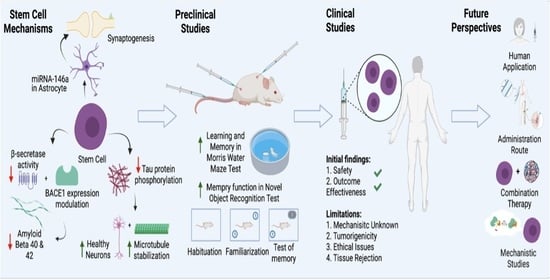
Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease
Abstract
:1. Introduction
2. Etiology of Alzheimer’s Disease
3. Current Treatments for Alzheimer’s Disease
4. Alternative Strategies for the Treatment of Alzheimer’s Disease
5. Outline of the Review
6. Therapeutic Potential of Stem Cells
7. Preclinical Research on Stem Cell Therapies in AD
8. Clinical Studies of Stem Cell Implantation in Diseases Other Than AD
9. Clinical Research on Stem Cell Therapies in AD
10. Mechanisms of Stem Cell Therapy in AD
11. Limitations and Future Perspectives
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Balázs, N.; Kovács, T. Heterogeneity of Alzheimer’s disease. Orv. Hetil. 2021, 162, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Emmady, P.D.; Tadi, P.; Del Pozo, E. Dementia (Nursing). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ganguli, M.; Hendrie, H.C. Screening for cognitive impairment and depression in ethnically diverse older populations. Alzheimer Dis. Assoc. Disord. 2005, 19, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Z.; Wen, H.; Hong, X.; Hong, Z.; Qu, Q.; Tang, M.; Wu, J.; Xu, Q.; Li, H.; et al. Incidence of dementia and subtypes: A cohort study in four regions in China. Alzheimers Dement. 2016, 12, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerreiro, R.; Bras, J. The age factor in Alzheimer’s disease. Genome Med. 2015, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.; Thomas, A.J. Depression and dementia: Cause, consequence or coincidence? Maturitas 2014, 79, 184–190. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Chapter 13—Alzheimer’s disease. In Handbook of Clinical Neurology; Dekosky, S.T., Asthana, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 231–255. [Google Scholar]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Pike, C.J. Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Pines, A. Alzheimer’s disease, menopause and the impact of the estrogenic environment. Climacteric 2016, 19, 430–432. [Google Scholar] [CrossRef]
- Davey, D.A. Alzheimer’s disease, dementia, mild cognitive impairment and the menopause: A ‘window of opportunity’? Womens Health 2013, 9, 279–290. [Google Scholar] [CrossRef]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Dang, H.; Hodis, H.N.; Henderson, V.W.; St John, J.A.; Mack, W.J.; Brinton, R.D. Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: Potential for detecting an at-Alzheimer’s risk metabolic phenotype. Neurobiol. Aging 2016, 40, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosconi, L.; Rahman, A.; Diaz, I.; Wu, X.; Scheyer, O.; Hristov, H.W.; Vallabhajosula, S.; Isaacson, R.S.; de Leon, M.J.; Brinton, R.D. Increased Alzheimer’s risk during the menopause transition: A 3-year longitudinal brain imaging study. PLoS ONE 2018, 13, e0207885. [Google Scholar] [CrossRef]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Lugliè, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dementia Fact Sheet 2020; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 22 June 2021).
- Liu, X.-Y.; Yang, L.-P.; Zhao, L. Stem cell therapy for Alzheimer’s disease. World J. Stem Cells 2020, 12, 787–802. [Google Scholar] [CrossRef]
- Boese, A.C.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for neurovascular injury in Alzheimer’s disease. Exp. Neurol. 2020, 324, 113112. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Akhondi, M.; Mousavi, N.S.; Haghparast, N.; Ghodsi, A.; Baharvand, H.; Ebrahimi, M.; Hassani, S.N. Pluripotent Stem Cells: Cancer Study, Therapy, and Vaccination. Stem Cell Rev. Rep. 2021, 1–18. [Google Scholar] [CrossRef]
- Wei, M.; Li, S.; Le, W. Nanomaterials modulate stem cell differentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Masliah, E.; Terry, R.D.; Mallory, M.; Alford, M.; Hansen, L.A. Diffuse plaques do not accentuate synapse loss in Alzheimer’s disease. Am. J. Pathol. 1990, 137, 1293–1297. [Google Scholar]
- Masliah, E.; Mallory, M.; Deerinck, T.; DeTeresa, R.; Lamont, S.; Miller, A.; Terry, R.D.; Carragher, B.; Ellisman, M. Re-evaluation of the structural organization of neuritic plaques in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1993, 52, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Roy, J.; Fung, M.L.; Heng, B.C.; Zhang, C.; Lim, L.W. Relationships between mitochondrial dysfunction and neurotransmission failure in Alzheimer’s disease. Aging Dis. 2020, 11, 1291. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; van Belle, G.; Morris, J.C.; Mohs, R.C.; Mirra, S.S.; Davis, P.C.; Tariot, P.N.; Silverman, J.M.; Clark, C.M.; Welsh-Bohmer, K.A.; et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The first twenty years. Alzheimers Dement. 2008, 4, 96–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihardja, M.; Roy, J.; Wong, K.Y.; Aquili, L.; Heng, B.C.; Chan, Y.S.; Fung, M.L.; Lim, L.W. Therapeutic potential of neurogenesis and melatonin regulation in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2020, 1478, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Nilsberth, C.; Westlind-Danielsson, A.; Eckman, C.B.; Condron, M.M.; Axelman, K.; Forsell, C.; Stenh, C.; Luthman, J.; Teplow, D.B.; Younkin, S.G.; et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat. Neurosci. 2001, 4, 887–893. [Google Scholar] [CrossRef]
- Tomiyama, T.; Shimada, H. APP Osaka Mutation in Familial Alzheimer’s Disease-Its Discovery, Phenotypes, and Mechanism of Recessive Inheritance. Int. J. Mol. Sci. 2020, 21, 1413. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.Y.; Frost, G.R.; Wu, X.; Crump, C.; Pan, S.J.; Wong, E.; Barros, M.; Li, T.; Nie, P.; Zhai, Y.; et al. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease. Nature 2020, 586, 735–740. [Google Scholar] [CrossRef]
- Armstrong, R.A. What causes alzheimer’s disease? Folia Neuropathol. 2013, 51, 169–188. [Google Scholar] [CrossRef]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril structure of amyloid-β(1-42) by cryo-electron microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer Dis. JAD 2010, 19, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Makin, S. The amyloid hypothesis on trial. Nature 2018, 559, S4–S7. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Hamano, T.; Enomoto, S.; Shirafuji, N.; Ikawa, M.; Yamamura, O.; Yen, S.H.; Nakamoto, Y. Autophagy and Tau Protein. Int. J. Mol. Sci. 2021, 22, 7475. [Google Scholar] [CrossRef]
