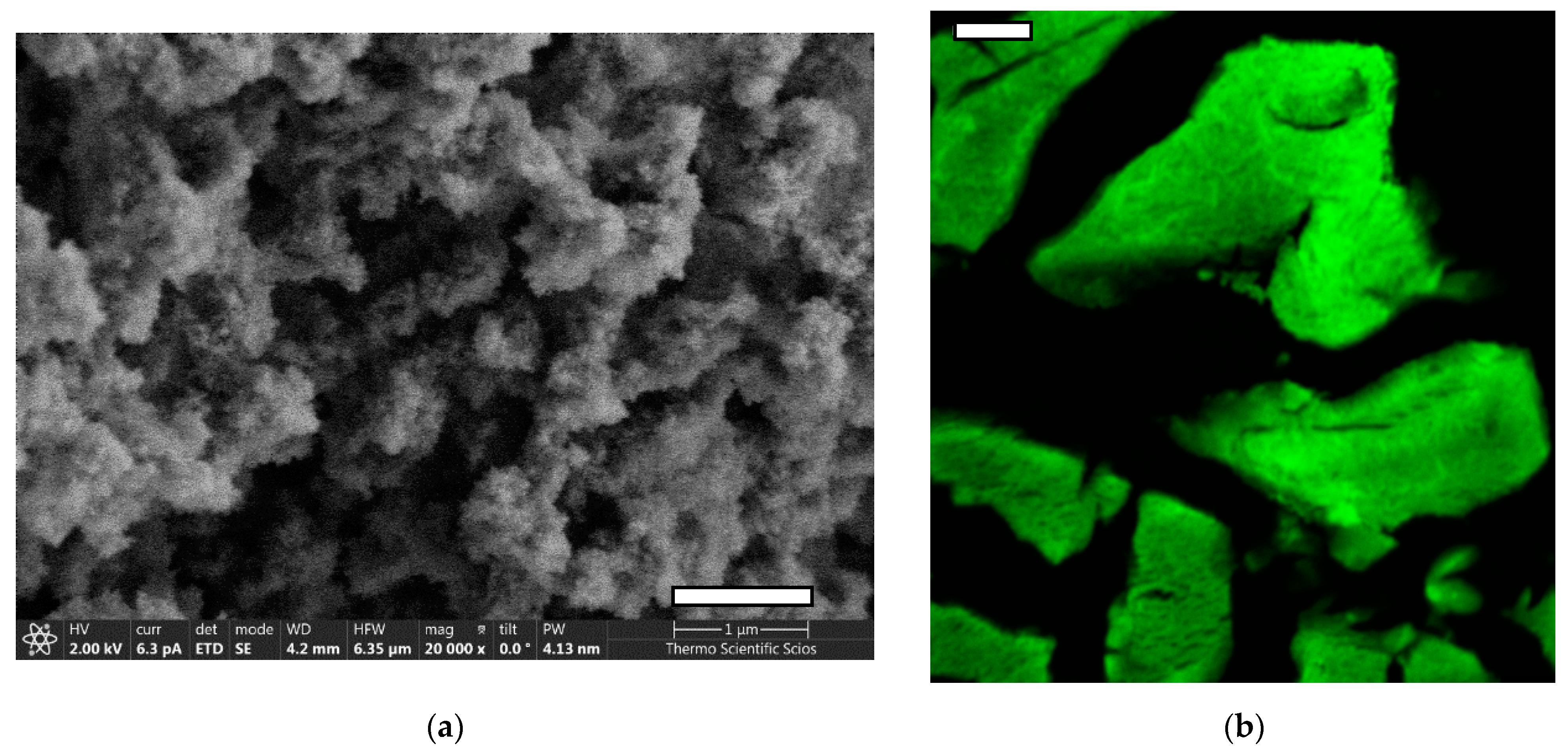
2.2. Preparation and Characterization of Fluorescein Functionalized Silica–Gelatin Aerogel
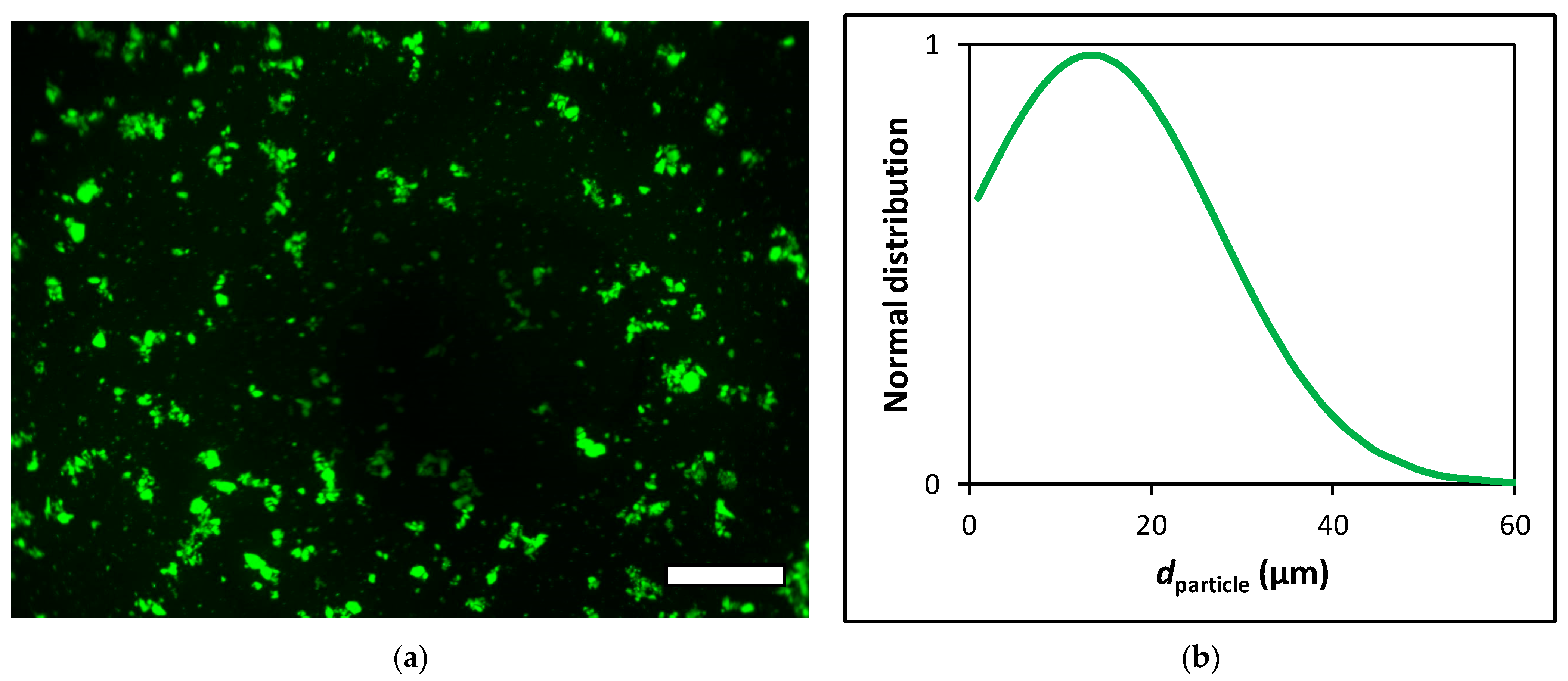
2.3. Sterilization and Micronization of the Aerogel
2.4. Animals
2.5. Aerogel Treatment and Autopsy
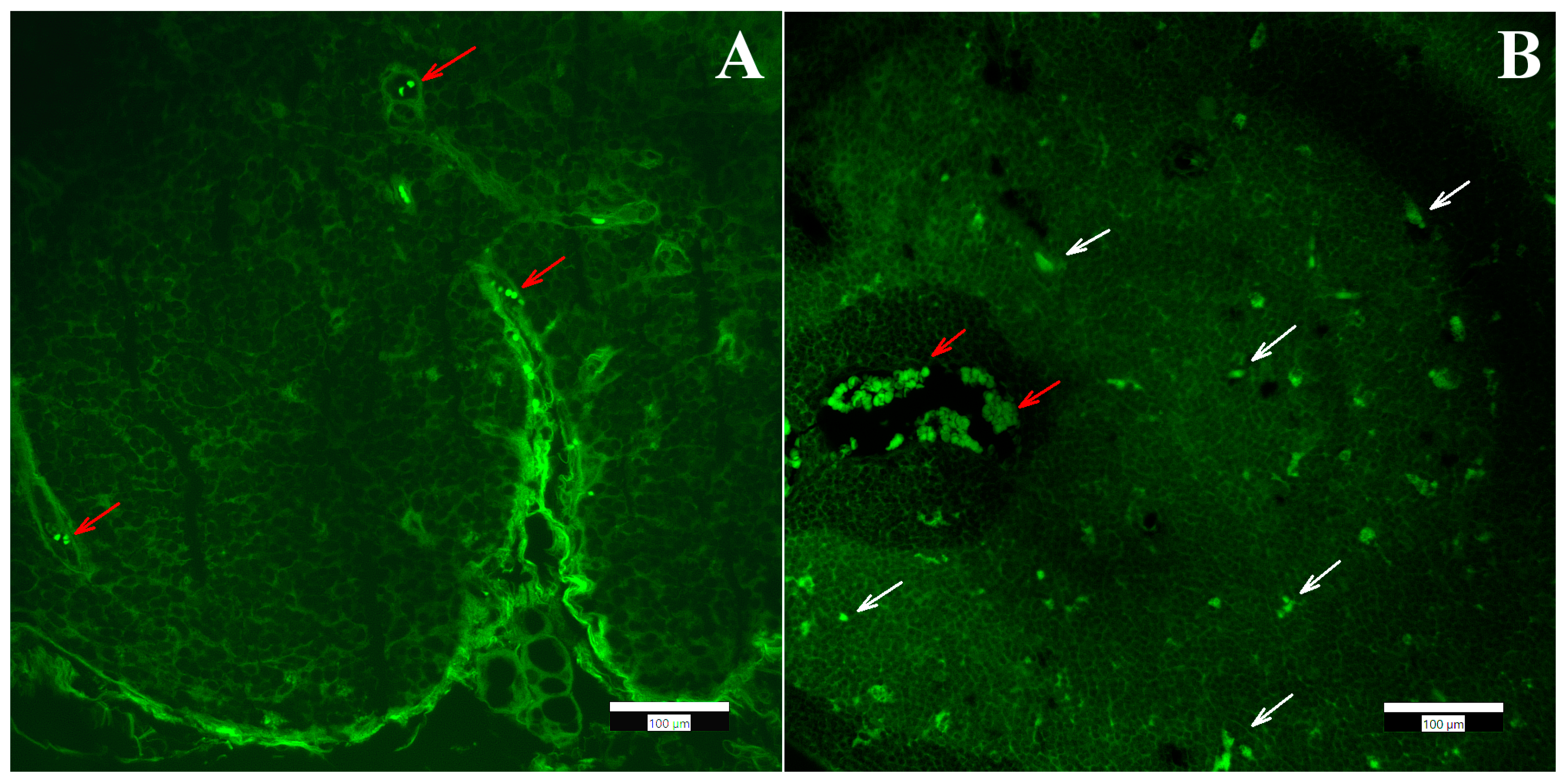
2.6. Fluorescence Microscopy
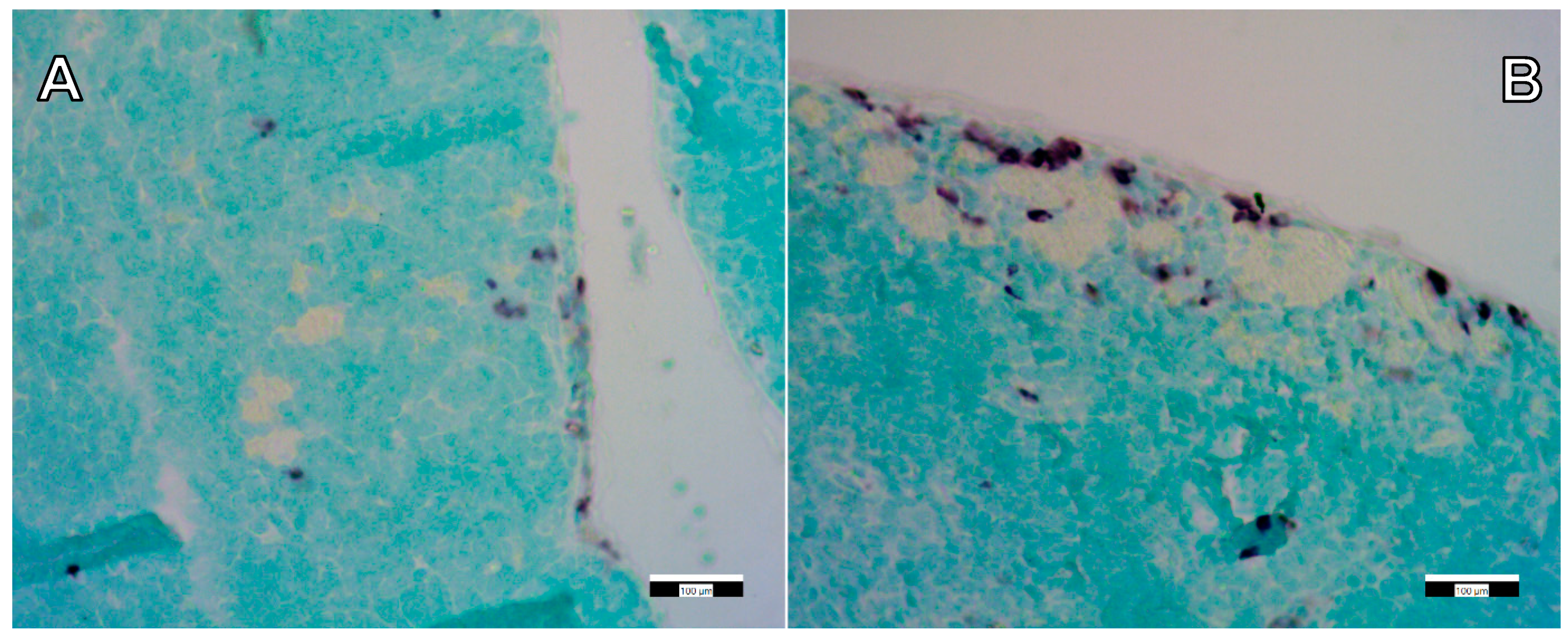
2.7. Immunohistochemistry
3. Results and Discussion
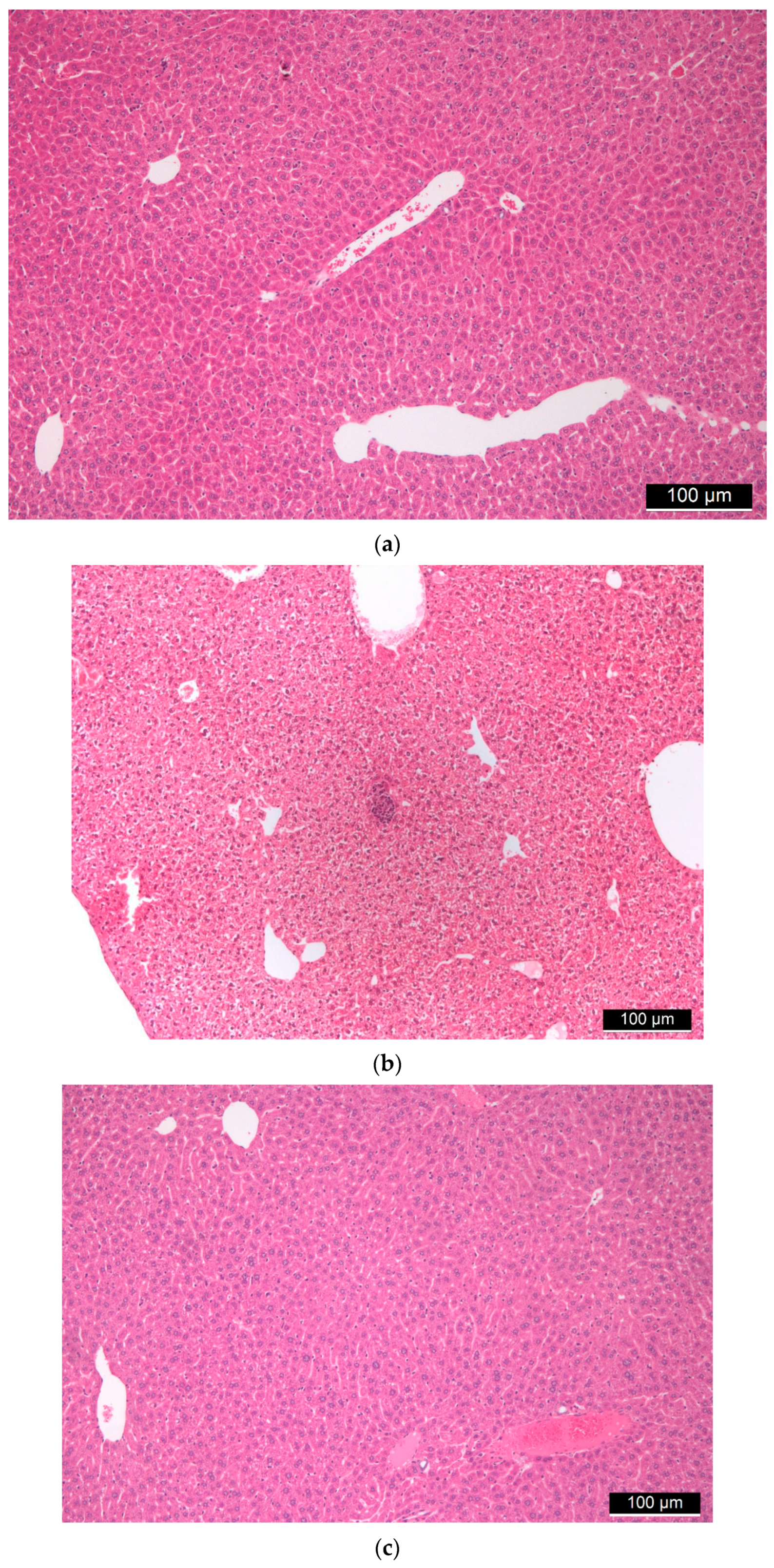
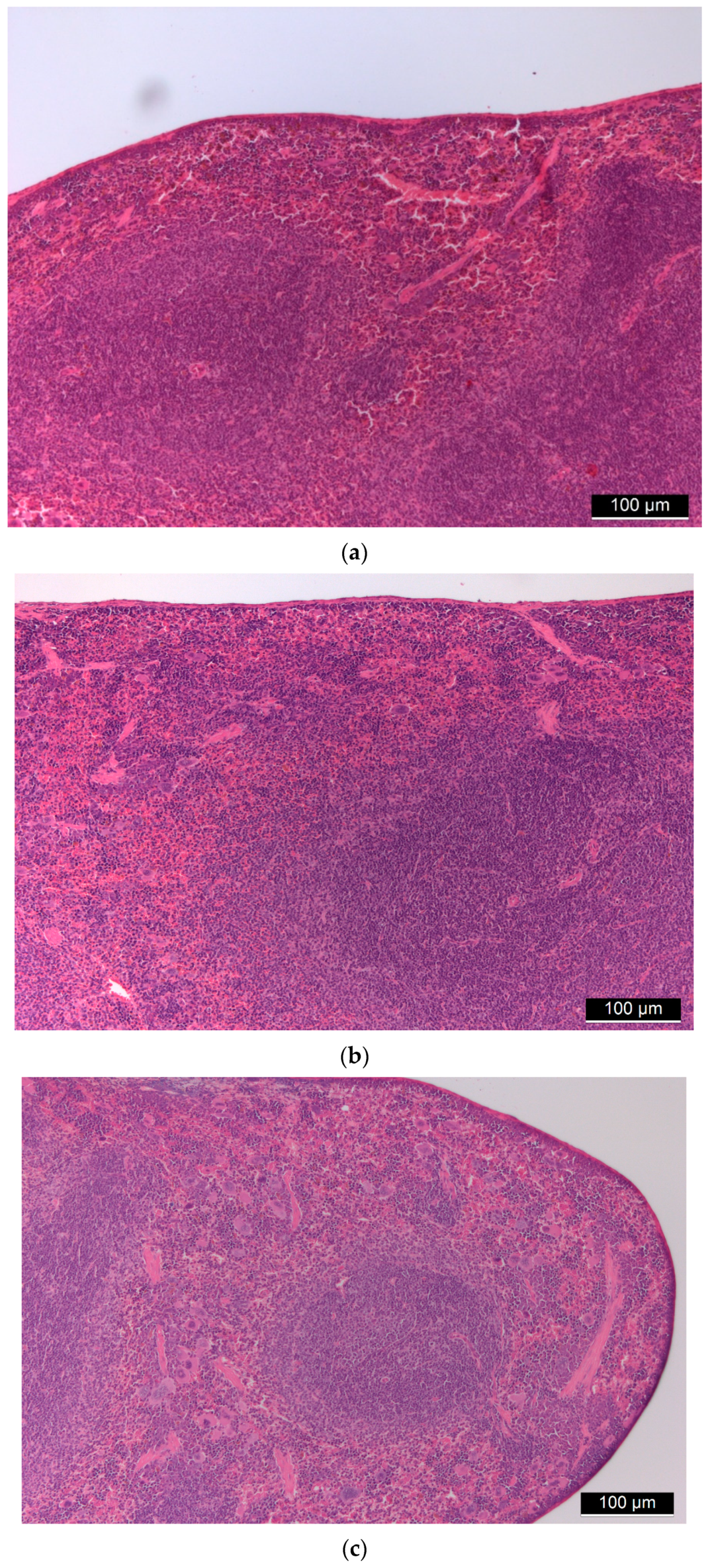
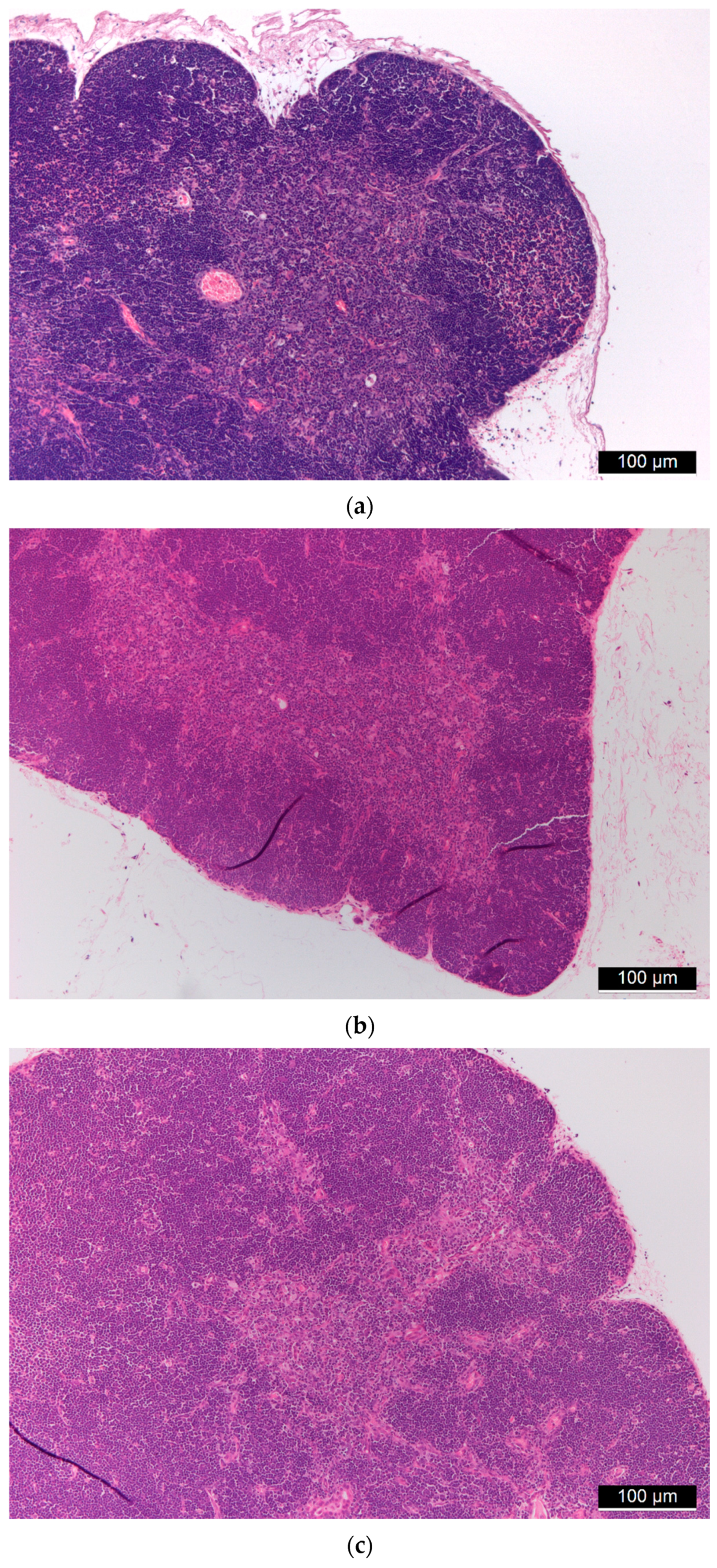
3.1. Biocompatibility of Aerogel Microparticles
3.2. Biodistribution of Aerogel Microparticles
3.3. Immunohistochemical Staining
3.4. Toxicity of Mesoporous Silica Nanoparticles and Aerogels as Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Gonzalez, C.A.; Budtova, T.; Duraes, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [Green Version]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.A.; Sosnik, A.; Kalmar, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Len, A.; Paladini, G.; Románszki, L.; Putz, A.M.; Almásy, L.; László, K.; Bálint, S.; Krajnc, A.; Kriechbaum, M.; Kuncser, A.; et al. Physicochemical characterization and drug release properties of methyl-substituted silica xerogels made by sol–gel process. Int. J. Mol. Sci. 2021, 22, 9197. