Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Clay Minerals and Pharmaceuticals
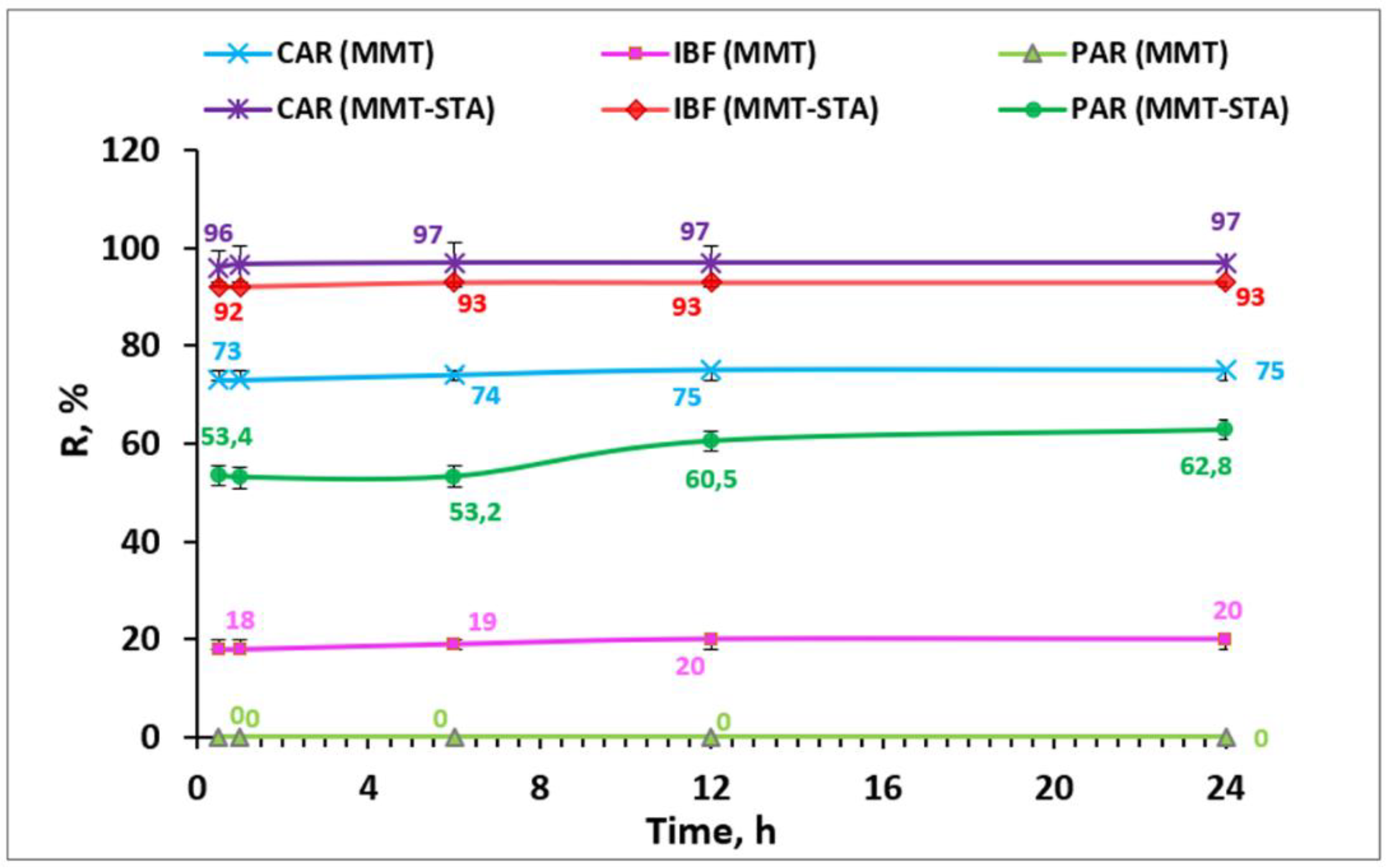
2.2. Effect of Contact Time
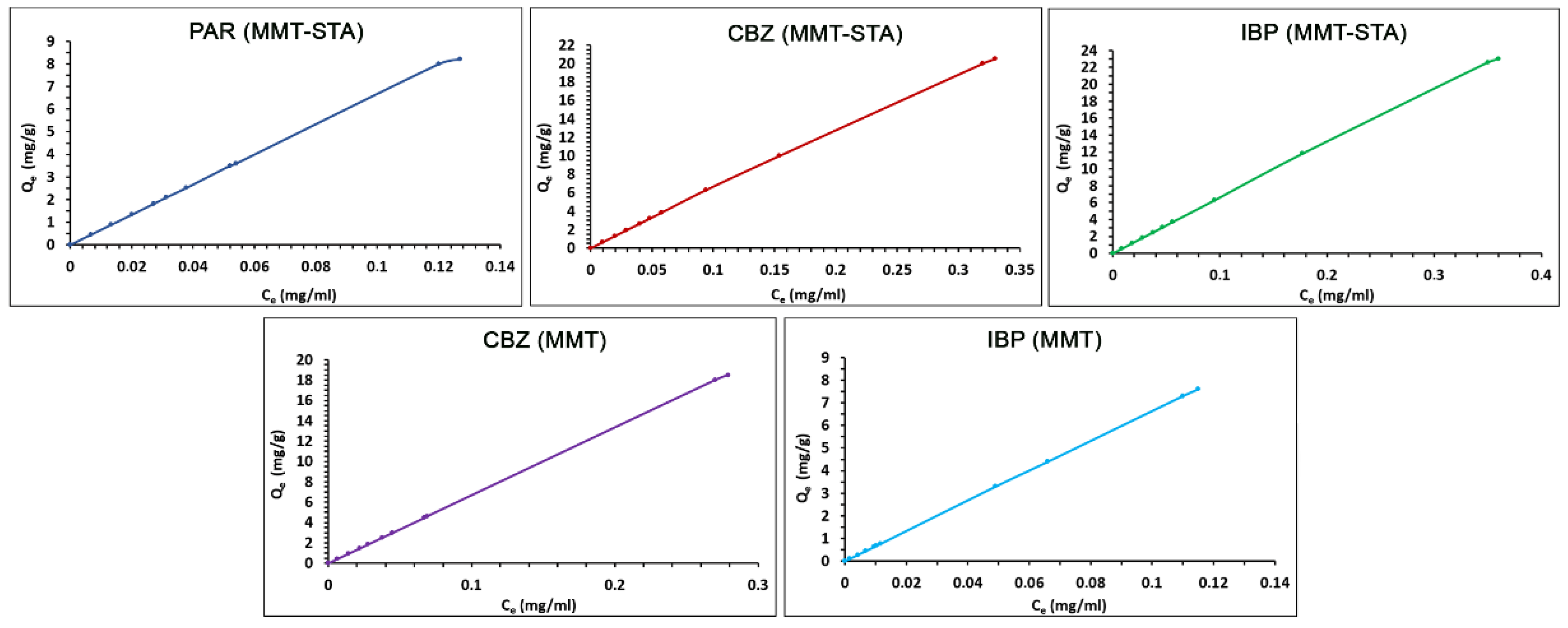
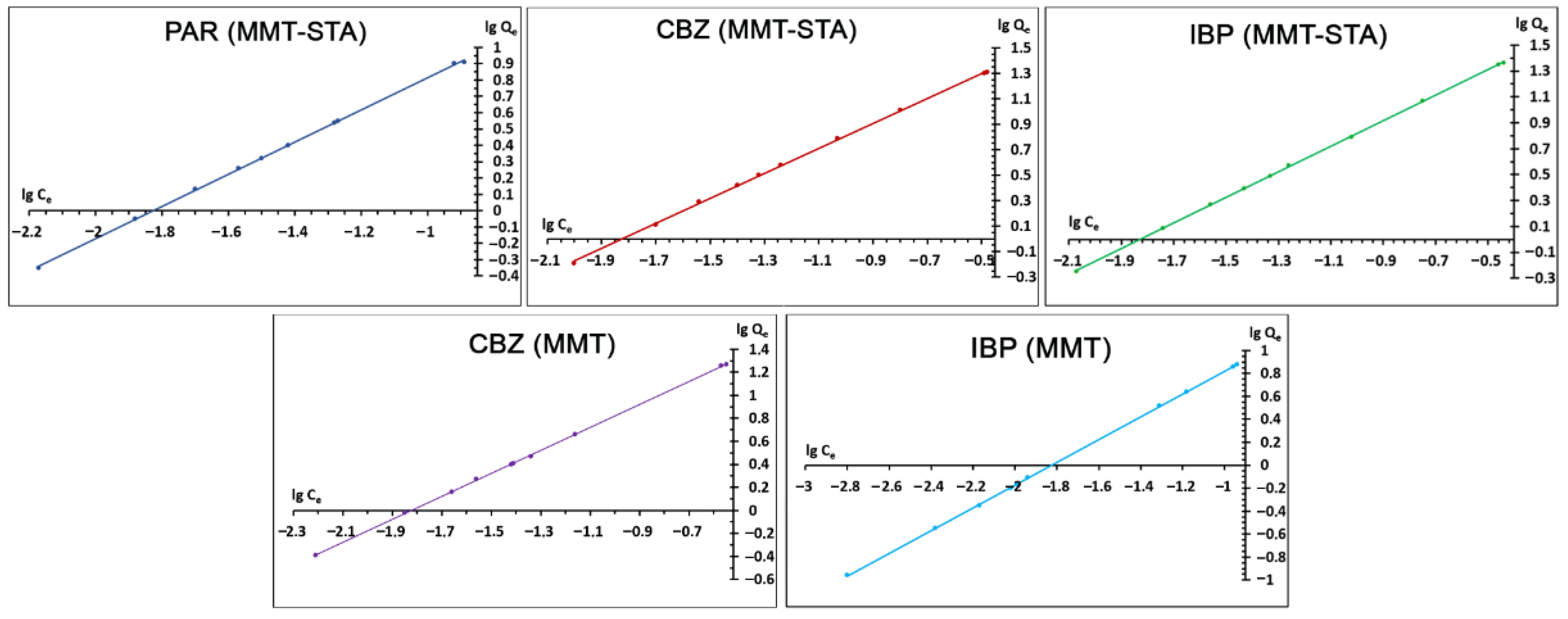
2.3. Adsorption Isotherms
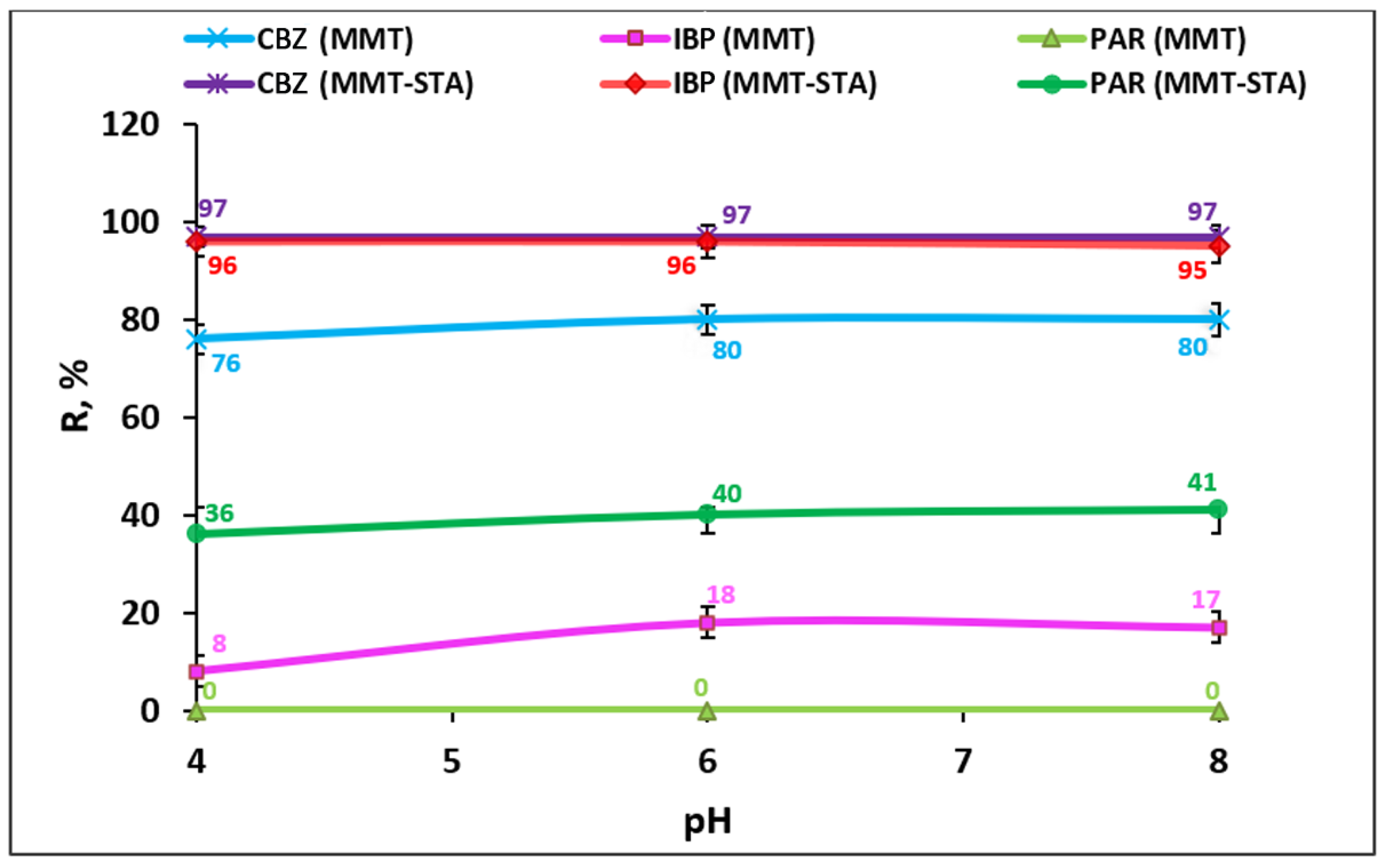
2.4. Effect of pH
2.5. Effect of Temperature
2.6. Removal Efficiency
3. Materials and Methods
3.1. Chemical Reagents and Adsorbents
3.2. Characterization of Clay Adsorbents
3.3. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.Q.; Yamamoto, K. Carbamazepine as a possible anthropogenic marker in water: Occurrences, toxicological effects, regulations and removal by wastewater treatment technologies. Water 2018, 2, 107. [Google Scholar] [CrossRef] [Green Version]
- Macías-García, A.; García-Sanz-Calcedo, J.; Carrasco-Amador, J.P.; Segura-Cruz, R. Adsorption of paracetamol in hospital wastewater through activated carbon filters. Sustainability 2019, 11, 2672. [Google Scholar] [CrossRef] [Green Version]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- Lewandowski, J.; Meinikmann, K.; Krause, S. Groundwater–surface water interactions: Recent advances and interdisciplinary challenges. Water 2020, 12, 296. [Google Scholar] [CrossRef] [Green Version]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Chander, V.; Sharma, B.; Negi, V.; Aswal, R.S.; Singh, P.; Singh, R.; Dobhal, R. Pharmaceutical compounds in drinking water. J. Xenobiot. 2016, 6, 5774. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Serna, R.; Petrovic, M.; Barcelo, D. Occurrence and distribution of multiclass pharmaceuticals and their active metabolites and transformation products in the Ebro River basin (NE Spain). Sci. Total Environ. 