Antistress Action of Melanocortin Derivatives Associated with Correction of Gene Expression Patterns in the Hippocampus of Male Rats Following Acute Stress
Abstract
:1. Introduction
2. Results
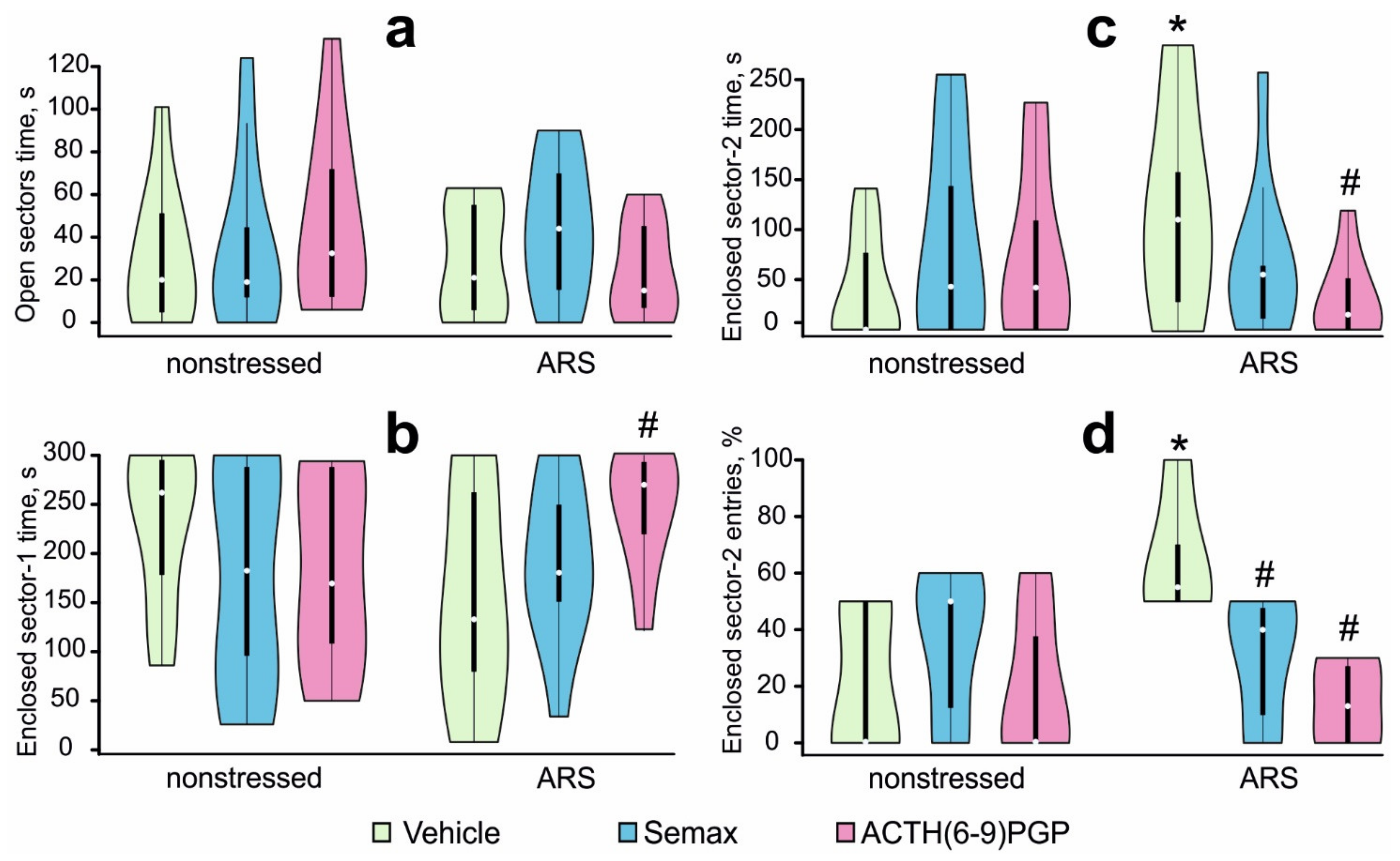
2.1. Effects of MC Derivatives on Behavioral Responses to ARS
2.2. RNA-Seq Analysis of the Effect of Semax and ACTH(6–9)PGP on the Hippocampal Transcriptome of Non-Stressed Rats
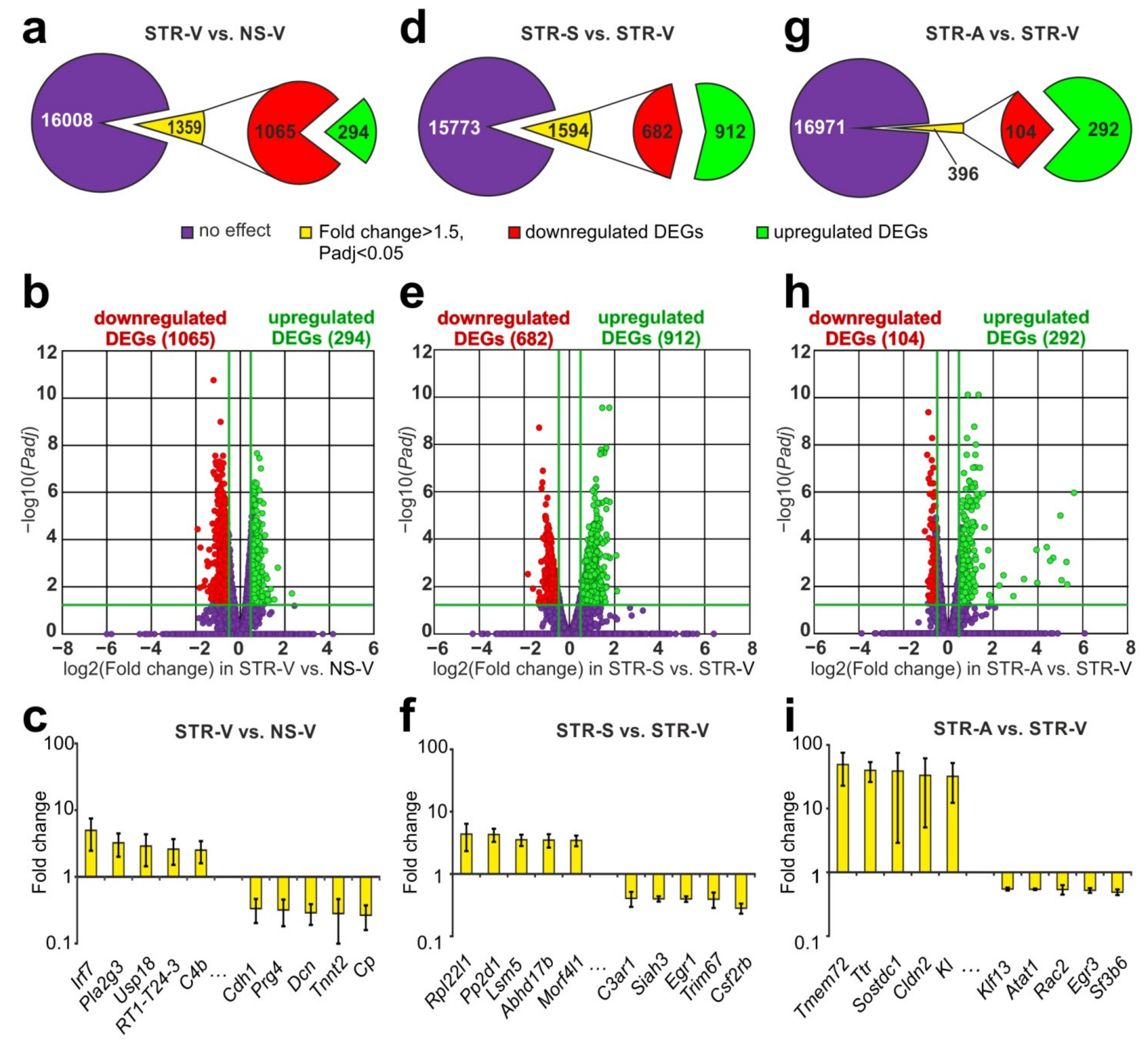
2.3. RNA-Seq Analysis of the Effect of ARS on the Hippocampal Transcriptome
2.4. RNA-Seq Analysis of the Effect of Semax and ACTH(6–9)PGP on the Effect of ARS on the Hippocampal Transcriptome
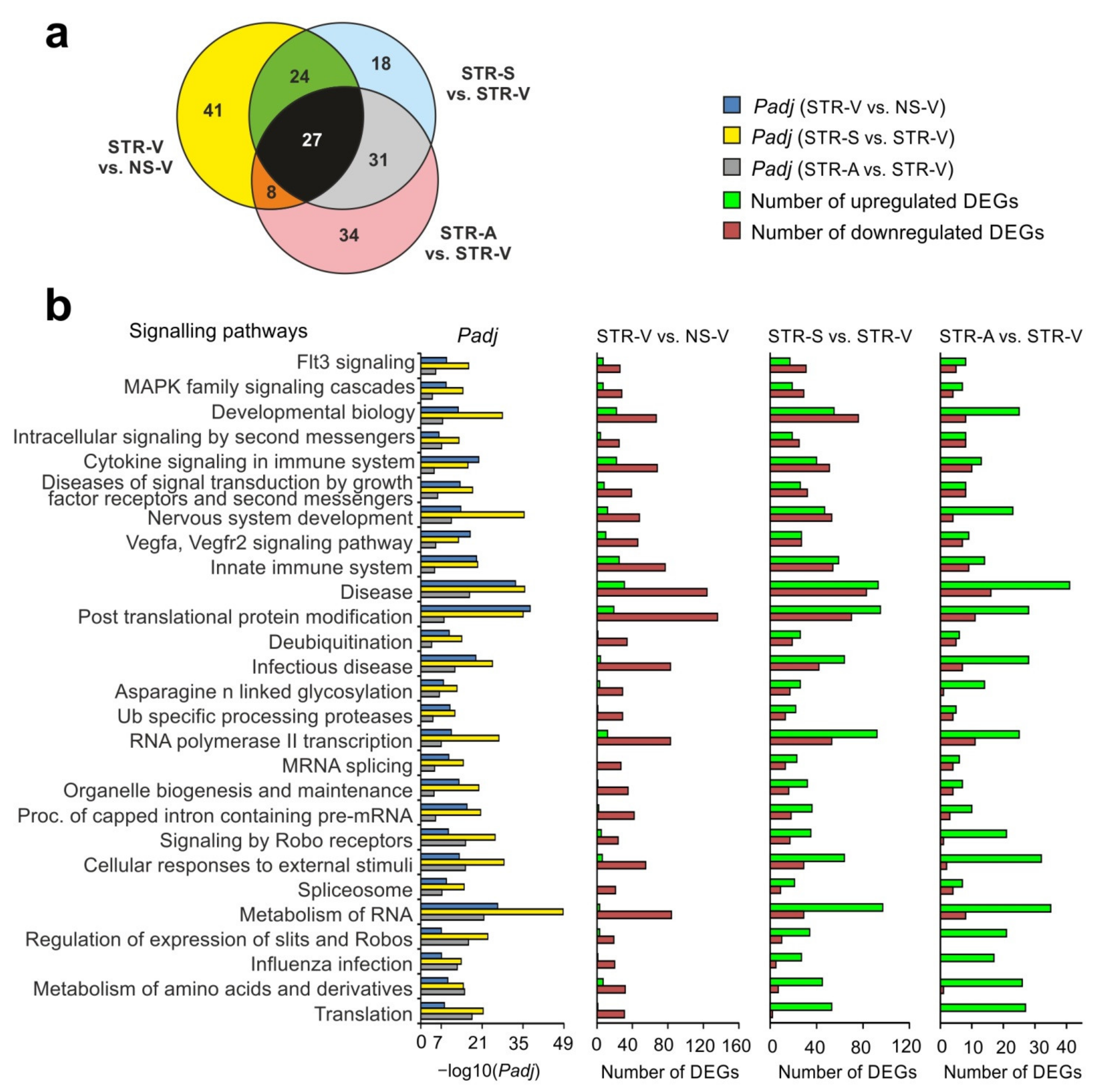
2.5. Analysis of RNA-Seq Results in Different Comparison Groups
2.6. Functional Annotations of DEGs Altered by Acute Stress and MC Peptides
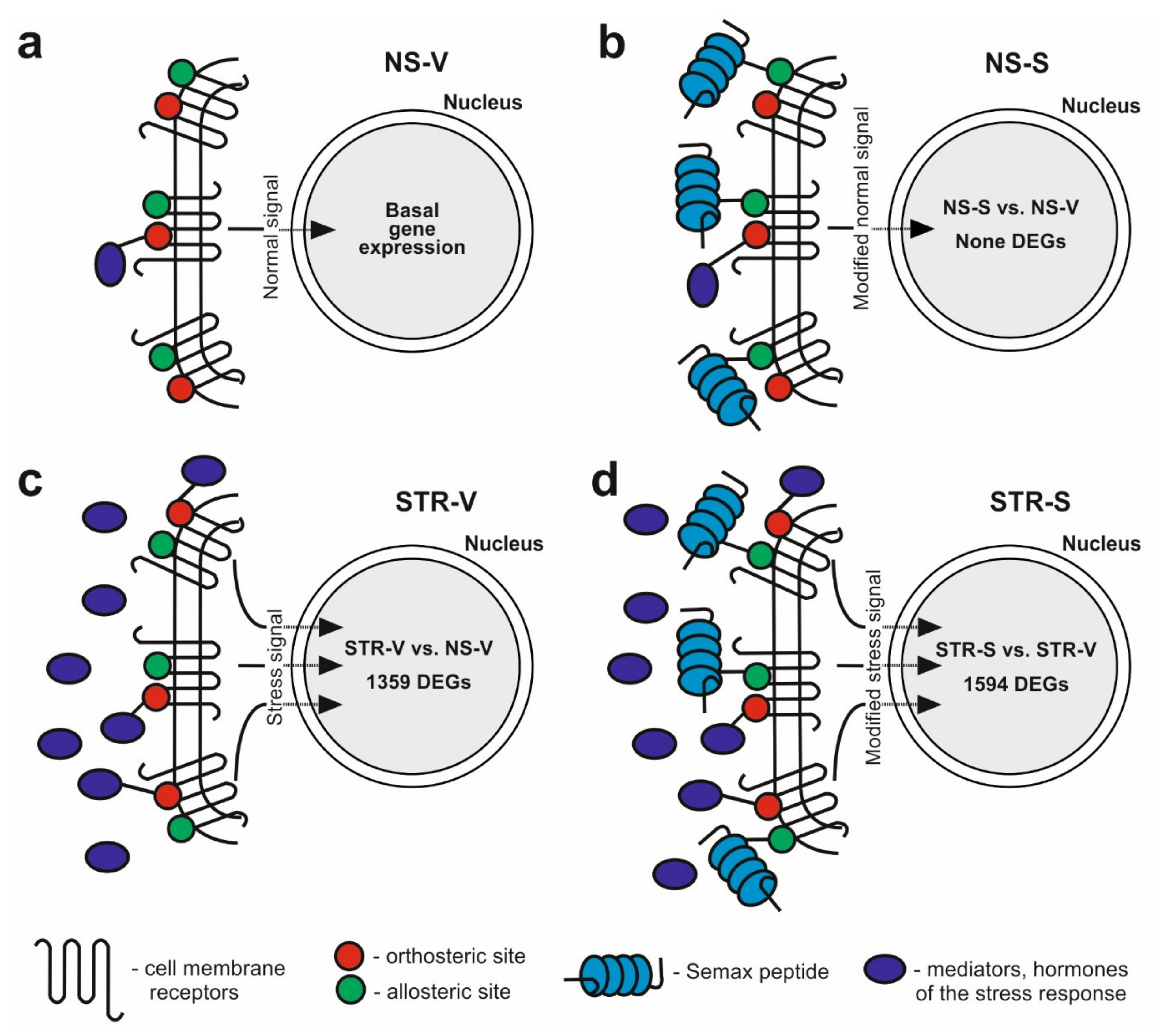
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Peptide Administration
4.4. Acute Restraint Stress
4.5. Behavioral Assessment
4.6. Statistical Analysis of the Behavioral Data
4.7. Sample Collection and RNA Isolation
4.8. RNA-Seq
4.9. RNA-Seq Data Analysis
