Distinct Effects of Escitalopram and Vortioxetine on Astroglial L-Glutamate Release Associated with Connexin43
Abstract
:1. Introduction
2. Results
2.1. Effects of Acute and Subchronic Administrations of Escitalopram and Vortioxetine on Astroglial L-Glutamate Release
2.2. Effects of Elective 5-HT Receptor Agents on Astroglial L-Glutamate Release
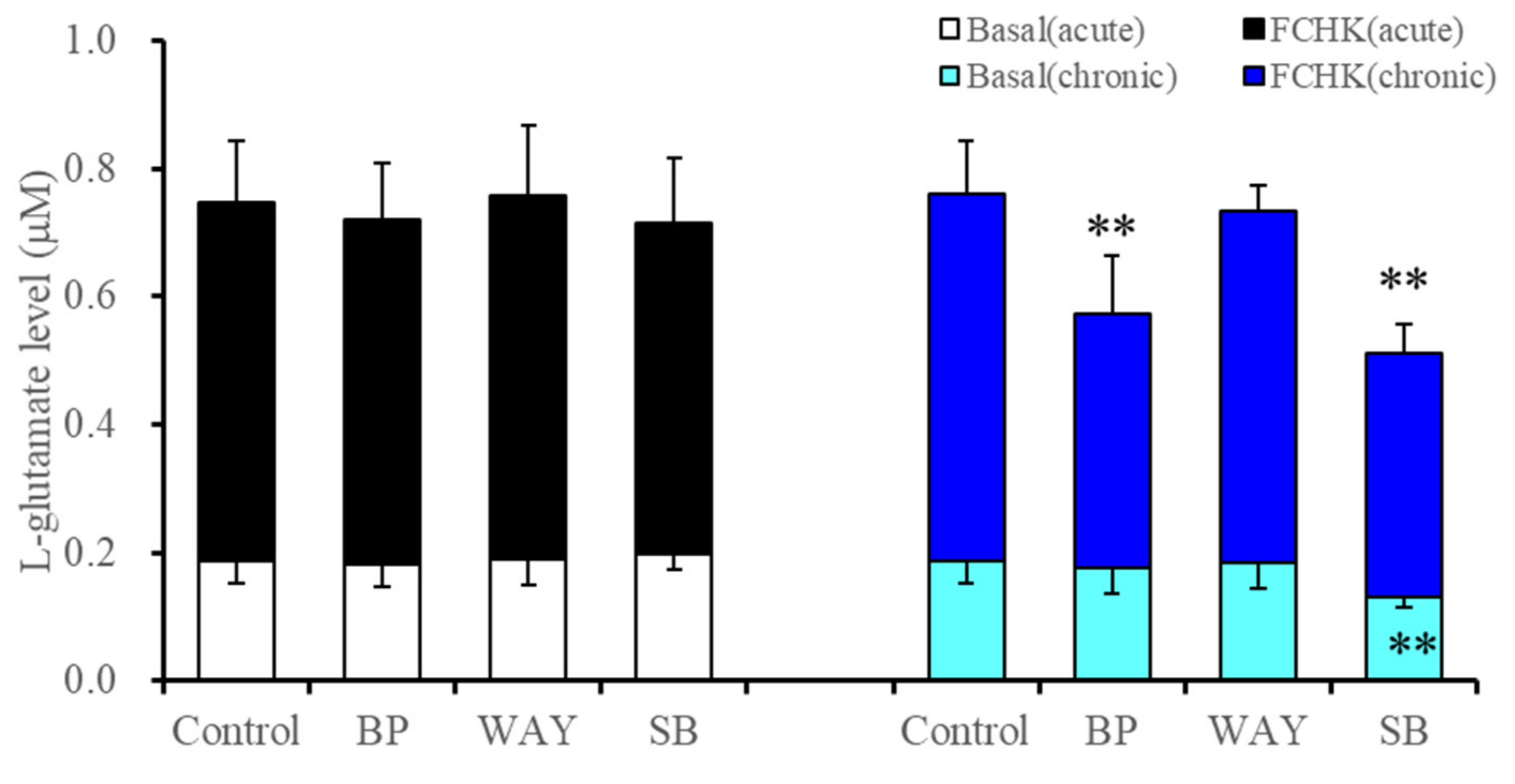
2.2.1. Effects of Acute and Subchronic Administration of 5-HT1AR and 5-HT7R Agents on Astroglial L-Glutamate Release during Resting Stage and through Activated Hemichannels (Study 3)
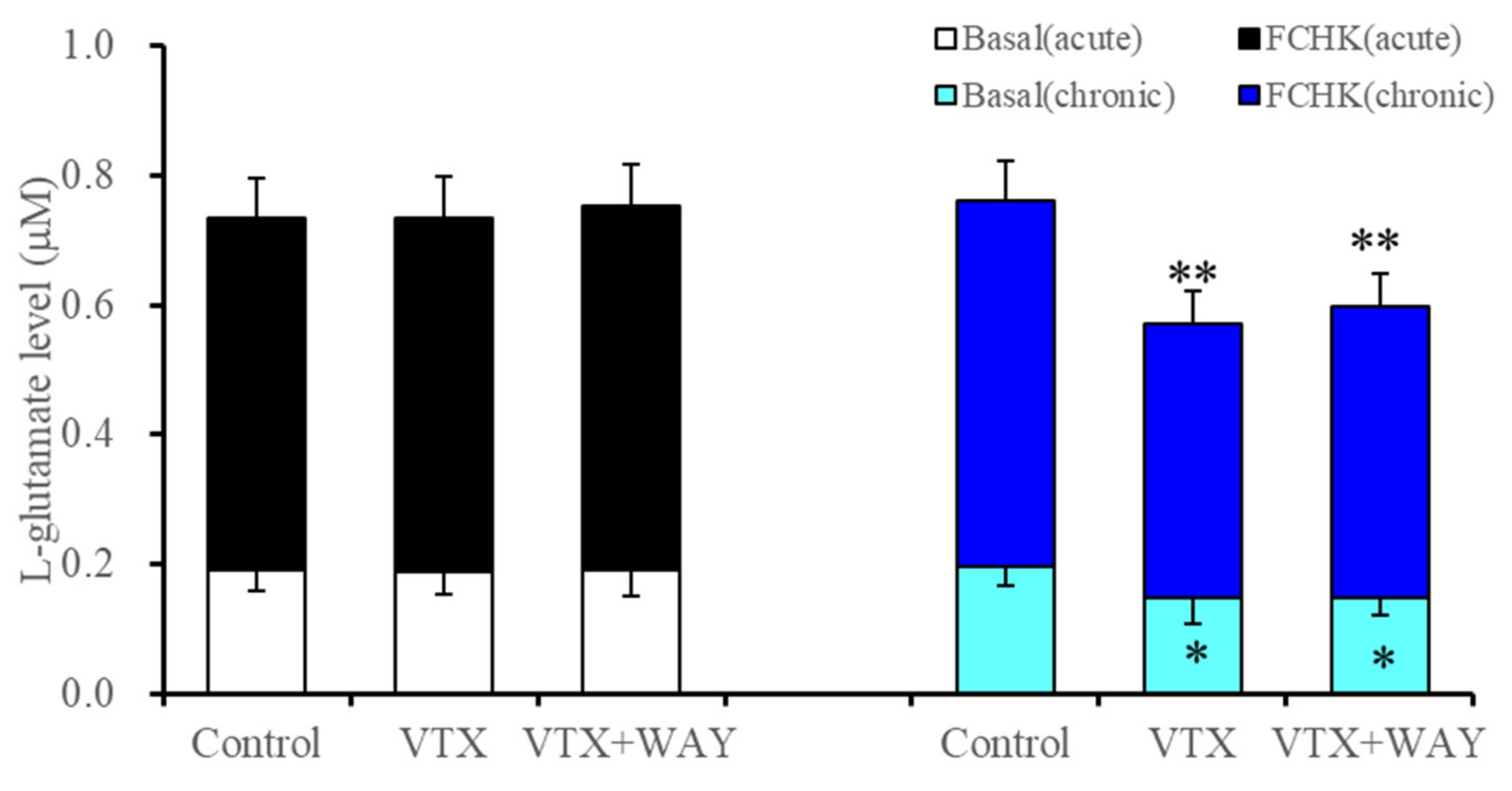
2.2.2. Interaction between Vortioxetine and 5-HT1AR Antagonist (WAY100635) on Basal Astroglial L-Glutamate Release and through Activated Hemichannels
2.3. Effects of Subchronic Administration of 5-HT Receptor Agents, Therapeutic-Relevant Concentrations of Escitalopram and Vortioxetine, on Expression of Proteins Associated with Astroglial L-Glutamate Release
2.3.1. Effects of Subchronic Administration of 5-HT Receptor Agents, Therapeutic-Relevant Concentrations of Escitalopram and Vortioxetine, on Cx43 Expression in the Cytosol and Plasma Membrane Fractions of Primary Cultured Astrocytes (Study 5)
2.3.2. Effects of Subchronic Administrations of 5-HT Receptor Agents, Therapeutic-Relevant Concentrations of Escitalopram and Vortioxetine, on Expression of 5-HT1AR and 5-HT7R in the Plasma Membrane Fractions of Primary Cultured Astrocytes
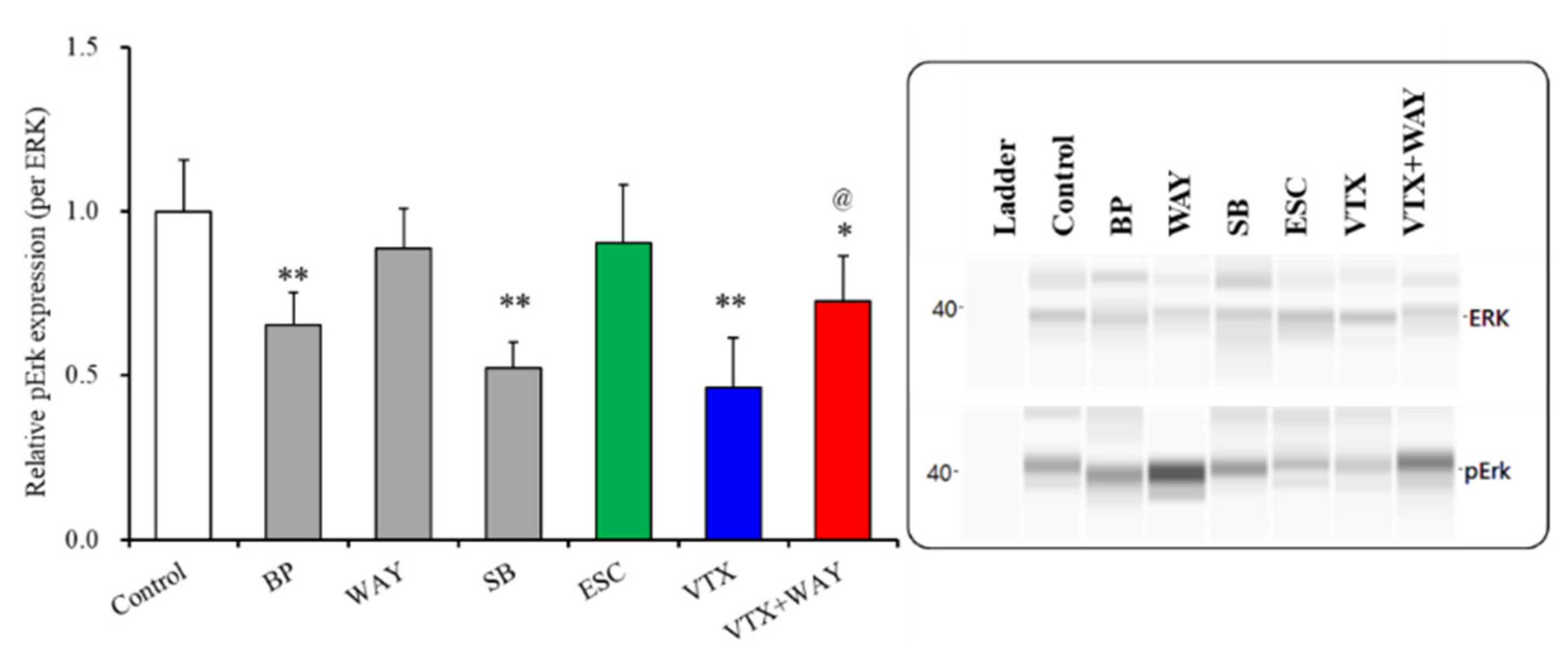
2.3.3. Effects of Subchronic Administrations of 5-HT Receptor Agents, Therapeutic-Relevant Concentrations of Escitalopram and Vortioxetine, on Phosphorylation of Extracellular Signal-Regulated Kinase (ERK) in the Plasma Membrane Fractions of Primary Cultured Astrocytes
3. Discussion
3.1. Effects of 5-HT Receptors on Astroglial L-Glutamate Release and Protein Expression Associated with Its Regulation Mechanisms in Astrocytes
3.2. Effects of Vortioxetine on Astroglial L-Glutamate Release and Protein Expression Associated with the Astroglial Serotonergic System
4. Materials and Methods
4.1. Preparation of Primary Astrocyte Culture
4.2. Ultra-High-Performance Liquid Chromatography (UHPLC)
4.3. Capillary Immunoblotting Analysis
4.4. Data Analysis
4.5. Chemical Agents
4.6. Nomenclature of Targets and Ligands
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Bowley, M.P.; Drevets, W.C.; Ongur, D.; Price, J.L. Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry 2002, 52, 404–412. [Google Scholar] [CrossRef]
- Chana, G.; Landau, S.; Beasley, C.; Everall, I.P.; Cotter, D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: Evidence for decreased neuronal somal size and increased neuronal density. Biol. Psychiatry 2003, 53, 1086–1098. [Google Scholar] [CrossRef]
- Maes, M.; Yirmyia, R.; Noraberg, J.; Brene, S.; Hibbeln, J.; Perini, G.; Kubera, M.; Bob, P.; Lerer, B.; Maj, M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009, 24, 27–53. [Google Scholar]
