Novel Strategies for Nanoparticle-Based Radiosensitization in Glioblastoma
Abstract
:1. Introduction
2. Nanoparticles
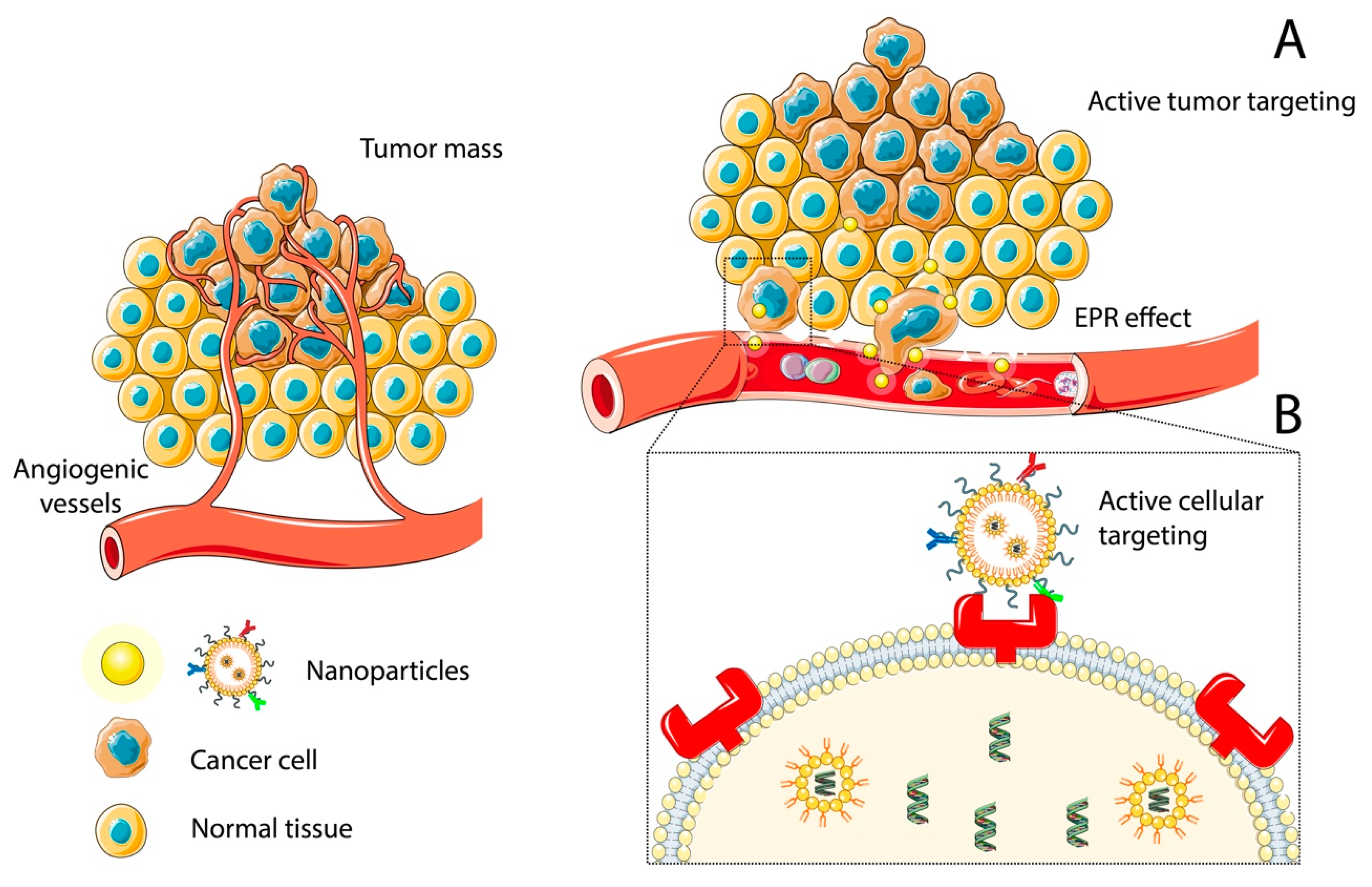
2.1. Nanoparticle Targeting
2.1.1. Passive Targeting: The Role of the Enhanced Permeability and Retention Effect and PEGylation
2.1.2. Active Targeting
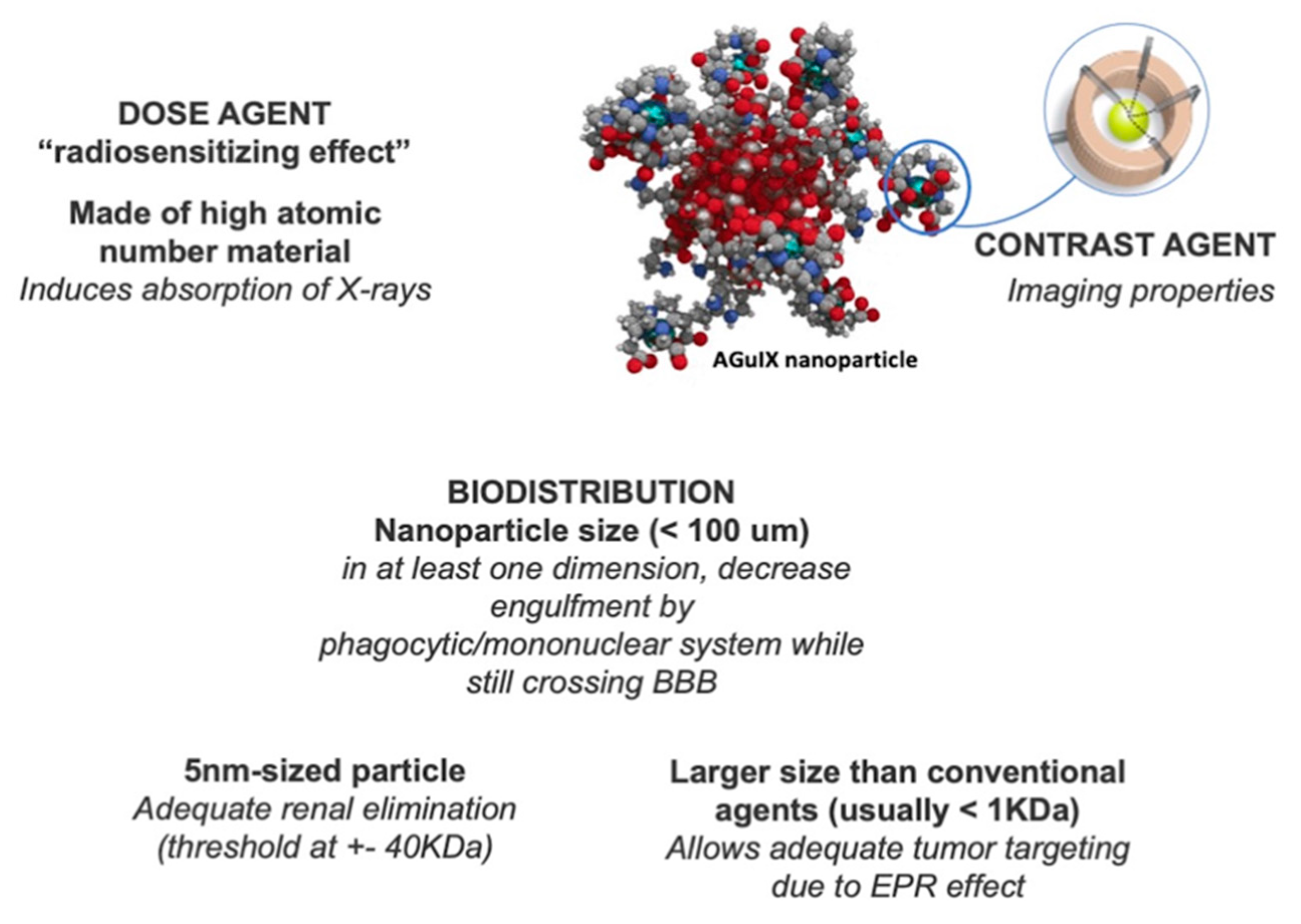
2.2. Nanoparticle Radiosensitization Efforts
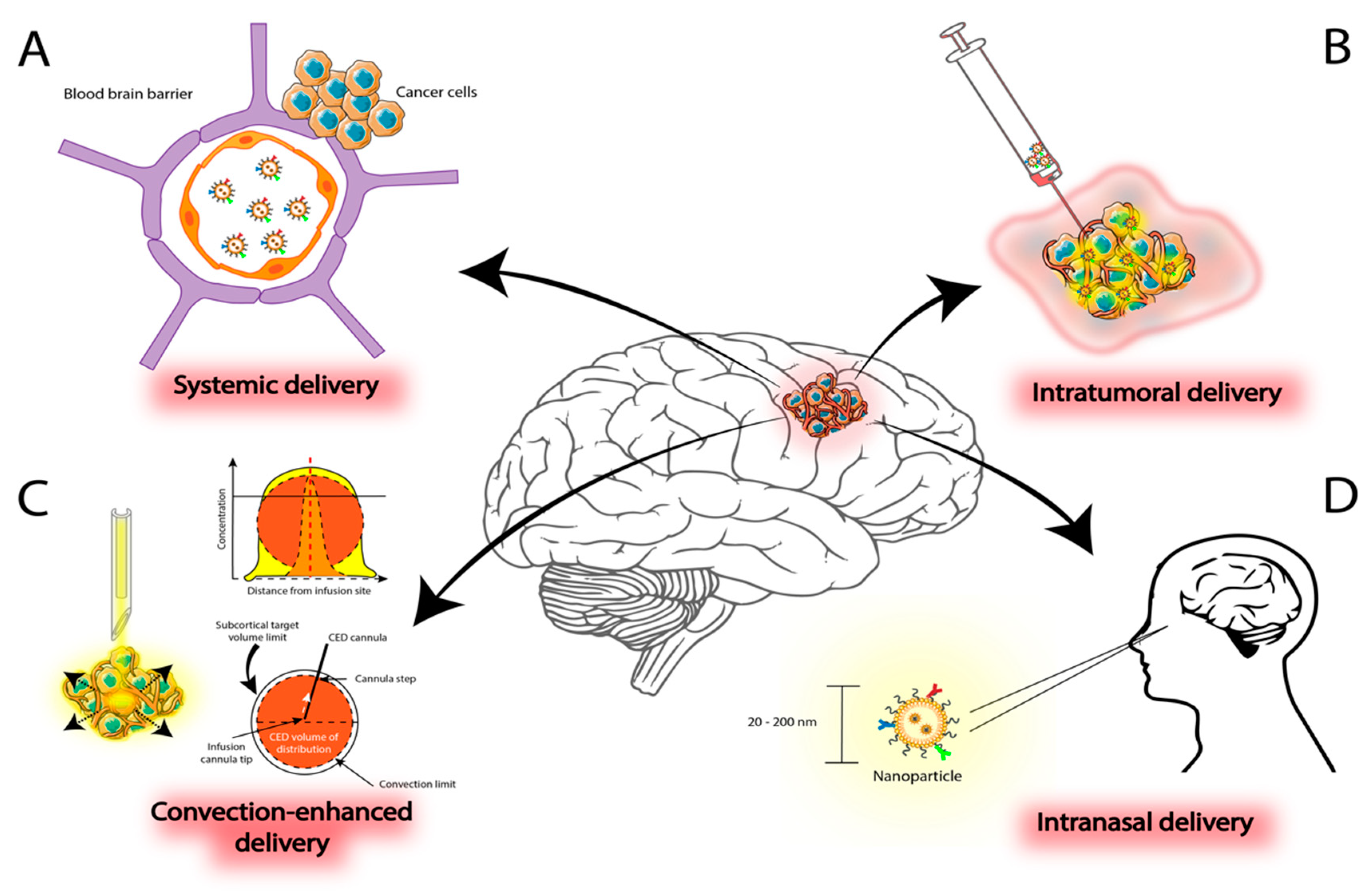
3. Delivery Methods for Nanoparticles in Malignant Gliomas
3.1. Systemic Administration
3.2. Intratumoral Delivery
4. Novel Strategies to Synergize with Nanoparticle-Based Radiosensitizers
4.1. Stem Cells
4.2. Stereotactic Radiosurgery
4.2.1. Potential Benefit of Radiosurgery in Glioblastoma
4.2.2. Nanoparticle Radiosensitization and Preoperative Radiation in GBM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Alexander, E., Jr.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S., Jr.; Mealey, J., Jr.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. 1978, 49, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chaichana, K.L.; Kleinberg, L.; Ye, X.; Quinones-Hinojosa, A.; Redmond, K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother. Oncol. 2015, 116, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, J.N.; Waddle, M.R.; Rotman, M.; Peterson, J.L.; Ruiz-Garcia, H.; Heckman, M.G.; Quinones-Hinojosa, A.; Rosenfeld, S.S.; Brown, P.D.; Trifiletti, D.M. Progress Toward Long-Term Survivors of Glioblastoma. Mayo Clin. Proc. 2019, 94, 1278–1286. [Google Scholar] [CrossRef]
- Walker, M.D.; Green, S.B.; Byar, D.P.; Alexander, E., Jr.; Batzdorf, U.; Brooks, W.H.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S., Jr.; Mealey, J., Jr.; et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 1980, 303, 1323–1329. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Devegowda, V.G.; Gupta, V.K.; Shivachar, A.; Bhosale, R.R.; Arunachalam, M.; Vaishnavi, T. Current perspectives on therapies, including drug delivery systems, for managing glioblastoma multiforme. ACS Chem. Neurosci. 2020, 11, 2962–2977. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [Green Version]
- The European Commission. Commission Recommendation of 18 October 2011 on the definition of nanomaterial 2011/696/EU. Off. J. Eur. Union 2011, 275, 38–40. [Google Scholar]
- Thimsen, E.; Johnson, M.; Zhang, X.; Wagner, A.J.; Mkhoyan, K.A.; Kortshagen, U.R.; Aydil, E.S. High electron mobility in thin films formed via supersonic impact deposition of nanocrystals synthesized in nonthermal plasmas. Nat. Commun. 2014, 5, 5822. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Warner, C.M.; Jin, H.E.; Barnes, E.; Poda, A.R.; Perkins, E.J.; Lee, S.W. Production of tunable nanomaterials using hierarchically assembled bacteriophages. Nat. Protoc. 2017, 12, 1999–2013. [Google Scholar] [CrossRef]