- Adams, J.D. Probable Causes of Alzheimer’s Disease. Science 2021, 3, 16. [Google Scholar] [CrossRef]
- Ma, K.; Ding, X.; Song, Q.; Han, Z.; Yao, H.; Ding, J.; Hu, G. Lactate enhances Arc/arg3.1 expression through hydroxycarboxylic acid receptor 1-β-arrestin2 pathway in astrocytes. Neuropharmacology 2020, 171, 108084. [Google Scholar] [CrossRef]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; De Melo Reis, R.A. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2018, 21, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Jafari Nasabian, P.; Inglis, J.E.; Reilly, W.; Kelly, O.J.; Ilich, J.Z. Aging human body: Changes in bone, muscle and body fat with consequent changes in nutrient intake. J. Endocrinol. 2017, 234, R37–R51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, S.J.; Korosi, A.; Layé, S.; Shukitt-Hale, B.; Barrientos, R.M. Food for thought: How nutrition impacts cognition and emotion. NPJ Sci. Food 2017, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Dyall, S.C. Interplay Between n-3 and n-6 Long-Chain Polyunsaturated Fatty Acids and the Endocannabinoid System in Brain Protection and Repair. Lipids 2017, 52, 885–900. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Yuyama, K.; Mitsutake, S.; Igarashi, Y. Pathological roles of ceramide and its metabolites in metabolic syndrome and Alzheimer’s disease. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2014, 1841, 793–798. [Google Scholar] [CrossRef]
- Adams, J.D., Jr. DNA, Nuclear Cell Signaling and Neurodegeneration. In Extracellular and Intracellular Signaling; Royal Society of Chemistry: London, UK, 2011; pp. 175–187. [Google Scholar]
- Adams, J.D., Jr.; Lien, E.J.; Parker, K. Extracellular and Intracellular Signaling—A New Approach to Diseases and Treatments. In Extracellular and Intracellular Signaling; Royal Society of Chemistry: London, UK, 2011; pp. 1–9. [Google Scholar]
- Adams, J.; James, D. Alzheimer’s disease, ceramide, visfatin and NAD. CNS Neurol Disord Drug Targets 2008, 7, 492–498. [Google Scholar] [CrossRef]
- Adams, J. The Treatment of Brain Inflammation in Alzheimer’s Disease. Can Traditional Medicines Help? Front. Clin. Drug Res.—Alzheimer Disord. 2017, 6, 1. [Google Scholar]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanctôt, K.L.; Rajaram, R.D.; Herrmann, N. Therapy for Alzheimer’s Disease: How Effective are Current Treatments? Ther. Adv. Neurol. Disord. 2009, 2, 163–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New pharmacological strategies for treatment of Alzheimer’s disease: Focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijpma, A.; Meulenbroek, O.; Olde Rikkert, M.G. Cholinesterase inhibitors and add-on nutritional supplements in Alzheimer’s disease: A systematic review of randomized controlled trials. Ageing Res. Rev. 2014, 16, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Vécsei, L. Novel Pharmaceutical Approaches in Dementia. In NeuroPsychopharmacotherapy; Riederer, P., Laux, G., Nagatsu, T., Le, W., Riederer, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W. Alzheimer’s disease: Early diagnosis and treatment. Hong Kong Med. J. 2012, 18, 228–237. [Google Scholar] [PubMed]
- Mendiola-Precoma, J.; Berumen, L.C.; Padilla, K.; Garcia-Alcocer, G. Therapies for Prevention and Treatment of Alzheimer’s Disease. Biomed. Res. Int. 2016, 2016, 2589276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.; Tsui, K.C.; Ng, J.; Fung, M.-L.; Lim, L.W. Regulation of Melatonin and Neurotransmission in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 6841. [Google Scholar] [CrossRef]
- Moss, D.E. Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: Are irreversible inhibitors the future? Int. J. Mol. Sci. 2020, 21, 3438. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Kornhuber, J.; Weller, M.; Schoppmeyer, K.; Riederer, P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J. Neural Transm. Suppl. 1994, 43, 91–104. [Google Scholar] [PubMed]
- Kornhuber, J.; Bormann, J.; Hübers, M.; Rusche, K.; Riederer, P. Effects of the 1-amino-adamantanes at the MK-801-binding site of the NMDA-receptor-gated ion channel: A human postmortem brain study. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 206, 297–300. [Google Scholar] [CrossRef]
- Kornhuber, J.; Bormann, J.; Retz, W.; Hübers, M.; Riederer, P. Memantine displaces [3H] MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur. J. Pharmacol. 1989, 166, 589–590. [Google Scholar] [CrossRef]
- Rogers, M.B. Anti-Agitation Drug Comes Up Short in Phase 3. 2019. Available online: https://www.alzforum.org/news/research-news/anti-agitation-drug-comes-short-phase-3 (accessed on 28 August 2021).
- BI 425809. 2020. Available online: https://www.alzforum.org/therapeutics/bi-425809 (accessed on 28 August 2021).
- Sacco, R.L.; DeRosa, J.T.; Haley, E.C., Jr.; Levin, B.; Ordronneau, P.; Phillips, S.J.; Rundek, T.; Snipes, R.G.; Thompson, J.L.; Glycine Antagonist in Neuroprotection Americas Investigators. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: A randomized controlled trial. JAMA 2001, 285, 1719–1728. [Google Scholar] [CrossRef]
- AXS-05. 2020. Available online: https://www.alzforum.org/therapeutics/axs-05 (accessed on 28 August 2021).
- Uddin, M.; Kabir, M.; Rahman, M.; Behl, T.; Jeandet, P.; Ashraf, G.M.; Najda, A.; Bin-Jumah, M.N.; El-Seedi, H.R.; Abdel-Daim, M.M. Revisiting the amyloid cascade hypothesis: From anti-Aβ therapeutics to auspicious new ways for Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 5858. [Google Scholar] [CrossRef]
- Solanezumab. 2021. Available online: https://www.alzforum.org/therapeutics/solanezumab (accessed on 29 August 2021).
- Aduhelm. 2021. Available online: https://www.alzforum.org/therapeutics/aduhelm (accessed on 29 August 2021).
- Crenezumab. 2019. Available online: https://www.alzforum.org/therapeutics/crenezumab (accessed on 29 August 2021).
- Therapeutics. 2021. Available online: https://www.alzforum.org/therapeutics/gv-971%3e (accessed on 29 August 2021).