- Ernst, C.; Nagy, C.; Kim, S.; Yang, J.P.; Deng, X.; Hellstrom, I.C.; Choi, K.H.; Gershenfeld, H.; Meaney, M.J.; Turecki, G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol. Psychiatry 2011, 70, 312–319. [Google Scholar] [CrossRef]
- Bernard, R.; Kerman, I.A.; Thompson, R.C.; Jones, E.G.; Bunney, W.E.; Barchas, J.D.; Schatzberg, A.F.; Myers, R.M.; Akil, H.; Watson, S.J. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol. Psychiatry 2011, 16, 634–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel-Hidalgo, J.J.; Wilson, B.A.; Hussain, S.; Meshram, A.; Rajkowska, G.; Stockmeier, C.A. Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. J. Psychiatr. Res. 2014, 55, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, C.; Torres-Platas, S.G.; Mechawar, N.; Turecki, G. Repression of astrocytic connexins in cortical and subcortical brain regions and prefrontal enrichment of h3k9me3 in depression and suicide. Int. J. Neuropsychopharmacol. 2017, 20, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Nagy, C.; Suderman, M.; Yang, J.; Szyf, M.; Mechawar, N.; Ernst, C.; Turecki, G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol. Psychiatry 2015, 20, 320–328. [Google Scholar] [CrossRef]
- Ongur, D.; Drevets, W.C.; Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willner, P.; Scheel-Kruger, J.; Belzung, C. The neurobiology of depression and antidepressant action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef]
- Mulders, P.C.; van Eijndhoven, P.F.; Schene, A.H.; Beckmann, C.F.; Tendolkar, I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015, 56, 330–344. [Google Scholar] [CrossRef]
- Okada, M.; Oka, T.; Nakamoto, M.; Fukuyama, K.; Shiroyama, T. Astroglial connexin43 as a potential target for a mood stabiliser. Int. J. Mol. Sci. 2020, 22, 339. [Google Scholar] [CrossRef] [PubMed]
- Czeh, B.; Simon, M.; Schmelting, B.; Hiemke, C.; Fuchs, E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 2006, 31, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Banasr, M.; Chowdhury, G.M.; Terwilliger, R.; Newton, S.S.; Duman, R.S.; Behar, K.L.; Sanacora, G. Glial pathology in an animal model of depression: Reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol. Psychiatry 2010, 15, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Quesseveur, G.; Gardier, A.M.; Guiard, B.P. The monoaminergic tripartite synapse: A putative target for currently available antidepressant drugs. Curr. Drug Targets 2013, 14, 1277–1294. [Google Scholar] [CrossRef]
- Kikuoka, R.; Miyazaki, I.; Kubota, N.; Maeda, M.; Kagawa, D.; Moriyama, M.; Sato, A.; Murakami, S.; Kitamura, Y.; Sendo, T.; et al. Mirtazapine exerts astrocyte-mediated dopaminergic neuroprotection. Sci. Rep. 2020, 10, 20698. [Google Scholar] [CrossRef]
- Stroth, N.; Svenningsson, P. S100b interacts with the serotonin 5-ht7 receptor to regulate a depressive-like behavior. Eur. Neuropsychopharmacol. 2015, 25, 2372–2380. [Google Scholar] [CrossRef]