- Portney, N.G.; Ozkan, M. Nano-oncology: Drug delivery, imaging, and sensing. Anal. Bioanal. Chem. 2006, 384, 620–630. [Google Scholar] [CrossRef]
- Yeini, E.; Ofek, P.; Albeck, N.; Rodriguez Ajamil, D.; Neufeld, L.; Eldar-Boock, A.; Kleiner, R.; Vaskovich, D.; Koshrovski-Michael, S.; Dangoor, S.I.; et al. Targeting glioblastoma: Advances in drug delivery and novel therapeutic approaches. Adv. Ther. 2021, 4, 2000124. [Google Scholar] [CrossRef]
- Villaverde, G.; Baeza, A. Targeting strategies for improving the efficacy of nanomedicine in oncology. Beilstein J. Nanotechnol. 2019, 10, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Kunjachan, S.; Pola, R.; Gremse, F.; Theek, B.; Ehling, J.; Moeckel, D.; Hermanns-Sachweh, B.; Pechar, M.; Ulbrich, K.; Hennink, W.E.; et al. Passive versus active tumor targeting using RGD- and NGR-modified polymeric nanomedicines. Nano Lett. 2014, 14, 972–981. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Greish, K. Enhanced permeability and retention of macromolecular drugs in solid tumors: A royal gate for targeted anticancer nanomedicines. J. Drug Target. 2007, 15, 457–464. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Caro, C.; Pozo, D. Polysaccharide colloids as smart vehicles in cancer therapy. Curr. Pharm. Des. 2015, 21, 4822–4836. [Google Scholar] [CrossRef]
- Ruiz-Garcia, H.; Alvarado-Estrada, K.; Krishnan, S.; Quinones-Hinojosa, A.; Trifiletti, D.M. Nanoparticles for stem cell therapy bioengineering in glioma. Front. Bioeng. Biotechnol. 2020, 8, 558375. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mondal, S.K.; Tzeng, S.Y.; Rui, Y.; Al-kharboosh, R.; Kozielski, K.K.; Bhargav, A.G.; Garcia, C.A.; Quiñones-Hinojosa, A.; Green, J.J. Poly(ethylene glycol)-Poly(beta-amino ester)-Based Nanoparticles for Suicide Gene Therapy Enhance Brain Penetration and Extend Survival in a Preclinical Human Glioblastoma Orthotopic Xenograft Model. ACS Biomater. Sci. Eng. 2020, 6, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Daou, T.J.; Li, L.; Reiss, P.; Josserand, V.; Texier, I. Effect of poly(ethylene glycol) length on the in vivo behavior of coated quantum dots. Langmuir 2009, 25, 3040–3044. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Pernia Leal, M.; Caro, C.; García-Martín, M.L. Shedding light on zwitterionic magnetic nanoparticles: Limitations for in vivo applications. Nanoscale 2017, 9, 8176–8184. [Google Scholar] [CrossRef]