4.10. cDNA Synthesis
4.11. Real-Time Reverse Transcription Polymerase Chain Reaction (RT–PCR)
4.12. Data Analysis of Real-Time RT–PCR and Statistics
4.13. Functional Analysis
4.14. Availability of Data and Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domingues, M.; Casaril, A.M.; Birmann, P.T.; Bampi, S.R.; Lourenço, D.D.A.; Vieira, B.M.; Dapper, L.H.; Lenardao, E.J.; Sonego, M.; Collares, T.; et al. Effects of a selanylimidazopyridine on the acute restraint stress-induced depressive- and anxiety-like behaviors and biological changes in mice. Behav. Brain Res. 2019, 366, 96–107. [Google Scholar] [CrossRef]
- Schwabe, L. Memory under stress: From single systems to network changes. Eur. J. Neurosci. 2016, 45, 478–489. [Google Scholar] [CrossRef]
- Floriou-Servou, A.; von Ziegler, L.; Stalder, L.; Sturman, O.; Privitera, M.; Rassi, A.; Cremonesi, A.; Thöny, B.; Bohacek, J. Distinct Proteomic, Transcriptomic, and Epigenetic Stress Responses in Dorsal and Ventral Hippocampus. Biol. Psychiatry 2018, 84, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Narendran, R.; Tollefson, S.; Fasenmyer, K.; Paris, J.; Himes, M.L.; Lopresti, B.; Ciccocioppo, R.; Mason, N.S. Decreased