- Okubo, R.; Hasegawa, T.; Fukuyama, K.; Shiroyama, T.; Okada, M. Current limitations and candidate potential of 5-ht7 receptor antagonism in psychiatric pharmacotherapy. Front. Psychiatry 2021, 12, 623684. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Bennett, M.V.; Contreras, J.E.; Bukauskas, F.F.; Saez, J.C. New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends Neurosci. 2003, 26, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girao, H. Role of connexin 43 in different forms of intercellular communication—Gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, M. Can rodent models elucidate pathomechanisms of genetic epilepsy? Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Fukuyama, K.; Ueda, Y.; Okada, M. Effects of carbamazepine, lacosamide and zonisamide on gliotransmitter release associated with activated astroglial hemichannels. Pharmaceuticals 2020, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Ueda, Y. Carbamazepine attenuates astroglial L-glutamate release induced by pro-inflammatory cytokines via chronically activation of adenosine a2a receptor. Int. J. Mol. Sci. 2019, 20, 3727. [Google Scholar] [CrossRef] [Green Version]
- Galinsky, R.; Davidson, J.O.; Dean, J.M.; Green, C.R.; Bennet, L.; Gunn, A.J. Glia and hemichannels: Key mediators of perinatal encephalopathy. Neural Regen. Res. 2018, 13, 181–189. [Google Scholar]
- Walrave, L.; Vinken, M.; Leybaert, L.; Smolders, I. Astrocytic connexin43 channels as candidate targets in epilepsy treatment. Biomolecules 2020, 10, 1578. [Google Scholar] [CrossRef]
- Tanti, A.; Lutz, P.-E.; Kim, J.; O’leary, L.; Théroux, J.-F.; Turecki, G.; Mechawar, N.J.N. Evidence of decreased gap junction coupling between astrocytes and oligodendrocytes in the anterior cingulate cortex of depressed suicides. Neuropsychopharmacology 2019, 44, 2099–2111. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Murata, M. A working hypothesis regarding identical pathomechanisms between clinical efficacy and adverse reaction of clozapine via the activation of connexin43. Int. J. Mol. Sci. 2020, 21, 7019. [Google Scholar] [CrossRef]
- Portal, B.; Delcourte, S.; Rovera, R.; Lejards, C.; Bullich, S.; Malnou, C.E.; Haddjeri, N.; Deglon, N.; Guiard, B.P. Genetic and pharmacological inactivation of astroglial connexin 43 differentially influences the acute response of antidepressant and anxiolytic drugs. Acta Physiol. 2020, 229, e13440. [Google Scholar] [CrossRef]
- Sun, J.-D.; Liu, Y.; Yuan, Y.-H.; Li, J.; Chen, N.-H. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 2012, 37, 1305–1320. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Pandian, T.; Braun, N.N.; Haug, K. Chronic psychotropic drug treatment causes differential expression of connexin 43 and gfap in frontal cortex of rats. Schizophr. Res. 2008, 104, 127–134. [Google Scholar] [CrossRef]
- Mostafavi, H.; Khaksarian, M.; Joghataei, M.T.; Hassanzadeh, G.; Soleimani, M.; Eftekhari, S.; Soleimani, M.; Mousavizadeh, K.; Hadjighassem, M.R. Fluoxetin upregulates connexin 43 expression in astrocyte. Basic Clin. Neurosci. 2014, 5, 74–79. [Google Scholar] [PubMed]