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccharide-Based Aerogel Production for Biomedical Applications: A Comparative Review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; Garcia-Gonzalez, C.A.; Oliveira, A.L. Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.; Garcia-Gonzalez, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluid 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Mehling, T.; Smirnova, I.; Guenther, U.; Neubert, R.H.H. Polysaccharide-based aerogels as drug carriers. J. Non-Cryst. Solids 2009, 355, 2472–2479. [Google Scholar] [CrossRef]
- Lopez-Iglesias, C.; Barros, J.; Ardao, I.; Gurikov, P.; Monteiro, F.J.; Smirnova, I.; Alvarez-Lorenzo, C.; Garcia-Gonzalez, C.A. Jet Cutting Technique for the Production of Chitosan Aerogel Microparticles Loaded with Vancomycin. Polymers 2020, 12, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Gurikov, P.; Smirnova, I.; Leopold, C.S. Pulmonary drug delivery with aerogels: Engineering of alginate and alginate-hyaluronic acid microspheres. Pharm. Dev. Technol. 2021, 26, 509–521. [Google Scholar] [CrossRef]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluid. 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Nagy, G.; Kiraly, G.; Veres, P.; Lazar, I.; Fabian, I.; Banfalvi, G.; Juhasz, I.; Kalmar, J. Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth. Int. J. Pharm. 2019, 558, 396–403. [Google Scholar] [CrossRef]
- Veres, P.; Kiraly, G.; Nagy, G.; Lazar, I.; Fabian, I.; Kalmar, J. Biocompatible silica-gelatin hybrid aerogels covalently labeled with fluorescein. J. Non-Cryst. Solids 2017, 473, 17–25. [Google Scholar] [CrossRef]
- Kiraly, G.; Hargitai, Z.; Kovacs, I.; Szeman-Nagy, G.; Juhasz, I.; Banfalvi, G. Metastatic Spread from Abdominal Tumor Cells to Parathymic Lymph Nodes. Pathol. Oncol. Res. 2019, 25, 625–633. [Google Scholar] [CrossRef]
- Vareda, J.P.; Garcia-Gonzalez, C.A.; Valente, A.J.M.; Simon-Vazquez, R.; Stipetic, M.; Duraes, L. Insights on toxicity, safe handling and disposal of silica aerogels and amorphous nanoparticles. Environ. Sci. Nano 2021, 8, 1177–1195. [Google Scholar] [CrossRef]
- Tiryaki, E.; Elalmis, Y.B.; Ikizler, B.K.; Yucel, S. Novel organic/inorganic hybrid nanoparticles as enzyme-triggered drug delivery systems: Dextran and Dextran aldehyde coated silica aerogels. J. Drug. Deliv. Sci. Tec. 2020, 56, 101517. [Google Scholar] [CrossRef]