- Bauzon, J.; Lee, G.; Cummings, J. Repurposed agents in the Alzheimer’s disease drug development pipeline. Alzheimers Res. Ther. 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.; Llewellyn, D.; Isaac, M.; Tabet, N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2017, 4, Cd002854. [Google Scholar] [CrossRef]
- Masitinib. 2021. Available online: https://www.alzforum.org/therapeutics/masitinib (accessed on 29 August 2021).
- Hoyer, D.; Allen, A.; Jacobson, L.H. Hypnotics with novel modes of action. Br. J. Clin. Pharmacol. 2020, 86, 244–249. [Google Scholar] [CrossRef]
- Forbes, D.; Forbes, S.C.; Blake, C.M.; Thiessen, E.J.; Forbes, S. Exercise programs for people with dementia. Cochrane Database Syst. Rev. 2015, CD006489. [Google Scholar] [CrossRef] [Green Version]
- Pfaff, A.L.; Bubb, V.J.; Quinn, J.P.; Koks, S. An increased burden of highly active retrotransposition competent L1s is associated with Parkinson’s Disease risk and progression in the PPMI Cohort. Int. J. Mol. Sci. 2020, 21, 6562. [Google Scholar] [CrossRef]
- Baeken, M.W.; Moosmann, B.; Hajieva, P. Retrotransposon activation by distressed mitochondria in neurons. Biochem. Biophys. Res. Commun. 2020, 525, 570–575. [Google Scholar] [CrossRef]
- Taguchi, Y.; Wang, H. Exploring MicroRNA Biomarkers for Parkinson’s Disease from mRNA Expression Profiles. Cells 2018, 7, 245. [Google Scholar] [CrossRef] [Green Version]
- Brito, L.M.; Ribeiro-dos-Santos, Â.; Vidal, A.F.; de Araújo, G.S. Differential expression and mirna–gene interactions in early and late mild cognitive impairment. Biology 2020, 9, 251. [Google Scholar] [CrossRef]
- Catanesi, M.; d’Angelo, M.; Tupone, M.G.; Benedetti, E.; Giordano, A.; Castelli, V.; Cimini, A. MicroRNAs dysregulation and mitochondrial dysfunction in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 5986. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs in blood and cerebrospinal fluid as diagnostic biomarkers of multiple sclerosis and to monitor disease progression. Neural Regen. Res. 2020, 15, 606–619. [Google Scholar] [CrossRef]
- Lanfranco, M.F.; Ng, C.A.; Rebeck, G.W. ApoE lipidation as a therapeutic target in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 6336. [Google Scholar] [CrossRef] [PubMed]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Day, D.H. Calmodulin binding proteins and Alzheimer’s disease: Biomarkers, regulatory enzymes and receptors that are regulated by calmodulin. Int. J. Mol. Sci. 2020, 21, 7344. [Google Scholar] [CrossRef] [PubMed]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Jovanovic, F.; Candido, K.D.; Knezevic, N.N. The role of the kynurenine signaling pathway in different chronic pain conditions and potential use of therapeutic agents. Int. J. Mol. Sci. 2020, 21, 6045. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.C.; Cordeiro, T.M.E.; Robert, S.; de Dios, C.; Leal, V.A.C.; Soares, J.C.; Robert, D.; Antonio, T.; Sudhakar, S.M. Effect of mmune Activation on the Kynurenine Pathway and Depression Symptoms—A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Ulivieri, M.; Wierońska, J.M.; Lionetto, L.; Martinello, K.; Cieslik, P.; Chocyk, A.; Curto, M.; Di Menna, L.; Iacovelli, L.; Traficante, A. The trace kynurenine, cinnabarinic acid, displays potent antipsychotic-like activity in mice and its levels are reduced in the prefrontal cortex of individuals affected by schizophrenia. Schizophr. Bull. 2020, 46, 1471–1481. [Google Scholar] [CrossRef]
- Török, N.; Maszlag-Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single nucleotide polymorphisms of Indoleamine 2, 3-Dioxygenase 1 influenced the age onset of Parkinson’s disease. Preprints 2020, 2020100172. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Muntsant, A.; Jiménez-Altayó, F.; Puertas-Umbert, L.; Jiménez-Xarrie, E.; Vila, E.; Giménez-Llort, L. Sex-Dependent End-of-Life Mental and Vascular Scenarios for Compensatory Mechanisms in Mice with Normal and AD-Neurodegenerative Aging. Biomedicines 2021, 9, 111. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Rodríguez de Fonseca, F. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef]
- Hamani, C.; McAndrews, M.P.; Cohn, M.; Oh, M.; Zumsteg, D.; Shapiro, C.M.; Wennberg, R.A.; Lozano, A.M. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann. Neurol. 2008, 63, 119–123. [Google Scholar] [CrossRef]
- Liu, A.; Jain, N.; Vyas, A.; Lim, L.W. Ventromedial prefrontal cortex stimulation enhances memory and hippocampal neurogenesis in the middle-aged rats. eLife 2015, 4, e04803. [Google Scholar] [CrossRef]
- Tan, S.Z.K.; Du, R.; Perucho, J.A.U.; Chopra, S.S.; Vardhanabhuti, V.; Lim, L.W. Dropout in Neural Networks Simulates the Paradoxical Effects of Deep Brain Stimulation on Memory. Front. Aging Neurosci. 2020, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.K.; Neoh, J.; Lawrence, A.J.; Wu, E.X.; Lim, L.W. Prelimbic Cortical Stimulation Improves Spatial Memory Through Distinct Patterns of Hippocampal Gene Expression in Aged Rats. Neurotherapeutics 2020, 17, 2054–2068. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.K.; Poon, C.H.; Chan, Y.S.; Lim, L.W. Prelimbic cortical stimulation disrupts fear memory consolidation through ventral hippocampal dopamine D2 receptors. Br. J. Pharmacol. 2021, 178, 3587–3601. [Google Scholar] [CrossRef] [PubMed]
- Hescham, S.; Jahanshahi, A.; Meriaux, C.; Lim, L.W.; Blokland, A.; Temel, Y. Behavioral effects of deep brain stimulation of different areas of the Papez circuit on memory- and anxiety-related functions. Behav. Brain Res. 2015, 292, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Hescham, S.; Lim, L.W.; Jahanshahi, A.; Blokland, A.; Temel, Y. Deep brain stimulation in dementia-related disorders. Neurosci. Biobehav. Rev. 2013, 37, 2666–2675. [Google Scholar] [CrossRef]
- Hescham, S.; Lim, L.W.; Jahanshahi, A.; Steinbusch, H.W.; Prickaerts, J.; Blokland, A.; Temel, Y. Deep brain stimulation of the forniceal area enhances memory functions in experimental dementia: The role of stimulation parameters. Brain Stimul. 2013, 6, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.W.; Janssen, M.L.; Kocabicak, E.; Temel, Y. The antidepressant effects of ventromedial prefrontal cortex stimulation is associated with neural activation in the medial part of the subthalamic nucleus. Behav. Brain Res. 2015, 279, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.W.; Prickaerts, J.; Huguet, G.; Kadar, E.; Hartung, H.; Sharp, T.; Temel, Y. Electrical stimulation alleviates depressive-like behaviors of rats: Investigation of brain targets and potential mechanisms. Transl. Psychiatry 2015, 5, e535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, G.; Bullock, R. Defining optimal treatment with cholinesterase inhibitors in Alzheimer’s disease. Alzheimers Dement. 2011, 7, 177–184. [Google Scholar] [CrossRef]