Nociceptin Receptors Are Related to Resilience and Recovery in College Women Who Have Experienced Sexual Violence: Therapeutic Implications for Posttraumatic Stress Disorder. Biol. Psychiatry 2019, 85, 1056–1064. [Google Scholar] [CrossRef]
- Carrive, P. Orexin, Stress and Central Cardiovascular Control. A Link with Hypertension? Neurosci. Biobehav. Rev. 2017, 74, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Kolomin, T.A.; Agapova, T.I.; Agniullin, I.V.; Shram, S.I.; Shradina, M.I.; Slominskiĭ, P.A.; Limborskaia, S.A.; Miasoedov, I.F. Transcriptome alteration in hippocampus under the treatment of tuftsin analog Selank. Zhurnal Vyss. Nervn. Deiatelnosti Im. I P Pavlov. 2013, 63, 365–374. (In Russian) [Google Scholar] [CrossRef]
- Koenig, S.; Luger, T.A.; Scholzen, T.E. Monitoring neuropeptide-specific proteases: Processing of the proopiomelanocortin peptides adrenocorticotropin and α-melanocyte-stimulating hormone in the skin. Exp. Dermatol. 2006, 15, 751–761. [Google Scholar] [CrossRef]
- Wikberg, J.E. Melanocortin receptors: Perspectives for novel drugs. Eur. J. Pharmacol. 1999, 375, 295–310. [Google Scholar] [CrossRef]
- Giuliani, D.; Ottani, A.; Neri, L.; Zaffe, D.; Grieco, P.; Jochem, J.; Cavallini, G.M.; Catania, A.; Guarini, S. Multiple beneficial effects of melanocortin MC4 receptor agonists in experimental neurodegenerative disorders: Therapeutic perspectives. Prog. Neurobiol. 2017, 148, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, M. Novel approaches to the design of bioavailable melanotropins. Expert Opin. Drug Discov. 2017, 12, 1023–1030. [Google Scholar] [CrossRef]
- Eipper, B.A.; Mains, R.E. Structure and Biosynthesis of Pro-Adrenocorticotropin/Endorphin and Related Peptides. Endocr. Rev. 1980, 1, 1–27. [Google Scholar] [CrossRef]
- Fridmanis, D.; Roga, A.; Klovins, J. ACTH receptor (MC2R) specificity: What do we know about underlying molecular mechanisms? Front. Endocrinol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Levitskaya, N.G.; Glazova, N.Y.; Sebentsova, E.A.; Manchenko, D.M.; Andreeva, L.A.; Kamensky, A.A.; Myasoedov, N.F. Nootropic and anxiolytic effects of heptapeptide ACTH6-9Pro-Gly-Pro. Russ. J. Physiol. 2019, 6, 761–770. [Google Scholar]