2012, 440, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Zhang, Z.L.; Banks, E.; Grover, D.; Jiang, J.Q. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; d’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; de Alda, M.L. Study of pharmaceuticals in surface and wastewater from cuernavaca, morelos, mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, L.H.; Helen, A.C.; Hooper, L.; Connon, R.; Hutchinson, T.H.; Maund, S.J.; Sibly, R.M. Chronic toxicity of ibuprofen to Daphnia magna: Effects on life history traits and population dynamics. Toxicol. Lett. 2007, 172, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Antunes, S.C.; Santos, J.; Martins, L.; Castro, B.B. Toxic potential of paracetamol to freshwater organisms: A headache to environmental regulators? Ecotoxicol. Environ. Saf. 2014, 107, 178–185. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sanchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Pirra, A.; Basto, M.C.P.; Almeida, C.M.R. Activated sludge systems removal efficiency of veterinary pharmaceuticals from slaughterhouse wastewater. Environ. Sci. Pollut. Res. 2013, 20, 8790–8800. [Google Scholar] [CrossRef]
- Radjenovic, J.; Petrovic, M.; Barcelo, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; López-Muñoz, M.J.; Aguado, J.; Melero, J.A.; Arsuaga, J.; Sotto, A.; Molina, R.; Segura, Y.; Pariente, M.I.; Revilla, A.; et al. Coupling membrane separation and photocatalytic oxidation processes for the degradation of pharmaceutical pollutants. Water Res. 2013, 47, 5647–5658. [Google Scholar] [CrossRef] [PubMed]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of pharmaceutical from water with an electrocoagulation process; effect of various parameters and studies of isotherm and kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, W.; Zhang, Z.; Yang, Z.; Wang, Y. Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. J. Environ. Sci. 2015, 31, 226–234. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.J.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B-Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G. Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: A review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Styszko, K.; Nosek, K.; Motak, M.; Bester, K. Preliminary selection of clay minerals for the removal of pharmaceuticals, bisphenol A and triclosan in acidic and neutral aqueous solutions. Comptes Rendus Chim. 2015, 18, 1134–1142. [Google Scholar] [CrossRef]
- Silva, A.R.; Cavaleiro, A.J.; Soares, O.S.G.P.; Braga, C.S.N.; Salvador, A.F.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Detoxification of ciprofloxacin in an anaerobic bioprocess supplemented with magnetic carbon nanotubes: Contribution of adsorption and biodegradation mechanisms. Int. J. Mol. Sci. 2021, 22, 2932. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Panchal, A.; Fu, Y.; Fakhrullin, R.; Kryuchkova, M.; Batasheva, S.; Stavitskaya, A.; Glotov, A.; Vinokurov, V. Interfacial self-assembly in halloysite nanotube composites. Langmuir 2019, 35, 8646–8657. [Google Scholar] [CrossRef] [PubMed]
- Thiebault, T. Raw and modified clays and clay minerals for the removal of pharmaceutical products from aqueous solutions: State of the art and future perspectives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1451–1514. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Fougere, L.; Destandau, E.; Monnin, L.; Milbeau, C. Clay minerals for the removal of pharmaceuticals: Initial investigations of their adsorption properties in real wastewater effluents. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100266. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The use of some clay minerals as natural resources for drug carrier applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J. Mol. Liq. 2017, 241, 1091–1113. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Z.; Hong, H.; Yin, K.; Tie, L. Adsorption and intercalation of ciprofloxacin on montmorillonite. Appl. Clay Sci. 2010, 50, 204–211. [Google Scholar] [CrossRef]
- Liu, N.; Wang, M.X.; Liu, M.M.; Liu, F.; Weng, L.; Koopal, L.K.; Tan, W.F. Sorption of Tetracycline on Organo-Montmorillonites. J. Hazard. Mater. 2012, 225–226, 28–35. [Google Scholar] [CrossRef]
- Martín, J.; del Mar Orta, M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Evaluation of a modified mica and montmorillonite for the adsorption of ibuprofen from aqueous media. Appl. Clay Sci. 2019, 171, 29–37. [Google Scholar] [CrossRef]
- Oiwa, M.; Yamaguchi, K.; Hayashi, H.; Saitoh, T. Rapid sorption of fenitrothion on didodecyldimethylammonium bromidemontmorillonite organoclay followed by the degradation into less toxic 3-methyl-4- nitrophenolate. J. Environ. Chem. Eng. 2020, 8, 104000. [Google Scholar] [CrossRef]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Le Milbeau, C.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, Y.; Boyd, S.A.; Teppen, B.J.; Li, H. Sorption and desorption of carbamazepine from water by smectite clays. Chemosphere 2010, 81, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Karaman, R.; Khamis, M.; Abbadi, J.; Amro, A.; Qurie, M.; Ayyad, I.; Ayyash, F.; Hamarsheh, O.; Yaqmour, R.; Nir, S.; et al. Paracetamol biodegradation by activated sludge and photocatalysis and its removal by a micelle–clay complex, activated charcoal, and reverse osmosis membranes. Environ. Technol. 