- Blanco-Silvente, L.; Castells, X.; Saez, M.; Barceló, M.A.; Garre-Olmo, J.; Vilalta-Franch, J.; Capellà, D. Discontinuation, Efficacy, and Safety of Cholinesterase Inhibitors for Alzheimer’s Disease: A Meta-Analysis and Meta-Regression of 43 Randomized Clinical Trials Enrolling 16 106 Patients. Int. J. Neuropsychopharmacol. 2017, 20, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M. Grafting genetically modified cells to the damaged brain: Restorative effects of NGF expression. Science 1988, 242, 1575–1578. [Google Scholar] [CrossRef]
- Cattaneo, E.; McKay, R. Identifying and manipulating neuronal stem cells. Trends Neurosci. 1991, 14, 338–340. [Google Scholar] [CrossRef]
- Gage, F.H.; Kawaja, M.D.; Fisher, L.J. Genetically modified cells: Applications for intracerebral grafting. Trends Neurosci. 1991, 14, 328–333. [Google Scholar] [CrossRef]
- Wang, Q.; Matsumoto, Y.; Shindo, T.; Miyake, K.; Shindo, A.; Kawanishi, M.; Kawai, N.; Tamiya, T.; Nagao, S. Neural stem cells transplantation in cortex in a mouse model of alzheimer’s disease. J. Med. Investig. 2006, 53, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog. Neurobiol. 2019, 173, 1–17. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Winkler, J.; Kempermann, G.; Thal, L.J.; Gage, F.H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997, 17, 5820–5829. [Google Scholar] [CrossRef]
- Teramoto, T.; Qiu, J.; Plumier, J.C.; Moskowitz, M.A. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J. Clin. Investig. 2003, 111, 1125–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salie, S.; Labuschagné, A.; Walters, A.; Geyer, S.; Jardine, A.; Jacobs, M.; Hsu, N.J. In vitro and in vivo toxicity evaluation of non-neuroleptic phenothiazines, antitubercular drug candidates. Regul. Toxicol. Pharmacol. 2019, 109, 104508. [Google Scholar] [CrossRef]
- Da Silva Siqueira, L.; Majolo, F.; da Silva, A.P.B.; da Costa, J.C.; Marinowic, D.R. Neurospheres: A potential in vitro model for the study of central nervous system disorders. Mol. Biol. Rep. 2021, 48, 3649–3663. [Google Scholar] [CrossRef]
- Jensen, J.B.; Parmar, M. Strengths and limitations of the neurosphere culture system. Mol. Neurobiol. 2006, 34, 153–161. [Google Scholar] [CrossRef]
- Moghadam, F.H.; Alaie, H.; Karbalaie, K.; Tanhaei, S.; Nasr Esfahani, M.H.; Baharvand, H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation 2009, 78, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, H.; Matthaei, K.I. Culture condition difference for establishment of new embryonic stem cell lines from the C57BL/6 and BALB/c mouse strains. In Vitro Cell. Dev. Biol.—Anim. 2004, 40, 76–81. [Google Scholar] [CrossRef]
- Liu, Y.; Weick, J.P.; Liu, H.; Krencik, R.; Zhang, X.; Ma, L.; Zhou, G.M.; Ayala, M.; Zhang, S.C. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol. 2013, 31, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.-Y.; Zhang, S.-C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009, 4, 1295–1304. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical Stimulation Promotes Stem Cell Neural Differentiation in Tissue Engineering. Stem Cells Int. 2021, 2021, 6697574. [Google Scholar] [CrossRef]
- Gholamigeravand, B.; Shahidi, S.; Afshar, S.; Gholipour, P.; Samzadeh-Kermani, A.; Amiri, K.; Majidi, M.; Abbasalipourkabir, R.; Arabestani, M.R.; Soleimani Asl, S. Synergistic effects of adipose-derived mesenchymal stem cells and selenium nanoparticles on streptozotocin-induced memory impairment in the rat. Life Sci. 2021, 272, 119246. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [Green Version]
- McGinley, L.M.; Kashlan, O.N.; Bruno, E.S.; Chen, K.S.; Hayes, J.M.; Kashlan, S.R.; Raykin, J.; Johe, K.; Murphy, G.G.; Feldman, E.L. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer’s disease. Sci. Rep. 2018, 8, 14776. [Google Scholar] [CrossRef]
- Venugopal, C.; Demos, C.M.; Rao, K.S.; Pappolla, M.A.; Sambamurti, K. Beta-secretase: Structure, function, and evolution. CNS Neurol. Disord. Drug Targets 2008, 7, 278–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.Y.; Suh, Y.H.; Chang, K.A. Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2658. [Google Scholar] [CrossRef] [Green Version]
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, H.N.; Park, H.J.; Shin, J.Y.; Lee, P.H. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer’s Disease Model. Cell Transplant. 2015, 24, 1097–1109. [Google Scholar] [CrossRef] [Green Version]
- Li, L.Y.; Li, J.T.; Wu, Q.Y.; Li, J.; Feng, Z.T.; Liu, S.; Wang, T.H. Transplantation of NGF-gene-modified bone marrow stromal cells into a rat model of Alzheimer’ disease. J. Mol. Neurosci. 2008, 34, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kubota, K.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Konari, N.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci. Rep. 2020, 10, 10772. [Google Scholar] [CrossRef]
- Lu, M.H.; Ji, W.L.; Chen, H.; Sun, Y.Y.; Zhao, X.Y.; Wang, F.; Shi, Y.; Hu, Y.N.; Liu, B.X.; Wu, J.W.; et al. Intranasal Transplantation of Human Neural Stem Cells Ameliorates Alzheimer’s Disease-Like Pathology in a Mouse Model. Front. Aging Neurosci. 2021, 13, 650103. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Sun, X.; Zuo, F.; Lei, J.; Wang, Z.; Bao, X.; Wang, R. Human Neural Stem Cell Transplantation Rescues Cognitive Defects in APP/PS1 Model of Alzheimer’s Disease by Enhancing Neuronal Connectivity and Metabolic Activity. Front. Aging Neurosci. 2016, 8, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.-S.; Jung, K.; Kim, I.-S.; Lee, H.; Kim, M.; Yun, S.; Hwang, K.; Shin, J.E.; Park, K.I. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol. Neurodegener. 2015, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; La Ferla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Zhang, N.; Hu, N.; Jiang, R.; Lu, H.; Xuan, A.; Long, D.; Chen, Y. Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2020, 21, 1172–1180. [Google Scholar] [CrossRef]
- Clinical Trial with Human ES Cells for Congenital Urea Cycle Disorder—World’s First Transplantation of Human ES Cell-Derived Hepatocytes into Humans. 2020. Available online: https://www.amed.go.jp/en/news/release_20200521.html (accessed on 29 August 2021).