- Morioka, N.; Suekama, K.; Zhang, F.F.; Kajitani, N.; Hisaoka-Nakashima, K.; Takebayashi, M.; Nakata, Y. Amitriptyline up-regulates connexin43-gap junction in rat cultured cortical astrocytes via activation of the p38 and c-fos/ap-1 signalling pathway. Br. J. Pharmacol. 2014, 171, 2854–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orellana, J.A.; Moraga-Amaro, R.; Diaz-Galarce, R.; Rojas, S.; Maturana, C.J.; Stehberg, J.; Saez, J.C. Restraint stress increases hemichannel activity in hippocampal glial cells and neurons. Front. Cell. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, Y.X.; Wang, Z.Z.; Xia, C.Y.; Mou, Z.; Ren, Q.; Liu, D.D.; Zhang, X.; Chen, N.H. The protective effect of ginsenoside rg1 on depression may benefit from the gap junction function in hippocampal astrocytes. Eur. J. Pharmacol. 2020, 882, 173309. [Google Scholar] [CrossRef]
- Jin, C.; Wang, Z.Z.; Zhou, H.; Lou, Y.X.; Chen, J.; Zuo, W.; Tian, M.T.; Wang, Z.Q.; Du, G.H.; Kawahata, I.; et al. Ginsenoside rg1-induced antidepressant effects involve the protection of astrocyte gap junctions within the prefrontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 183–191. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Moulana, M.; Deloach, P.H.; Rajkowska, G. Chronic unpredictable stress reduces immunostaining for connexins 43 and 30 and myelin basic protein in the rat prelimbic and orbitofrontal cortices. Chronic Stress 2018, 2, 2470547018814186. [Google Scholar] [CrossRef]
- Xia, C.Y.; Chu, S.F.; Zhang, S.; Gao, Y.; Ren, Q.; Lou, Y.X.; Luo, P.; Tian, M.T.; Wang, Z.Q.; Du, G.H.; et al. Ginsenoside rg1 alleviates corticosterone-induced dysfunction of gap junctions in astrocytes. J. Ethnopharmacol. 2017, 208, 207–213. [Google Scholar] [CrossRef]
- Zmudzka, E.; Salaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jie, W.; Liu, J.H.; Yang, J.M.; Gao, T.M. An astroglial basis of major depressive disorder? An overview. Glia 2017, 65, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H. Inflammation effects on brain glutamate in depression: Mechanistic considerations and treatment implications. Curr. Top. Behav. Neurosci. 2017, 31, 173–198. [Google Scholar]
- Okada, M.; Kawano, Y.; Fukuyama, K.; Motomura, E.; Shiroyama, T. Candidate strategies for development of a rapid-acting antidepressant class that does not result in neuropsychiatric adverse effects: Prevention of ketamine-induced neuropsychiatric adverse reactions. Int. J. Mol. Sci. 2020, 21, 7951. [Google Scholar] [CrossRef]
- Belanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.M.; Swanson, R.A. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef]
- Tanahashi, S.; Yamamura, S.; Nakagawa, M.; Motomura, E.; Okada, M. Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br. J. Pharmacol. 2012, 165, 1543–1555. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, S.; Hoshikawa, M.; Dai, K.; Saito, H.; Suzuki, N.; Niwa, O.; Okada, M. Ono-2506 inhibits spike-wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation. Br. J. Pharmacol. 2013, 168, 1088–1100. [Google Scholar] [CrossRef]