- Bertrand, N.; Leroux, J.C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kawakami, S.; Akimoto, N.; Niidome, T.; Yamashita, F.; Hashida, M. PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J. Control. Release 2006, 116, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harder, B.G.; Blomquist, M.R.; Wang, J.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A.; Loftus, J.C.; Tran, N.L. Developments in blood-brain barrier penetrance and drug repurposing for improved treatment of glioblastoma. Front. Oncol. 2018, 8, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Carter, P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer 2001, 1, 118–129. [Google Scholar] [CrossRef]
- Zhang, P.; Miska, J.; Lee-Chang, C.; Rashidi, A.; Panek, W.K.; An, S.; Zannikou, M.; Lopez-Rosas, A.; Han, Y.; Xiao, T.; et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2019, 116, 23714–23723. [Google Scholar] [CrossRef]
- Kim, D.H.; Rozhkova, E.A.; Ulasov, I.V.; Bader, S.D.; Rajh, T.; Lesniak, M.S.; Novosad, V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2010, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, T.; Chen, T. Overcoming blood-brain barrier by HER2-targeted nanosystem to suppress glioblastoma cell migration, invasion and tumor growth. J. Mater. Chem. B 2018, 6, 568–579. [Google Scholar] [CrossRef]
- Ruiz-Garcia, H.; Alvarado-Estrada, K.; Schiapparelli, P.; Quinones-Hinojosa, A.; Trifiletti, D.M. Engineering Three-Dimensional Tumor Models to Study Glioma Cancer Stem Cells and Tumor Microenvironment. Front. Cell. Neurosci. 2020, 14, 558381. [Google Scholar] [CrossRef]
- Hua, L.; Wang, Z.; Zhao, L.; Mao, H.; Wang, G.; Zhang, K.; Liu, X.; Wu, D.; Zheng, Y.; Lu, J.; et al. Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy. Theranostics 2018, 8, 5088–5105. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Shirai, K.; Chakravarti, A. Recent advancements in multimodality treatment of gliomas. Future Oncol. 2011, 7, 1169–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernsen, H.J.; Rijken, P.F.; Peters, H.; Raleigh, J.A.; Jeuken, J.W.; Wesseling, P.; van der Kogel, A.J. Hypoxia in a human intracerebral glioma model. J. Neurosurg. 2000, 93, 449–454. [Google Scholar] [CrossRef]
- Shah, J.L.; Li, G.; Shaffer, J.L.; Azoulay, M.I.; Gibbs, I.C.; Nagpal, S.; Soltys, S.G. Stereotactic Radiosurgery and Hypofractionated Radiotherapy for Glioblastoma. Neurosurgery 2018, 82, 24–34. [Google Scholar] [CrossRef]
- Kazmi, F.; Vallis, K.A.; Vellayappan, B.A.; Bandla, A.; Yukun, D.; Carlisle, R. Megavoltage radiosensitization of gold nanoparticles on a glioblastoma cancer cell line using a clinical platform. Int. J. Mol. Sci. 2020, 21, 429. [Google Scholar] [CrossRef] [Green Version]