- Mendonça, M.V.; Larocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [Green Version]
- Alzheimer’s Association. 2015 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef]
- Jinfeng, L.; Yunliang, W.; Xinshan, L.; Shanshan, W.; Chunyang, X.; Peng, X.; Xiaopeng, Y.; Zhixiu, X.; Honglei, Y.; Xia, C.; et al. The Effect of MSCs Derived from the Human Umbilical Cord Transduced by Fibroblast Growth Factor-20 on Parkinson’s Disease. Stem Cells Int. 2016, 2016, 5016768. [Google Scholar] [CrossRef] [Green Version]
- Bhansali, A.; Upreti, V.; Khandelwal, N.; Marwaha, N.; Gupta, V.; Sachdeva, N.; Sharma, R.R.; Saluja, K.; Dutta, P.; Walia, R.; et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009, 18, 1407–1416. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Wild, E.J. Huntington’s Disease Clinical Trials Corner: August 2018. J. Huntingt. Dis. 2018, 7, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.L. Regenerative medicine: Could Parkinson’s be the first neurodegenerative disease to be cured? Future Sci. OA 2019, 5, Fso418. [Google Scholar] [CrossRef] [Green Version]
- Poulos, J. The limited application of stem cells in medicine: A review. Stem Cell Res. Ther. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guadix, J.A.; Zugaza, J.L.; Gálvez-Martín, P. Characteristics, applications and prospects of mesenchymal stem cells in cell therapy. Med. Clin. 2017, 148, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Medipost Co Ltd. The Safety and The Efficacy Evaluation of NEUROSTEM®-AD in Patients With Alzheimer’s Disease; Medipost Co Ltd.: Seongnam-si, Korea, 2011. [Google Scholar]
- Colagiuri, B.; Schenk, L.A.; Kessler, M.D.; Dorsey, S.G.; Colloca, L. The placebo effect: From concepts to genes. Neuroscience 2015, 307, 171–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.-w.; Zhang, B.; Chen, H. Safety and efficacy of human umbilical cord-derived mesenchymal stem cells in patients with Alzheimer′s disease: Study protocol for an open-label self-control trial. Clin. Transl. Degener. Dis. 2016, 1, 1. [Google Scholar] [CrossRef]
- Oliva, A.; Baumel, B.; Brody, M.; Agronin, M.; Drouillard, A.; Peña, A.; McClain-Moss, L.; Page, S.; Perez, C.; Landman, J.; et al. Progress of the phase I clinical trial to evaluate Longeveron allogeneic mesenchymal stem cells (LMSCS) as a potential therapeutic for Alzheimer’s disease. Alzheimer Dement. 2019, 15, P586. [Google Scholar] [CrossRef]
- Martello, G.; Smith, A. The nature of embryonic stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 647–675. [Google Scholar] [CrossRef]
- Jin, X.; Lin, T.; Xu, Y. Stem Cell Therapy and Immunological Rejection in Animal Models. Curr. Mol. Pharmacol. 2016, 9, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Teng, J.; Takei, Y.; Oguchi, K.; Hirokawa, N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 2002, 158, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, K.T.; Eroglu, C. Molecular mechanisms of astrocyte-induced synaptogenesis. Curr. Opin. Neurobiol. 2017, 45, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Müller, F.J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Date, I.; Kawamura, K.; Nakashima, H. Histological signs of immune reactions against allogeneic solid fetal neural grafts in the mouse cerebellum depend on the MHC locus. Exp. Brain Res. 1988, 73, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Juengst, E.; Fossel, M. The Ethics of Embryonic Stem Cells—Now and Forever, Cells Without End. JAMA 2000, 284, 3180–3184. [Google Scholar] [CrossRef]
- Tan, S.Z.K.; Lim, L.W. A practical approach to the ethical use of memory modulating technologies. BMC Med. Ethics 2020, 21, 89. [Google Scholar] [CrossRef]
- Aguila, J.C.; Hedlund, E.; Sanchez-Pernaute, R. Cellular Programming and Reprogramming: Sculpting Cell Fate for the Production of Dopamine Neurons for Cell Therapy. Stem Cells Int. 2012, 2012, 412040. [Google Scholar] [CrossRef] [Green Version]
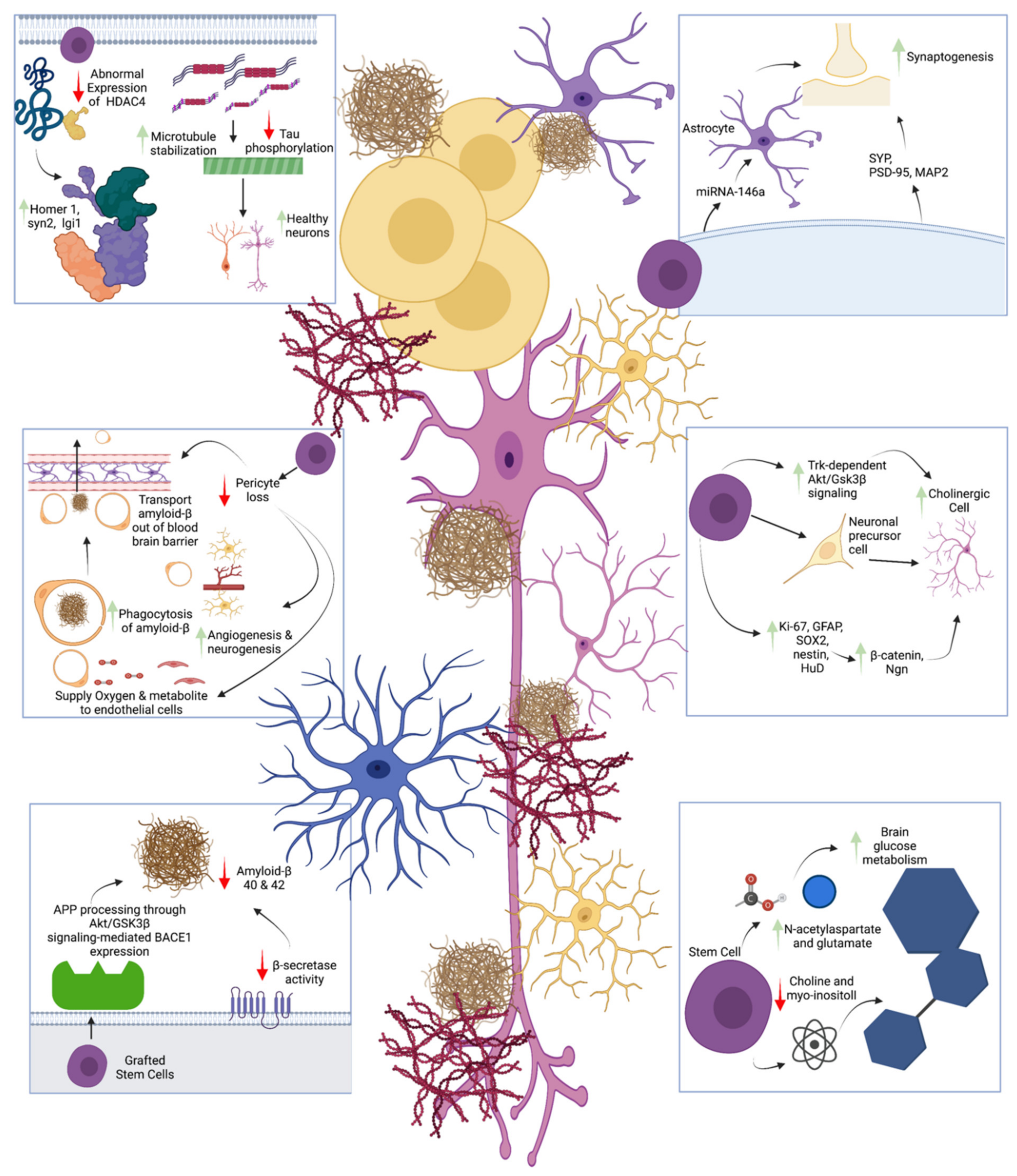
| Authors | Models and Transgene | Dosage and Injection Site | Type of Stem Cells | Behavioral Outcome | Mechanisms & Physiological Effects |
|---|---|---|---|---|---|
| Farshad et al., 2009 [124] | Sprague–Dawley rats with nucleus basalis of Meynert lesion | One surgery injection into coordinates from bregma: AP = −0.9, L = 2.8, V = 6.8 with 2 × 105 cells in 2 μL. | Embryonic stem cells | Learning, memory, and spatial cognition improved in the MWM test | Successful in vitro differentiation of ESC to neuronal precursor cells. |
| Liu et al., 2013 [126] | p75-saporin lesion models (8 to 10 weeks old) | One surgery injection into ventricle with about 100,000 cells | hESCs (line H9, passages 18–35; line H1 passages 30–36) | Learning, memory, and spatial cognition improved in the MWM test | hESCs differentiated into primitive neuroepithelia, MGE-like progenitors, then into cholinergic spinal motor neurons in vitro. MGE progenitors efficiently induced by SHH, and ISL1, OLIG2, and ASCL1 (expressed in lateral ganglionic eminence (LGE) and MGE cells in vivo). Grafted neural progenitor cells produced neurons and glia. |