- Sani, N.S.; Malek, N.A.N.N.; Jemon, K.; Kadir, M.R.A.; Hamdan, H. In vitro bioactivity and osteoblast cell viability studies of hydroxyapatite-incorporated silica aerogel. J. Sol Gel Sci. Technol. 2020, 96, 166–177. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Feng, S.; Zhang, Z.; Wu, C.; Zhang, X.; Kang, F. Nano-Porous Silica Aerogels as Promising Biomaterials for Oral Drug Delivery of Paclitaxel. J. Biomed. Nanotechnol. 2019, 15, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; He, Y.; Zhao, X.; Zhang, T.; Qin, Y.; Du, A. Preparation, Characterization, and In Vitro Sustained Release Profile of Resveratrol-Loaded Silica Aerogel. Molecules 2020, 25, 2752. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Forgacs, A.; Horvath, A.; Kiraly, G.; Nagy, G.; Len, A.; Dudas, Z.; Papp, V.; Balogh, Z.; Moldovan, K.; et al. Mechanism of hydration of biocompatible silica-casein aerogels probed by NMR and SANS reveal backbone rigidity. Appl. Surf. Sci. 2020, 531, 147232. [Google Scholar] [CrossRef]
- Al-Najjar, M.A.A.; Athamneh, T.; AbuTayeh, R.; Basheti, I.; Leopold, C.; Gurikov, P.; Smirnova, I. Evaluation of the orally administered calcium alginate aerogel on the changes of gut microbiota and hepatic and renal function of Wistar rats. PLoS ONE 2021, 16, e0247633. [Google Scholar] [CrossRef]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluid. 