- Okada, M.; Matsumoto, R.; Yamamoto, Y.; Fukuyama, K. Effects of subchronic administrations of vortioxetine, lurasidone, and escitalopram on thalamocortical glutamatergic transmission associated with serotonin 5-ht7 receptor. Int. J. Mol. Sci. 2021, 22, 1351. [Google Scholar] [CrossRef]
- Okada, M.; Okubo, R.; Fukuyama, K. Vortioxetine subchronically activates serotonergic transmission via desensitization of serotonin 5-ht1a receptor with 5-ht3 receptor inhibition in rats. Int. J. Mol. Sci. 2019, 20, 6235. [Google Scholar] [CrossRef] [Green Version]
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Grunder, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in psychiatry and neurology: A comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Ueda, Y. Brivaracetam prevents astroglial L-glutamate release associated with hemichannel through modulation of synaptic vesicle protein. Biomed. Pharmacother. 2021, 138, 111462. [Google Scholar] [CrossRef]
- Fukuyama, K.; Fukuzawa, M.; Ruri, O.; Okada, M. Upregulated connexin 43 induced by loss-of-functional s284l-mutant alpha4 subunit of nicotinic ach receptor contributes to pathomechanisms of autosomal dominant sleep-related hypermotor epilepsy. Pharmaceuticals 2020, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, K.; Fukuzawa, M.; Okada, M. Upregulated and hyperactivated thalamic connexin 43 plays important roles in pathomechanisms of cognitive impairment and seizure of autosomal dominant sleep-related hypermotor epilepsy with s284l-mutant α4 subunit of nicotinic ach receptor. Pharmaceuticals 2020, 13, 99. [Google Scholar] [CrossRef]
- Mork, A.; Montezinho, L.P.; Miller, S.; Trippodi-Murphy, C.; Plath, N.; Li, Y.; Gulinello, M.; Sanchez, C. Vortioxetine (lu aa21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol. Biochem. Behav. 2013, 105, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang-Andersen, B.; Ruhland, T.; Jorgensen, M.; Smith, G.; Frederiksen, K.; Jensen, K.G.; Zhong, H.; Nielsen, S.M.; Hogg, S.; Mork, A.; et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (lu aa21004): A novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 2011, 54, 3206–3221. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Ueda, Y. Lurasidone inhibits nmda antagonist-induced functional abnormality of thalamocortical glutamatergic transmission via 5-ht7 receptor blockade. Br. J. Pharmacol. 2019, 176, 4002–4018. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Okubo, R.; Shiroyama, T.; Ueda, Y. Lurasidone sub-chronically activates serotonergic transmission via desensitization of 5-ht1a and 5-ht7 receptors in dorsal raphe nucleus. Pharmaceuticals 2019, 12, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, P.R.; Francois, B.L. Modifying 5-ht1a receptor gene expression as a new target for antidepressant therapy. Front. Neurosci 2010, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, K.; Okada, M. Age-dependent and sleep/seizure-induced pathomechanisms of autosomal dominant sleep-related hypermotor epilepsy. Int. J. Mol. Sci. 2020, 21, 8142. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-ht1a receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Tempio, A.; Niso, M.; Laera, L.; Trisolini, L.; Favia, M.; Ciranna, L.; Marzulli, D.; Petrosillo, G.; Pierri, C.L.; Lacivita, E.; et al. Mitochondrial membranes of human sh-sy5y neuroblastoma cells express serotonin 5-ht7 receptor. Int. J. Mol. Sci. 2020, 21, 9629. [Google Scholar] [CrossRef] [PubMed]