- Sancey, L.; Lux, F.; Kotb, S.; Roux, S.; Dufort, S.; Bianchi, A.; Crémillieux, Y.; Fries, P.; Coll, J.L.; Rodriguez-Lafrasse, C.; et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br. J. Radiol. 2014, 87, 20140134. [Google Scholar] [CrossRef]
- Caban-Toktas, S.; Sahin, A.; Lule, S.; Esendagli, G.; Vural, I.; Karlı Oguz, K.; Soylemezoglu, F.; Mut, M.; Dalkara, T.; Khan, M.; et al. Combination of Paclitaxel and R-flurbiprofen loaded PLGA nanoparticles suppresses glioblastoma growth on systemic administration. Int. J. Pharm. 2020, 578, 119076. [Google Scholar] [CrossRef]
- Chung, K.; Ullah, I.; Kim, N.; Lim, J.; Shin, J.; Lee, S.C.; Jeon, S.; Kim, S.H.; Kumar, P.; Lee, S.K. Intranasal delivery of cancer-targeting doxorubicin-loaded PLGA nanoparticles arrests glioblastoma growth. J. Drug Target. 2020, 28, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, A.G.; Mondal, S.K.; Garcia, C.A.; Green, J.J.; Quiñones-Hinojosa, A. Nanomedicine Revisited: Next Generation Therapies for Brain Cancer. Adv. Ther. 2020, 3, 2000118. [Google Scholar] [CrossRef]
- Lux, F.; Tran, V.L.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F.; et al. AGuIX(®) from bench to bedside-Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2019, 92, 20180365. [Google Scholar] [CrossRef] [PubMed]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR0102. [Google Scholar] [CrossRef]
- Lacombe, S.; Porcel, E.; Scifoni, E. Particle therapy and nanomedicine: State of art and research perspectives. Cancer Nanotechnol. 2017, 8, 9. [Google Scholar] [CrossRef]
- Kefayat, A.; Ghahremani, F.; Motaghi, H.; Amouheidari, A. Ultra-small but ultra-effective: Folic acid-targeted gold nanoclusters for enhancement of intracranial glioma tumors’ radiation therapy efficacy. Nanomedicine 2019, 16, 173–184. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef]
- Kunoh, T.; Shimura, T.; Kasai, T.; Matsumoto, S.; Mahmud, H.; Khayrani, A.C.; Seno, M.; Kunoh, H.; Takada, J. Use of DNA-generated gold nanoparticles to radiosensitize and eradicate radioresistant glioma stem cells. Nanotechnology 2019, 30, 055101. [Google Scholar] [CrossRef]
- Séhédic, D.; Chourpa, I.; Tétaud, C.; Griveau, A.; Loussouarn, C.; Avril, S.; Legendre, C.; Lepareur, N.; Wion, D.; Hindré, F.; et al. Locoregional confinement and major clinical benefit of 188re-loaded CXCR4-Targeted nanocarriers in an orthotopic human to mouse model of glioblastoma. Theranostics 2017, 7, 4517–4536. [Google Scholar] [CrossRef]
- Barbero, S.; Bonavia, R.; Bajetto, A.; Porcile, C.; Pirani, P.; Ravetti, J.L.; Zona, G.L.; Spaziante, R.; Florio, T.; Schettini, G. Stromal cell-derived factor 1α stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003, 63, 1969. [Google Scholar]