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Sabri, F.; Sebelik, M.E.; Meacham, R.; Boughter, J.D., Jr.; Challis, M.J.; Leventis, N. In vivo ultrasonic detection of polyurea crosslinked silica aerogel implants. PLoS ONE 2013, 8, e66348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabri, F.; Boughter, J.D., Jr.; Gerth, D.; Skalli, O.; Phung, T.C.; Tamula, G.R.; Leventis, N. Histological evaluation of the biocompatibility of polyurea crosslinked silica aerogel implants in a rat model: A pilot study. PLoS ONE 2012, 7, e50686. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Ai, F.; Shen, W.; Yang, Y.; Zhou, Y.; Deng, J.; Li, C.; Ding, X.; Xin, H.; Wang, X. Microstructural Orientation and Precise Regeneration: A Proof-of-Concept Study on the Sugar-Cane-Derived Implants with Bone-Mimetic Hierarchical Structure. ACS Biomater. Sci. Eng. 2018, 4, 4331–4337. [Google Scholar] [CrossRef]
- Chen, T.J.; Hou, H.; Lu, J.H.; Zhang, K.; Li, B.F. Protective effect of gelatin and gelatin hydrolysate from salmon skin on UV irradiation-induced photoaging of mice skin. J. Ocean Univ. China 2016, 15, 711–718. [Google Scholar] [CrossRef]