| Ra et al., 2011 [135] | SCID mice (91 males and 91 females, 6 weeks old) | One injection of different MSC dosages in the tail vein: Saline control Low dose (5 × 106 hAdMSCs/kg B.W.) Medium dose (3.5 × 107 hAdMSCs/kg B.W.) High dose (2.5 × 108 hAdMSCs/kg B.W.) | MSCs | No abnormal side effects | No specific pathological changes were observed in mice organs including lungs. No significant changes in the hematology, urine, ophthalmic test, and blood chemistry results. |
| Se et al., 2015 [136] | Male C57BL/6 mice receiving Aβ1—42 injection (6 weeks old, not transgenic) | One intracerebroventricular injection of 1.0 × 106 cells/mouse | MSCs | Behavioral analysis of the radial Arm Maze test showed the MSC treatment significantly improved cognitive memory performance | Short-term treatment with MSCs in Aβ-treated NPCs slightly increased expression of proliferation marker Ki-67 and neuronal progenitor markers GFAP, SOX2, and nestin without changing HuD expression. Long-term treatment significantly increased expression of the proliferation marker, neuronal progenitor markers, and the neuronal marker HuD compared to Aβ treatment alone. In Aβ-treated NPCs co-cultured with MSCs, long-term MSC treatment significantly increased expression of β-catenin and Ngn1 compared to Aβ treatment alone, showing Wnt/β-catenin signaling is involved in MSC-induced increased survival and neuronal differentiation in NPCs. MSCs enhanced expression of β-catenin and Ngn1 in AD animal models. Double-stained BrdU and Ngn1 cells in the hippocampus were frequently observed in MSC-treated AD animals. |
| Li et al., 2007 [137] | Sprague–Dawley rats with synthetic Aβ 1–40 amyloid protein injected | One injection with 2–3 × 105 cells into the hippocampus | BMSCs | Cognition improved in the MWM | Transplanted cells expressing NGF survived and differentiated into ChAT-like neurons. |
| Gholamigeravand et al., 2021 [129] | Adult male Wistar rats with streptozotocin-induced memory impairment | Five groups:
| Adipose-derived mesenchymal stem cells (AMSCs) | NOR test showed cognitive improvements | Synergistic effects of SeNPs and AMSCs on memory function in STZ-injected rats with decreased Aβ deposition. Administration of SeNPs enhanced migration, survival, and BDNF hippocampus concentration of transplanted AMSCs rats. |
| Losurdo et al., 2020 [134] | Triple-transgenic 3xTg mice | PBS solution or extracellular vesicles in ∼5 μL spurts per nostril | MSCs | Not available | MSC-EVs decreased microglia activation in 3xTg AD mice. MSC-EVs increased dendritic spine density in 3xTg mice. |
| Kim et al., 2020 [133] | Tg2576 mice expressing mutant human APP containing the Swedish (K670N/M671L) mutation | Not given | hAESCs | Spatial learning and memory were improved in MWM test | Transplantation of hAESCs reduced amyloid burden. |
| Chen et al., 2021 [138] | J20 transgenic mouse model of C57BL/6 type (JAX-006293) and age-matched control group | One intravenous injection of 50 μg of purified MSC-exosomes (number not specified) | MSCsh | Cognitive function improved in the NOR test | MSC-exosomes decreased Aβ levels. MSC-exosomes prevented the downregulation of neuronal memory/synaptic plasticity-related genes such as Bdnf IV, SYP, GluR2, and GRIN2B. MSC-exosomes improved brain glucose metabolism as shown in the [18f] FDG-PET. MSC-exosomes inhibited astrocyte activation, upregulated neuronal memory, and synapse-related genes; levels of bdnfiv, syp, and glur1 were increased. HDAC4 expression decreased after treatment, and expressions of HDAC4 target genes Homer1 (Homer protein homolog 1), Syn2 (Synapsin II), and Lgi1 (leucine-rich glioma inactivated 1) were elevated. |
| Masako et al., 2020 [139] | Protein/presenilin 1 (APP/PS1) mice | Two times at a 2-week interval with 1 × 105 BM-MSCs per mouse via intracerebroventricular injection | BM-MSCs | Improved learning and memory impairment shown by the probe test and hidden platform MWM | BM-MSCs decreased glial fibrillary acidic protein (GFAP)- and tumor necrosis factor (TNF) α-positive areas in astrocytes. BM-MSCs decreased M1 type activated microglia and increased M2 type activated microglia in AD model mice. MSC-CM secreted C-X-C Motif Chemokine Ligand 5 (CXCL5), monocyte chemoattractant protein-1 (MCP-1), beta-nerve growth factor (β-NGF), tissue inhibitor of metalloproteinase-1 (TIMP-1), and vascular endothelial growth factor-A (VEGF-A). The numbers of F4/80-positive macrophages and the intensity of transthyretin (TTR) were increased in CP. The expression of miR-146a in the hippocampus was significantly upregulated. Expression of TRAF6 in the subiculum area was significantly decreased. BM-MSC-derived exosomes suppressed the expression of NF-κB by transferring miR-146a into astrocytes. BM-MSC-derived exosomal miR-146a were taken up into astrocytes leading to anti-inflammatory effects. |