- Le Duc, G.; Roux, S.; Paruta-Tuarez, A.; Dufort, S.; Brauer, E.; Marais, A.; Truillet, C.; Sancey, L.; Perriat, P.; Lux, F.; et al. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Jarrett, B.R.; Gustafsson, B.; Kukis, D.L.; Louie, A.Y. Synthesis of 64Cu-labeled magnetic nanoparticles for multimodal imaging. Bioconjug. Chem. 2008, 19, 1496–1504. [Google Scholar] [CrossRef] [Green Version]
- Badiyan, S.N.; Markovina, S.; Simpson, J.R.; Robinson, C.G.; DeWees, T.; Tran, D.D.; Linette, G.; Jalalizadeh, R.; Dacey, R.; Rich, K.M.; et al. Radiation therapy dose escalation for glioblastoma multiforme in the era of temozolomide. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 877–885. [Google Scholar] [CrossRef]
- Verry, C.; Sancey, L.; Dufort, S.; Le Duc, G.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]
- Nwagwu, C.D.; Immidisetti, A.V.; Bukanowska, G.; Vogelbaum, M.A.; Carbonell, A.M. Convection-Enhanced delivery of a first-in-class anti-β1 integrin Antibody for the treatment of high-grade glioma utilizing real-time imaging. Pharmaceutics 2020, 13, 40. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Derry, P.J.; Aziz, K.; Singh, P.K.; Khoo, A.M.; Chadha, A.S.; Liopo, A.; Zubarev, E.R.; Krishnan, S. Gold nanotriangles: Scale up and X-ray radiosensitization effects in mice. Nanoscale 2017, 9, 5085–5093. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tan, H.; Zhang, X.; Chen, F.; Zhou, Z.; Hu, X.; Chang, S.; Liu, P.; Zhang, H. Enhancement of radiotherapy efficacy by silver nanoparticles in hypoxic glioma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, S922–S930. [Google Scholar] [CrossRef] [Green Version]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Mahdavi, S.R.; Raoufi, N.J.; Motevalian, M.; Karimi, M.Y. Enhancement radiation-induced apoptosis in C6 glioma tumor-bearing rats via pH-responsive magnetic graphene oxide nanocarrier. J. Photochem. Photobiol. B Biol. 2020, 205, 111827. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 2020, 6, eaay5279. [Google Scholar] [CrossRef]
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000, 6, 2585–2597. [Google Scholar]
- Chang, S.M.; Theodosopoulos, P.; Lamborn, K.; Malec, M.; Rabbitt, J.; Page, M.; Prados, M.D. Temozolomide in the treatment of recurrent malignant glioma. Cancer 2004, 100, 605–611. [Google Scholar] [CrossRef]
- Zong, Z.; Hua, L.; Wang, Z.; Xu, H.; Ye, C.; Pan, B.; Zhao, Z.; Zhang, L.; Lu, J.; Mei, L.H.; et al. Self-Assembled angiopep-2 modified lipid-poly (hypoxic radiosensitized polyprodrug) nanoparticles delivery TMZ for glioma synergistic TMZ and RT therapy. Drug Deliv. 2019, 26, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Joh, D.Y.; Sun, L.; Stangl, M.; Al Zaki, A.; Murty, S.; Santoiemma, P.P.