- Li, D.; Chen, K.; Duan, L.; Fu, T.; Li, J.; Mu, Z.; Wang, S.; Zou, Q.; Chen, L.; Feng, Y.; et al. Strontium Ranelate Incorporated Enzyme-Cross-Linked Gelatin Nanoparticle/Silk Fibroin Aerogel for Osteogenesis in OVX-Induced Osteoporosis. ACS Biomater. Sci. Eng. 2019, 5, 1440–1451. [Google Scholar] [CrossRef]
- Veres, P.; Lopez-Periago, A.M.; Lazar, I.; Saurina, J.; Domingo, C. Hybrid aerogel preparations as drug delivery matrices for low water-solubility drugs. Int. J. Pharmaceut. 2015, 496, 360–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, I.; Fabian, I. A Continuous Extraction and Pumpless Supercritical CO2 Drying System for Laboratory-Scale Aerogel Production. Gels 2016, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Barnabei, M.S.; Palpant, N.J.; Metzger, J.M. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol. Genomics. 2010, 42a, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.G. Inhibition of spontaneous breast cancer formation in female C3H(Avy/a) mice by long-term treatment with dehydroepiandrosterone. Cancer Res. 1979, 39, 1129–1132. [Google Scholar] [PubMed]
- Abbring, S.; Engen, P.A.; Naqib, A.; Green, S.J.; Garssen, J.; Keshavarzian, A.; van Esch, B.C.A.M. Raw Milk-Induced Protection against Food Allergic Symptoms in Mice Is Accompanied by Shifts in Microbial Community Structure. Int. J. Mol. Sci. 2021, 22, 3417. [Google Scholar] [CrossRef]