| Lu et al., 2021 [140] | APP/PS1 double transgenic mice expressing mutant human amyloid precursor protein (APPswe) and presenilin 1 (PS1-dE9) under a mouse prion protein promoter | One injection with 8 μL (1 × 106, 4 μL/side) of hNSCs or saline on both sides of the nasal cavity | hNSCs | Cognition was improved in NOR and MWM tests | hNSCs attenuated Aβ40 and Aβ42 accumulation in APP/PS1 mice and promoted clearance via increased IDE and NEP levels. hNSC transplantation reduced the density of astrocytes and microglia, indicating an inhibition of neuroinflammation. Intranasal transplantation of hNSCs enhanced endogenous neurogenesis, ameliorated pericytes, and synaptic loss in the hippocampus of APP/PS1 mice. Increased number of pericytes increased Aβ clearance by phagocytosis and transportation of Aβ out of the CNS through the BBB, maintaining BBB permeability, and supplying oxygen and metabolites in endothelial cells. Increased number of pericytes modulated neurogenesis and angiogenesis correlated with cognition. |
| Li et al., 2016 [141] | APP/PS1 Tg mice | A Hamilton micro syringe fixed on the stereotaxic apparatus was inserted 2.5 mm under the dura, and a 4 μL NSC suspension (at 1 × 105/μL) or PBS was injected into the brain gently | hNSCs | Alleviated cognitive, learning, and memory but not anxiety deficits in NOR and MWM tests | Transplantation of hNSC reduced soluble Aβs, but not insoluble Aβs and plaque burden in AD mice brains. Transplantation of hNSC rescued neuronal loss and connectivity in AD mice brains. Improved neuronal metabolic activity, elevated NAA and Glu peaks, but lowered choline and myo-inositol. |
| Lee et al., 2015 [142] | NSE/APPswe transgenic mice | One injection of 5 μL of vehicle or hNSC suspension 1 × 105 cells/μL bilaterally into lateral ventricles (LVs; 0.1 mm caudal, 0.9 mm bilateral to bregma, and 2.0 mm ventral from the dura mater) | hNSCs | Spatial memory was improved in the MWM | Human NSC transplantation inhibited tau phosphorylation, activated Trk-dependent Akt/GSK3β signaling, and reduced Aβ42 levels. Human NSC transplantation altered APP processing by modulating BACE1 expression, and decreased astrogliosis and microgliosis. Human NSC transplantation attenuated microglial activation through cell-to-cell contact and secretory molecules. Transplantation of hNSC reduced soluble Aβs, but not insoluble Aβs and plaque burden, in AD mice brains. |
| McGinley et al., 2018 [131] | Male B6C3-Tg (APPswe/PSEN1ΔE9) 85Dbo/J (APP/PS1; n = 20) mice (stock #034829-JAX; Jackson Laboratory, Bar Harbor, ME) | Unknown number of stem cell injected at the following coordinates (bregma/lateral/ventral): −0.82/0.75/2.5, −1.46/2.3/2.9, −1.94/2.8/2.9 mm | NSCs | NSCs targeted to the fimbria fornix improved cognitive function in NOR and MWM tests | Transient NSC engraftment reduced Aβ plaque pathology. NSCs modulated microglial activation in vivo and in vitro. |
| Ager et al., 2018 [143] | APP/PS1 AD mouse model (8 weeks old) | 2-week interval with 3 × 105 cells per hippocampus | Unique line of human cortex-derived neural stem cells (NSCs; NSI-HK532-IGF-1) | 3xTg-AD-HuCNS-SC mice performed significantly better during the probe trial in MWM Significantly enhanced place-dependent memory performance with transplanted stem cell in the NOR task | Substantial survival of transplanted human cells in two distinct immunosuppressed transgenic models of AD-associated neurodegeneration. Increased synaptophysin (SYP), synapsin, and growth-associated protein-43 (GAP-43) in mice, indicating increased synaptic density. |
| Zhu et al., 2020 [144] | APP/PS1 (APPswe, PSEN1dE9) double transgenic mice (5 months old) | One injection of 5 µL × (1 × 105 cells/µL) in the hippocampus bilaterally | NSCs derived from the embryonic brain (E12.5–14.5 days) of pregnant EGFP-labeled mice | Improved spatial learning and memory ability in the MWM | Engrafted stem cells survived and partly remained at the injection site. Some engrafted stem cells migrated to surrounding regions including the corpus callosum and adjacent cortex. Some engrafted stem cells experienced morphologic changes and differentiated into GFAP+ and DCX+ cells, i.e., astrocytes. SYP, PSD-95 (postsynaptic density protein 95), and MAP-2 proteins significantly increased after stem cell implantation. Neural stem cell transplantation increased ChAT protein levels in the basal forebrain. |
| Authors | Disease, Clinical Phase, and Duration | Study Design | Type of Stem Cells and Implantation Route | Dosage and Concentration | Outcome Measures | Clinical Evaluation | Adverse Effects |
|---|---|---|---|---|---|---|---|
| “Clinical trial with human...” [145] | Congenital Urea Cycle Disorder Phase N.A. | 6-day-old baby | Human embryonic stem (HES) cell-derived hepatocytes Intravenous infusion | N.A. | Blood ammonia concentration | Liver transplantation | No complications from the surgical procedure. |
| Mendonça et al., 2014 [146] | Spinal Cord Injury Phase 1 6 months | Open-label, single-group assignment 14 patients (18 to 50 years old) | Bone marrow mesenchymal stem cells (BMMSC) Intralesional injection | 5 × 106 cells/cm3 single dose | Feasibility and Safety of BMMSC, Functional Improvement in muscle strength and sphincter control | Frankel Scale American Spinal Injury Association Impairment Scale (AIS) Somatosensory evoked potential (SEP) | One patient developed cerebrospinal fluid (CSF) leak due to intervention practices. No severe side effects or other complications. |