2016, 37, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, M.; Fan, X.; Sun, X.; He, G. Preparation and in vitro evaluation of hydrophobic-modified montmorillonite stabilized pickering emulsion for overdose acetaminophen removal. Can. J. Chem. Eng. 2018, 96, 11–19. [Google Scholar] [CrossRef]
- Rozhina, E.; Panchal, A.; Akhatova, F.; Lvov, Y.; Fakhrullin, R. Cytocompatibility and cellular uptake of alkylsilane-modified hydrophobic halloysite nanotubes. Appl. Clay Sci. 2020, 185, 105371. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 786, 3973–3993. [Google Scholar] [CrossRef]
- Brdar, M.; Šćiban, M.; Takači, A.; Došenović, T. Comparison of two and three parameters adsorption isotherm for Cr (VI) onto Kraft lignin. Chem. Eng. J. 2012, 183, 108–111. [Google Scholar] [CrossRef]
- Mahouachi, L.; Rastogi, T.; Palm, W.U.; Ghorbel-Abid, I.; Chehimi, D.B.; Kümmerer, K. Natural clay as a sorbent to remove pharmaceutical micropollutants from wastewater. Chemosphere 2020, 258, 127213. [Google Scholar] [CrossRef]
- Khazri, H.; Ghorbel-Abid, I.; Kalfat, R.; Trabelsi-Ayadi, M. Removal of ibuprofen, naproxen and carbamazepine in aqueous solution onto natural clay: Equilibrium, kinetics, and thermodynamic study. Appl. Water Sci. 2017, 7, 3031–3040. [Google Scholar] [CrossRef] [Green Version]