- Committee, U. United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia. Br. J. Cancer 1998, 58, 109–113. [Google Scholar]
- Sainte-Marie, G. The Autofluorescent Cells of the Lymphocytic Tissues of the Rat. Anat. Rec. 1965, 151, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.S.; Sergeeva, V.E.; Smorodchenko, A.T.; Kirillov, N.A.; Petrova, T.L.; Olangin, O.I.; Spirin, I.V. Fluorescent granular cells of the thymus can be identified as dendritic macrophages. Bull. Exp. Biol. Med. 2001, 132, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [Green Version]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef]
- Craighead, J.E. Diseases associated with exposure to silica and nonfibrous silicate minerals. Arch. Pathol. Lab. Med. 1988, 112, 673–720. [Google Scholar]
- Shi, Y.; Miller, M.L.; Di Pasqua, A.J. Biocompatibility of mesoporous silica nanoparticles? Comments Inorg. Chem. 2016, 36, 61–80. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jana, S.C. Synergistic hybrid organic–inorganic aerogels. ACS Appl. Mater. Interfaces 2013, 5, 6423–6429. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Király, G.; Egu, J.C.; Hargitai, Z.; Kovács, I.; Fábián, I.; Kalmár, J.; Szemán-Nagy, G. Mesoporous Aerogel Microparticles Injected into the Abdominal Cavity of Mice Accumulate in Parathymic Lymph Nodes. Int. J. Mol. Sci. 2021, 22, 9756. https://doi.org/10.3390/ijms22189756
Király G, Egu JC, Hargitai Z, Kovács I, Fábián I, Kalmár J, Szemán-Nagy G. Mesoporous Aerogel Microparticles Injected into the Abdominal Cavity of Mice Accumulate in Parathymic Lymph Nodes. International Journal of Molecular Sciences. 2021; 22(18):9756. https://doi.org/10.3390/ijms22189756
Chicago/Turabian StyleKirály, Gábor, John Chinonso Egu, Zoltán Hargitai, Ilona Kovács, István Fábián, József Kalmár, and Gábor Szemán-Nagy. 2021. "Mesoporous Aerogel Microparticles Injected into the Abdominal Cavity of Mice Accumulate in Parathymic Lymph Nodes" International Journal of Molecular Sciences 22, no. 18: 9756. https://doi.org/10.3390/ijms22189756
APA StyleKirály, G., Egu, J. C., Hargitai, Z., Kovács, I., Fábián, I., Kalmár, J., & Szemán-Nagy, G. (2021). Mesoporous Aerogel Microparticles Injected into the Abdominal Cavity of Mice Accumulate in Parathymic Lymph Nodes. International Journal of Molecular Sciences, 22(18), 9756. https://doi.org/10.3390/ijms22189756