- Levitskaya, N.G.; Glazova, N.Y.; Sebentsova, E.A.; Manchenko, D.M.; Vilensky, D.A.; Andreeva, L.A.; Kamensky, A.A.; Myasoedov, N.F. Investigation of the Spectrum of Physiological Activities of the Heptapeptide Semax, an ACTH 4–10 Analogue. Neurochem. J. 2008, 2, 95–101. [Google Scholar] [CrossRef]
- Ashmarin, I.; Nezavibatko, V.; Levitskaya, N.; Koshelev, V.; Kamensky, A. Design and Investigation of an ACTH(4-10) Analog Lacking D-Amino Acids and Hydrophobic Radicals. Neurosci. Res. Commun. 1995, 16, 105–112. [Google Scholar]
- Glazova, N.Y.; Sebentsova, E.A.; Manchenko, D.M.; Andreeva, L.A.; Dergunova, L.V.; Levitskaya, N.G.; Limborska, S.A.; Myasoedov, N.F. The Protective Effect of Semax in a Model of Stress-Induced Impairment of Memory and Behavior in White Rats. Biol. Bull. 2018, 45, 394–399. [Google Scholar] [CrossRef]
- Yatsenko, K.A.; Glazova, N.Y.; Inozemtseva, L.S.; Andreeva, L.A.; Kamensky, A.A.; Grivennikov, I.A.; Levitskaya, N.; Dolotov, O.V.; Myasoedov, N.F. Heptapeptide semax attenuates the effects of chronic unpredictable stress in rats. Dokl. Biol. Sci. 2013, 453, 353–357. [Google Scholar] [CrossRef]