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Bhang, D.; et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS ONE 2013, 8, e62425. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Yang, W.; Bao, Y.; Xu, H.; Qin, S.; Tu, Y. BSA capped Au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. RSC Adv. 2015, 5, 40514–40520. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Ryzhov, V.A.; Yakovleva, L.Y.; Marchenko, Y.Y.; Parr, M.A.; Rolich, V.I.; Mikhrina, A.L.; Dobrodumov, A.V.; Pitkin, E.; et al. Ionizing radiation improves glioma-specific targeting of superparamagnetic iron oxide nanoparticles conjugated with cmHsp70.1 monoclonal antibodies (SPION–cmHsp70.1). Nanoscale 2015, 7, 20652–20664. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zheng, X.; Ren, Q.; Bu, W.; Ge, W.; Xiao, Q.; Zhang, S.; Wei, C.; Qu, H.; Wang, Z.; et al. Computed tomography imaging-guided radiotherapy by targeting upconversion nanocubes with significant imaging and radiosensitization enhancements. Sci. Rep. 2013, 3, 1751. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kaol, G.D.; Tsourkas, A.; et al. Theranostic Application of mixed gold and superparamagnetic iron oxide nanoparticle micelles in glioblastoma multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Huang, Z.; Chen, Z.; Xu, R.; Wu, H.; Zang, F.; Wang, C.; Gu, N. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale 2013, 5, 11829–11836. [Google Scholar] [CrossRef] [PubMed]
- Brachi, G.; Ruiz-Ramírez, J.; Dogra, P.; Wang, Z.; Cristini, V.; Ciardelli, G.; Rostomily, R.C.; Ferrari, M.; Mikheev, A.M.; Blanco, E.; et al. Intratumoral injection of hydrogel-embedded nanoparticles enhances retention in glioblastoma. Nanoscale 2020, 12, 23838–23850. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, J.; Ouyang, X.; Wang, J.; Sun, X.; Lv, Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cheng, F.; Wei, Y.; Zhang, L.; Guo, D.; Wang, B.; Li, W. Inhibition of TAZ contributes radiation-induced senescence and growth arrest in glioma cells. Oncogene 2019, 38, 2788–2799. [Google Scholar] [CrossRef]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; Weichselbaum, R.R.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef]
- Lehrer, E.J.; McGee, H.M.; Peterson, J.L.; Vallow, L.; Ruiz-Garcia, H.; Zaorsky, N.G.; Sharma, S.; Trifiletti, D.M. Stereotactic radiosurgery and immune checkpoint inhibitors in the management of brain metastases. Int. J. Mol. Sci. 2018, 19, 3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Klein, B.; Loven, D.; Lurie, H.; Rakowsky, E.; Nyska, A.; Levin, I.; Klein, T. The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J. Neurosurg. 1994, 80, 1074–1077. [Google Scholar] [CrossRef] [Green Version]