- Andressen, K.W.; Manfra, O.; Brevik, C.H.; Ulsund, A.H.; Vanhoenacker, P.; Levy, F.O.; Krobert, K.A. The atypical antipsychotics clozapine and olanzapine promote down-regulation and display functional selectivity at human 5-ht7 receptors. Br. J. Pharmacol. 2015, 172, 3846–3860. [Google Scholar] [CrossRef] [Green Version]
- La Cour, C.M.; El Mestikawy, S.; Hanoun, N.; Hamon, M.; Lanfumey, L. Regional differences in the coupling of 5-hydroxytryptamine-1a receptors to g proteins in the rat brain. Mol. Pharmacol. 2006, 70, 1013–1021. [Google Scholar] [CrossRef]
- Valdizán, E.M.; Castro, E.; Pazos, A. Agonist-dependent modulation of g-protein coupling and transduction of 5-ht1a receptors in rat dorsal raphe nucleus. Int. J. Neuropsychopharmacol. 2010, 13, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Albert, P.R.; Lembo, P.; Storring, J.M.; Charest, A.; Saucier, C. The 5-ht1a receptor: Signaling, desensitization, and gene transcription. Neuropsychopharmacology 1996, 14, 19–25. [Google Scholar] [CrossRef]
- Riad, M.; Kobert, A.; Descarries, L.; Boye, S.; Rompré, P.-P.; Lacaille, J.-C. Chronic fluoxetine rescues changes in plasma membrane density of 5-ht1a autoreceptors and serotonin transporters in the olfactory bulbectomy rodent model of depression. Neuroscience 2017, 356, 78–88. [Google Scholar] [CrossRef]
- Kushwaha, N.; Albert, P.R. Coupling of 5-ht1a autoreceptors to inhibition of mitogen-activated protein kinase activation via gβγ subunit signaling. Eur. J. Neurosci. 2005, 21, 721–732. [Google Scholar] [CrossRef]
- Adayev, T.; El-Sherif, Y.; Barua, M.; Penington, N.J.; Banerjee, P. Agonist stimulation of the serotonin1a receptor causes suppression of anoxia-induced apoptosis via mitogen-activated protein kinase in neuronal hn2-5 cells. J. Neurochem. 1999, 72, 1489–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marazziti, D.; Palego, L.; Giromella, A.; Rosa Mazzoni, M.; Borsini, F.; Mayer, N.; Giuseppe Naccarato, A.; Lucacchini, A.; Battista Cassano, G. Region-dependent effects of flibanserin and buspirone on adenylyl cyclase activity in the human brain. Int. J. Neuropsychopharmacol. 2002, 5, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.; Spatuzza, M.; D’Antoni, S.; Bonaccorso, C.M.; Trovato, C.; Musumeci, S.A.; Leopoldo, M.; Lacivita, E.; Catania, M.V.; Ciranna, L. Activation of 5-ht7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and fmr1 knockout mice, a model of fragile x syndrome. Biol. Psychiatry 2012, 72, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Mahe, C.; Loetscher, E.; Feuerbach, D.; Muller, W.; Seiler, M.P.; Schoeffter, P. Differential inverse agonist efficacies of sb-258719, sb-258741 and sb-269970 at human recombinant serotonin 5-ht7 receptors. Eur. J. Pharmacol. 2004, 495, 97–102. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Effects of atypical antipsychotics, clozapine, quetiapine and brexpiprazole on astroglial transmission associated with connexin43. Int. J. Mol. Sci. 2021, 22, 5623. [Google Scholar] [CrossRef]