| Díaz, 2019 [151] | Parkinson’s Disease Phase 1 1 year | Open-label, single-group assignment 20 participants (40 to 60 years old) | Umbilical cord blood-derived mesenchymal stem cells (UC-MSCs) Intravenous infusion | 10–20 million cells once a week for 3 weeks | N.A. | Unified Parkinson’s Disease Rating Scale (UPDRS) MMSE Hoehn and Yahr staging (H-Y) Hamilton Depression Scale 24 (HAMD 24) Hamilton Anxiety Scale 14 (HAMA-14) Adverse reaction | N.A. |
| Bhansali et al., 2009 [149] | Diabetes Mellitus Phase II 6 months | Open-label, single-group assignment 10 patients (30 to 75 years old) | Autologous bone marrow-derived stem cells (ABMSCs) Angiographically | N.A. | Reduction of insulin requirement by > 50% Increment in glucagon stimulated C-peptide levels Reduction of insulin requirement Improvement of HbA1c levels compared to baseline | Glucagon stimulated C-peptide levels Insulin dosage HbA1c levels | No serious adverse effects were noted. |
| Rodrigues & Edward, 2018 [150] | Huntington’s Disease Phase I/II 36 months | Open-label, single-group assignment 50 participants (35 to 44 years old) | Bone marrow-derived autologous mononuclear cells (BMAMNCs) Intrathecal transplantation | 100 million stem cells per dose | Improvement in cognitive and psychiatric symptoms Improvement in neuropsychiatric behavior Increase in life expectancy Improvement in writhing motions or abnormal posturing Improvement in compulsive behavior | N.A. | N.A. |
| Authors | Clinical Phase & Duration | Study Design | Type of Stem Cells and Implantation Route | Dosage and Concentration | Outcome Measures | Clinical Evaluation | Adverse Effects |
|---|---|---|---|---|---|---|---|
| Liu et al. [20] | Phase I active 65 weeks | Open-label, prospective, single-group assignment 50 to 85 years old | Umbilical cord-derived, allogeneic hMSCs Intravenous infusion | 100 million cells per infusion | N.A. | Adverse events evaluation ADAS-Cog MMSE Geriatric Depression Scale (GDS) Odor identification test Alzheimer’s Disease Related Quality of Life (ADRQL-40) Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS-ADL) Neuropsychiatric Inventory-Q (NPI-Q) | N.A. |
| Oliva et al., 2019 [157] | Phase I completed Active for Phase II 1 year | Randomized controlled trial 50 to 80 years old | Longeveron MSCs Intravenous infusion | Once for 20 million LMSCs (low-dose), 100 million LMSCs (high-dose), or placebo | N.A. | Cognitive assessments Patient-reported outcomes (PROs) Biomarkers (serum, CSF, and MRI). | No serious adverse events |
| Ra et al., 2011 [135] | Phase 1 7 months | Single group 8 male patients (19 to 60 years old) | Autologous adipose-derived MSCs Intravenous infusion | 4 × 108 autologous hAdMSCs per patient | Improvement in some of the cases | Blood chemistry, HBV/HCV, hematology, and urinalysis, and were screened for HIV, and VDRL. A pulmonary function test, chest X-ray, spinal cord independence measure (SCIM), visual analog scale, electrophysiological examination of motor, spinal magnetic resonance imaging, somatosensory evoked potentials (MEP and SEP, respectively), and neurological examinations using ASIA were obtained for each patient. | 19 adverse events were observed in 8 patients, including chest tightness, chest pain, and mild fever. |
| Niu et al., 2016 [156] | Phase I/II Active 1 year | Open-label, self-control, single-center prospective trial 30 patients (50 to 85 years old) | hUC-MSCs Intravenous into the median cubital vein | 0.5 × 106 hUC-MSCs/kg | N.A. | Adverse effects evaluation AD improvement, e.g., CIBIC, MMSE, ADL, and NPI | N.A. |
| Medipost Co Ltd. [154] | Phase I N.A. | Open-label, single-group assignment 9 patients (50 to 75 years old) | Human umbilical cord blood-derived MSCs | Dose A—250,000 cells per 5 µL per entry site, 3 million cells per brain Dose B—500,000 cells per 5 µL per entry site, 6 million cells per brain | N.A. | Adverse event evaluation Changes in ADAS-cog | N.A. |
| Liu et al. [20] | Phase N.A. 12 months | Open-label, non-randomized, parallel assignment 18 years and older | Bone marrow stem cells (BMSCs)
|
| N.A. | MMSE Autism Spectrum Quotient Exam (AQ) ADCS-ADL | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, H.J.; Yanshree; Roy, J.; Tipoe, G.L.; Fung, M.-L.; Lim, L.W. Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10151. https://doi.org/10.3390/ijms221810151
Chan HJ, Yanshree, Roy J, Tipoe GL, Fung M-L, Lim LW. Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(18):10151. https://doi.org/10.3390/ijms221810151
Chicago/Turabian StyleChan, Hau Jun, Yanshree, Jaydeep Roy, George Lim Tipoe, Man-Lung Fung, and Lee Wei Lim. 2021. "Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 18: 10151. https://doi.org/10.3390/ijms221810151
APA StyleChan, H. J., Yanshree, Roy, J., Tipoe, G. L., Fung, M.-L., & Lim, L. W. (2021). Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease. International Journal of Molecular Sciences, 22(18), 10151. https://doi.org/10.3390/ijms221810151