- Alaghmand, M.; Alizadeh-Saei, J.; Barkat, S. Adsorption and removal of a selected emerging contaminant, carbamazepine, using humic acid, humasorb and montmorillonite. equilibrium isotherms, kinetics and effect of the water matrix. J. Environ. Sci. Health A 2020, 55, 1534–1541. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Behera, S.K.; Oh, S.Y.; Park, H.S. Sorptive removal of ibuprofen from water using selected soil minerals and activated carbon. Int. J. Environ. Sci. Technol. 2012, 9, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Malvar, J.L.; Martín, J.; del Mar Orta, M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Simultaneous and individual adsorption of ibuprofen metabolites by a modified montmorillonite. Appl. Clay Sci. 2020, 189, 105529. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Tai, X.; Wang, G. Preparation of modified montmorillonite with different quaternary ammonium salts and application in Pickering emulsion. New J. Chem. 2019, 43, 11543–11548. [Google Scholar] [CrossRef]
- Vallova, S.; Plevova, E.; Smutna, K.; Sokolova, B.; Vaculikova, L.; Valovicova, V.; Hundakova, M.; Praus, P. Removal of analgesics from aqueous solutions onto montmorillonite KSF. J. Therm. Anal. Calorim. 2021. [CrossRef]
- Choi, J.; Shin, W.S. Removal of salicylic and ibuprofen by hexadecyltrimethylammonium-modified montmorillonite and zeolite. Minerals 2020, 10, 898. [Google Scholar] [CrossRef]
- Corbin, G.; Vulliet, E.; Lanson, B.; Rimola, A.; Mignon, P. Adsorption of pharmaceuticals onto smectite clay minerals: A combined experimental and theoretical study. Minerals 2021, 11, 62. [Google Scholar] [CrossRef]
- Chauhan, M.; Saini, V.K.; Suthar, S. Ti-pillared montmorillonite clay for adsorptive removal of amoxicillin, imipramine, diclofenac-sodium, and paracetamol from water. J. Hazard. Mater. 2020, 399, 122832. [Google Scholar] [CrossRef] [PubMed]