- Hirschhauser, C.; Lissoni, A.; Gorge, P.M.; Lampe, P.D.; Heger, J.; Schluter, K.D.; Leybaert, L.; Schulz, R.; Boengler, K. Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning. Basic Res. Cardiol. 2021, 116, 21. [Google Scholar] [CrossRef]
- Xia, C.Y.; Wang, Z.Z.; Yamakuni, T.; Chen, N.H. A novel mechanism of depression: Role for connexins. Eur. Neuropsychopharmacol. 2018, 28, 483–498. [Google Scholar] [CrossRef]
- Xia, C.Y.; Wang, Z.Z.; Zhang, Z.; Chen, J.; Wang, Y.Y.; Lou, Y.X.; Gao, Y.; Luo, P.; Ren, Q.; Du, G.H.; et al. Corticosterone impairs gap junctions in the prefrontal cortical and hippocampal astrocytes via different mechanisms. Neuropharmacology 2018, 131, 20–30. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Gao, K.; Chi, Y.; Mitsui, T.; Ihara, T.; Sawada, N.; Kamiyama, M.; Fan, J.; Takeda, M. Ampk suppresses connexin43 expression in the bladder and ameliorates voiding dysfunction in cyclophosphamide-induced mouse cystitis. Sci. Rep. 2016, 6, 19708. [Google Scholar] [CrossRef]
- Wein, M.N.; Foretz, M.; Fisher, D.E.; Xavier, R.J.; Kronenberg, H.M. Salt-inducible kinases: Physiology, regulation by camp, and therapeutic potential. Trends Endocrinol. Metab. 2018, 29, 723–735. [Google Scholar] [CrossRef]
- Jeanson, T.; Pondaven, A.; Ezan, P.; Mouthon, F.; Charveriat, M.; Giaume, C. Antidepressants impact connexin 43 channel functions in astrocytes. Front. Cell. Neurosci. 2015, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Garre, J.M.; Retamal, M.A.; Cassina, P.; Barbeito, L.; Bukauskas, F.F.; Saez, J.C.; Bennett, M.V.; Abudara, V. Fgf-1 induces atp release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc. Natl. Acad. Sci. USA 2010, 107, 22659–22664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuyama, K.; Fukuzawa, M.; Shiroyama, T.; Okada, M. Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with s284l-mutant alpha4 subunit of nicotinic ach receptor. Br. J. Pharmacol. 2020, 177, 2143–2162. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Effects of levetiracetam on astroglial release of kynurenine-pathway metabolites. Br. J. Pharmacol. 2018, 175, 4253–4265. [Google Scholar] [CrossRef] [Green Version]
- Okada, M.; Fukuyama, K.; Kawano, Y.; Shiroyama, T.; Ueda, Y. Memantine protects thalamocortical hyper-glutamatergic transmission induced by nmda receptor antagonism via activation of system xc−. Pharmacol Res. Perspect 2019, 7, e00457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, T.; Hasegawa, T.; Suzuki, D.; Motomura, E.; Okada, M. Amantadine combines astroglial system xc− activation with glutamate/nmda receptor inhibition. Biomolecules 2019, 9, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamura, S.; Ohoyama, K.; Hamaguchi, T.; Nakagawa, M.; Suzuki, D.; Matsumoto, T.; Motomura, E.; Tanii, H.; Shiroyama, T.; Okada, M. Effects of zotepine on extracellular levels of monoamine, gaba and glutamate in rat prefrontal cortex. Br. J. Pharmacol. 