- Newcomb, E.W.; Demaria, S.; Lukyanov, Y.; Shao, Y.; Schnee, T.; Kawashima, N.; Lan, L.; Dewyngaert, J.K.; Zagzag, D.; McBride, W.H.; et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin. Cancer Res. 2006, 12, 4730. [Google Scholar] [CrossRef] [Green Version]
- Golden, E.B.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2012, 2, 88. [Google Scholar] [CrossRef] [Green Version]
- Routman, D.M.; Yan, E.; Vora, S.; Peterson, J.; Mahajan, A.; Chaichana, K.L.; Laack, N.; Brown, P.D.; Parney, I.F.; Burns, T.C.; et al. Preoperative stereotactic radiosurgery for brain metastases. Front. Neurol. 2018, 9, 959. [Google Scholar] [CrossRef] [Green Version]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Tienhoven, G.V.; Versteijne, E.; Suker, M.; Groothuis, K.B.C.; Busch, O.R.; Bonsing, B.A.; Hingh, I.H.J.T.d.; Festen, S.; Patijn, G.A.; Vos-Geelen, J.d.; et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. J. Clin. Oncol. 2018, 36, LBA4002. [Google Scholar] [CrossRef]
- Deora, H.; Tripathi, M.; Tewari, M.K.; Ahuja, C.K.; Kumar, N.; Kaur, A.; Kamboj, P. Role of gamma knife radiosurgery in the management of intracranial gliomas. Neurol. India 2020, 68, 290–298. [Google Scholar] [CrossRef]
- Kievit, F.M.; Wang, K.; Ozawa, T.; Tarudji, A.W.; Silber, J.R.; Holland, E.C.; Ellenbogen, R.G.; Zhang, M. Nanoparticle-Mediated knockdown of DNA repair sensitizes cells to radiotherapy and extends survival in a genetic mouse model of glioblastoma. Nanomedicine 2017, 13, 2131–2139. [Google Scholar] [CrossRef]
- Bonvalot, S.; Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. First-in-Human Study testing a new radioenhancer using nanoparticles (NBTXR3) Activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin. Cancer Res. 2017, 23, 908. [Google Scholar] [CrossRef] [Green Version]
| Agent | Study Type | Treatment Regimen |
|---|---|---|
| Gold nanoparticles (NP) | Preclinical | Gold NP + clinical 6 MV (megavoltage) photon beam in U87 glioblastoma cells [44] |
| Gold NP | Preclinical | X-ray irradiation + Gold NP in U251MG glioblastoma cells [54] |
| Folic acid decorated gold nanoclusters | Preclinical | Gold nanoclusters + 6 Gy radiation dose in C6 rat glioma cells [52] |
| Gold nanotriangles | Preclinical | Gold nanotriangles + 250 kVp X-rays in U87MG human glioblastoma cells [64] |
| Silver NP | Preclinical | Silver NP + 6 MV X-rays beams with linear accelerator in U251 glioblastoma cells [65] |
| Silver NP | Preclinical | Silver NP + 6 MV X-rays beams with linear accelerator in U251 glioblastoma cells and C6 glioma cells [66] |
| Graphene oxide NP as carrier of IUdR | Preclinical | Graphene NP + 8 Gy radiation in C6 glioma cells [67] |
| Gadolinium-based AGuIX NP | Phase 1 clinical trial | AGuIX NP injected IV + WBRT3 (30 Gy/10 fractions) [68] |