- Berhane, T.M.; Levy, J.; Krekeler, M.P.S.; Danielson, N.D.; Stalcup, A. Sorption–desorption of carbamazepine bypalygorskite–montmorillonite (PM) filter medium. J. Hazard. Mater. 2015, 282, 183–193. [Google Scholar] [CrossRef]
- Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Rexing, D.J.; Snyder, S.A. Broad range analysis of endocrine disruptors and pharmaceuticals using gas chromatography and liquid chromatography tandem mass spectrometry. Chemosphere 2006, 65, 1990–1998. [Google Scholar] [CrossRef]
- Dzamukova, M.R.; Naumenko, E.A.; Lannik, N.I.; Fakhrullin, R.F. Surface-modified magnetic human cells for scaffold-free tissue engineering. Biomater. Sci. 2013, 1, 810–813. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Fakhrullin, R. Kaolin alleviates graphene oxide toxicity. Environ. Sci. Technol. Lett. 2018, 5, 295–300. [Google Scholar] [CrossRef]
- Akhatova, F.; Fakhrullina, G.; Khakimova, E.; Fakhrullin, R. Atomic force microscopy for imaging and nanomechanical characterisation of live nematode epicuticle: A comparative Caenorhabditis elegans and Turbatrix aceti study. Ultramicroscopy 2018, 194, 40–47. [Google Scholar] [CrossRef] [PubMed]
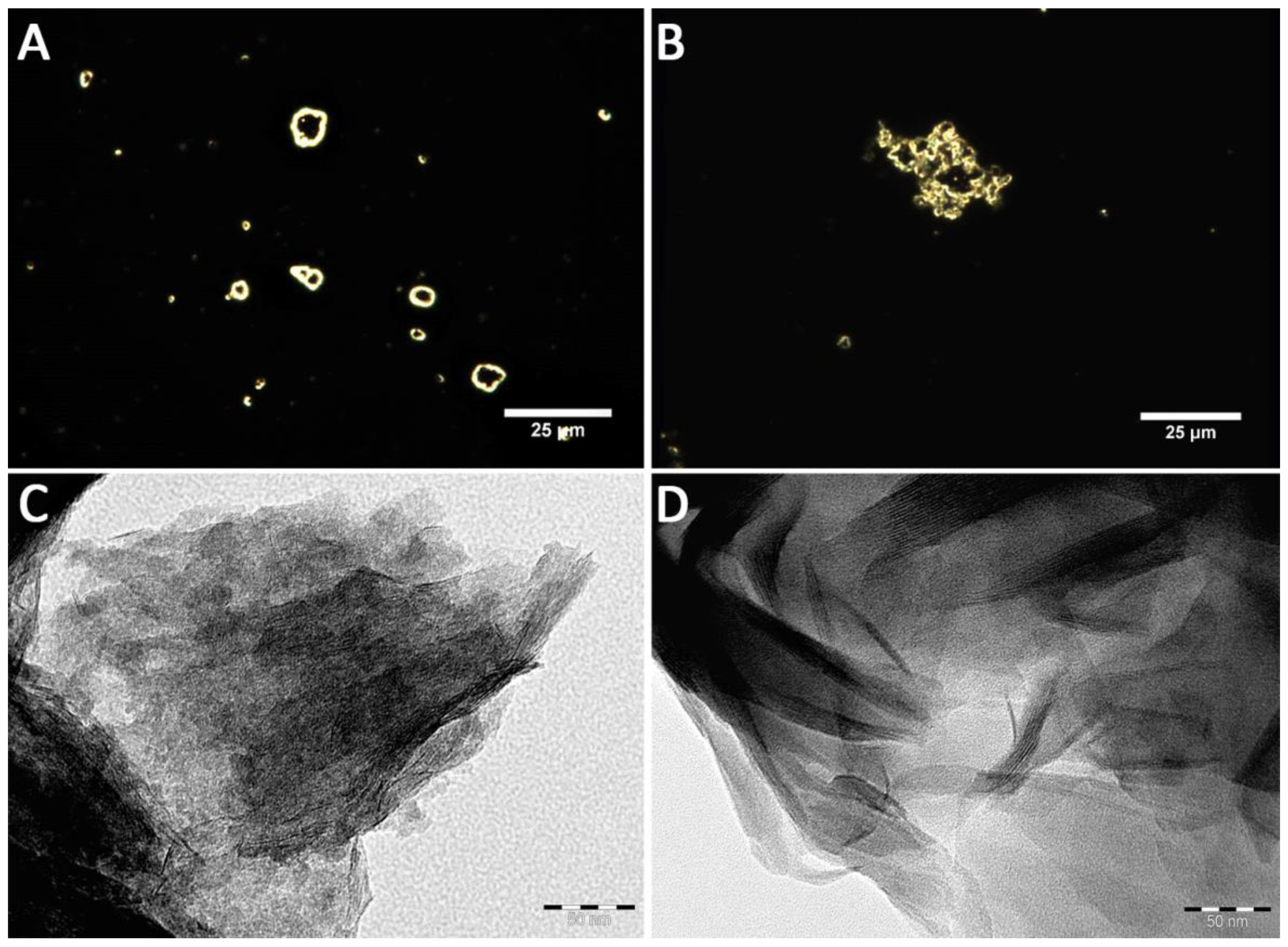



| Nanomaterials | Dh, nm | ζ, mV |
|---|---|---|
| MMT | 1723 ± 65.20 | −31.0 ± 1.23 |
| MMT-STA | 7002 ± 198.0 | 20.9 ± 0.25 |
| Carbamazepine | 612.0 ± 81.20 | −18.9 ± 6.12 |
| Ibuprofen | 1950 ± 764.5 | −3.64 ± 0.16 |
| Paracetamol | 997.2 ± 425.6 | −9.76 ± 4.03 |
| Fitting Parameters | Adsorption of Pharmaceuticals on Clays | ||||
|---|---|---|---|---|---|
| PAR (MMT-STA) | CBZ (MMT-STA) | IBP (MMT-STA) | CBZ (MMT) | IBP (MMT) | |
| Freundlich Equation | |||||
| Kf, (mg/g) (mL/mg) 1/n | 63.39 | 61.99 | 63.66 | 66.47 | 65.07 |
| n | 1.013 | 1.017 | 1.012 | 0.999 | 1.007 |