2009, 157, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Kawata, Y.; Okada, M.; Murakami, T.; Mizuno, K.; Wada, K.; Kondo, T.; Kaneko, S. Effects of zonisamide on K+ and Ca2+ evoked release of monoamine as well as K+ evoked intracellular Ca2+ mobilization in rat hippocampus. Epilepsy Res. 1999, 35, 173–182. [Google Scholar] [CrossRef]
- Okada, M.; Kawata, Y.; Kiryu, K.; Mizuno, K.; Wada, K.; Tasaki, H.; Kaneko, S. Effects of adenosine receptor subtypes on hippocampal extracellular serotonin level and serotonin reuptake activity. J. Neurochem. 1997, 69, 2581–2588. [Google Scholar] [CrossRef]
- Harding, S.D.; Sharman, J.L.; Faccenda, E.; Southan, C.; Pawson, A.J.; Ireland, S.; Gray, A.J.G.; Bruce, L.; Alexander, S.P.H.; Anderton, S.; et al. The iuphar/bps guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Res. 2018, 46, D1091–D1106. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176 (Suppl. 1), S21–S141. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| ESC | VTX | VTX+WAY (vs. VTX) | WAY | BP | SB | Figure | ||
|---|---|---|---|---|---|---|---|---|
| (L-glutamate release) | Administration | |||||||
| Basal | Acute | → | → | →(→) | → | → | → | Figure 1 and Figure 2 |
| Subchronic | → | ↓ | ↓(→) | → | → | ↓ | Figure 1 and Figure 2 | |
| FCHK-Evoked | Acute | → | → | →(→) | → | → | → | Figure 1 and Figure 2 |
| Subchronic | → | ↓ | ↓(→) | → | ↓ | ↓ | Figure 1 and Figure 3 | |
| Protein Expression | Fraction | |||||||
| Cx43 | Cytosol | → | ↑ | → (↓) | → | ↑ | → | Figure 4 |
| Plasma membrane | → | ↓ | ↓ (→) | → | → | ↓ | Figure 4 | |
| 5-HT1AR | Plasma membrane | → | ↓ | ↓ (↑) | → | ↓ | ↓ | Figure 5 |
| 5-HT7R | Plasma membrane | → | ↓ | ↓ (→) | → | ↓ | ↓ | Figure 5 |
| Phosphorylated ERK | Plasma membrane | → | ↓ | ↓ (↑) | → | ↓ | ↓ | Figure 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiroyama, T.; Fukuyama, K.; Okada, M. Distinct Effects of Escitalopram and Vortioxetine on Astroglial L-Glutamate Release Associated with Connexin43. Int. J. Mol. Sci. 2021, 22, 10013. https://doi.org/10.3390/ijms221810013
Shiroyama T, Fukuyama K, Okada M. Distinct Effects of Escitalopram and Vortioxetine on Astroglial L-Glutamate Release Associated with Connexin43. International Journal of Molecular Sciences. 2021; 22(18):10013. https://doi.org/10.3390/ijms221810013
Chicago/Turabian StyleShiroyama, Takashi, Kouji Fukuyama, and Motohiro Okada. 2021. "Distinct Effects of Escitalopram and Vortioxetine on Astroglial L-Glutamate Release Associated with Connexin43" International Journal of Molecular Sciences 22, no. 18: 10013. https://doi.org/10.3390/ijms221810013
APA StyleShiroyama, T., Fukuyama, K., & Okada, M. (2021). Distinct Effects of Escitalopram and Vortioxetine on Astroglial L-Glutamate Release Associated with Connexin43. International Journal of Molecular Sciences, 22(18), 10013. https://doi.org/10.3390/ijms221810013