| RRx-001 | Phase 1 clinical trial (ongoing)—NCT02871843 | RRx-001 + TMZ (temozolomide) + radiation |
| Sulfasalazine | Phase 1 clinical trial (ongoing)—NCT04205357 | Sulfasalazine + stereotactic radiotherapy |
| NVX-108 | Phase 2 clinical trial (ongoing)—NCT03862430 | NVX-108 + TMZ + radiotherapy |
| Carboplatin | Phase 1 & 2 clinical trial (ongoing)—NCT03672721 | Carboplatin + radiotherapy |
| Chloroquine | Phase 2 clinical trial (ongoing)—NCT02432417 | Chloroquine + TMZ + radiotherapy |
| Adavosertib | Phase 1 clinical trial (ongoing)—NCT01849146 | Adavosertib + TMZ + radiotherapy |
| AZD1390 | Phase 1 clinical trial (ongoing)—NCT03423628 | AZD1390 + radiotherapy |
| Problem | Main Challenges Ahead |
|---|---|
| Drug interactions | Experimental studies focused on improving radiosensitization of therapeutical agents and replication of antineoplastic effect within the microenvironment. |
| Delivery systems | Delivering agents to the tumor site more precisely, leveraging existing technologies to monitor response and effectiveness (e.g., post-treatment markers, advanced imaging techniques).Focus studies to assess the functioning of delivery systems and their efficacy against biological barriers, such as bypassing systemic metabolism and the blood–brain barrier. |
| Preclinical models | Adapting preclinical models of simulated tumor microenvironment for radiosensitizer assessment to produce more accurate tumor response data. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Garcia, H.; Ramirez-Loera, C.; Malouff, T.D.; Seneviratne, D.S.; Palmer, J.D.; Trifiletti, D.M. Novel Strategies for Nanoparticle-Based Radiosensitization in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 9673. https://doi.org/10.3390/ijms22189673
Ruiz-Garcia H, Ramirez-Loera C, Malouff TD, Seneviratne DS, Palmer JD, Trifiletti DM. Novel Strategies for Nanoparticle-Based Radiosensitization in Glioblastoma. International Journal of Molecular Sciences. 2021; 22(18):9673. https://doi.org/10.3390/ijms22189673
Chicago/Turabian StyleRuiz-Garcia, Henry, Cristopher Ramirez-Loera, Timothy D. Malouff, Danushka S. Seneviratne, Joshua D. Palmer, and Daniel M. Trifiletti. 2021. "Novel Strategies for Nanoparticle-Based Radiosensitization in Glioblastoma" International Journal of Molecular Sciences 22, no. 18: 9673. https://doi.org/10.3390/ijms22189673
APA StyleRuiz-Garcia, H., Ramirez-Loera, C., Malouff, T. D., Seneviratne, D. S., Palmer, J. D., & Trifiletti, D. M. (2021). Novel Strategies for Nanoparticle-Based Radiosensitization in Glioblastoma. International Journal of Molecular Sciences, 22(18), 9673. https://doi.org/10.3390/ijms22189673