| R2 | 0.99965 | 0.99952 | 0.99981 | 0.99983 | 0.99991 |
| Pharmaceutical Products (PP) | Molecular Formula | Chemical Structure | Mw (g.mol r−1) | Sw (mg. L−1) | pKa | log Kow |
|---|---|---|---|---|---|---|
| Carbamazepine (CBZ) | C15H12N2O | 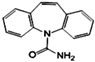 | 236.3 | 17.7 | 13.9 | 2.45 |
| Ibuprofen (IBP) | C13H18O2 | 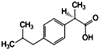 | 206.3 | 21.0 | 4.91 | 3.97 |
| Paracetamol (PAR) | C8H9NO2 | 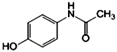 | 151.2 | 1.4 × 104 | 9.38 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryuchkova, M.; Batasheva, S.; Akhatova, F.; Babaev, V.; Buzyurova, D.; Vikulina, A.; Volodkin, D.; Fakhrullin, R.; Rozhina, E. Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay. Int. J. Mol. Sci. 2021, 22, 9670. https://doi.org/10.3390/ijms22189670
Kryuchkova M, Batasheva S, Akhatova F, Babaev V, Buzyurova D, Vikulina A, Volodkin D, Fakhrullin R, Rozhina E. Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay. International Journal of Molecular Sciences. 2021; 22(18):9670. https://doi.org/10.3390/ijms22189670
Chicago/Turabian StyleKryuchkova, Marina, Svetlana Batasheva, Farida Akhatova, Vasily Babaev, Daina Buzyurova, Anna Vikulina, Dmitry Volodkin, Rawil Fakhrullin, and Elvira Rozhina. 2021. "Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay" International Journal of Molecular Sciences 22, no. 18: 9670. https://doi.org/10.3390/ijms22189670
APA StyleKryuchkova, M., Batasheva, S., Akhatova, F., Babaev, V., Buzyurova, D., Vikulina, A., Volodkin, D., Fakhrullin, R., & Rozhina, E. (2021). Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay. International Journal of Molecular Sciences, 22(18), 9670. https://doi.org/10.3390/ijms22189670