- Potaman, V.; Alfeeva, L.; Kamensky, A.; Nezavibatko, V. Degradation of ACTH/MSH(4–10) and its synthetic analog semax by rat serum enzymes: An inhibitor study. Peptides 1993, 14, 491–495. [Google Scholar] [CrossRef]
- Shevchenko, K.V.; Dulov, S.A.; Andreeva, L.A.; Nagaev, I.Y.; Shevchenko, V.P.; Radilov, A.S.; Myasoedov, N.F. Stability of His-Phe-Arg-Trp-Pro-Gly-Pro to Leucine Aminopeptidase, Carboxypeptidase Y, and Rat Nasal Mucus, Blood, and Plasma. Russ. J. Bioorg. Chem. 2016, 42, 153–161. [Google Scholar] [CrossRef]
- Potaman, V.; Antonova, L.; Dubynin, V.; Zaitzev, D.; Kamensky, A.; Myasoedov, N.; Nezavibatko, V. Entry of the synthetic ACTH(4–10) analogue into the rat brain following intravenous injection. Neurosci. Lett. 1991, 127, 133–136. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Shevchenko, K.V.; Shevchenko, V.P.; Bobrov, M.Y.; Bezuglov, V.V.; Myasoedov, N.F. Binding of Regulatory Neuropeptide [3H] Semax, Labeled in Terminal Pro, to Plasma Membranes of the Rat Forebrain. Neurochem. J. 2006, 23, 57–62. [Google Scholar] [CrossRef]
- Shevchenko, K.V.; Nagaev, I.Y.; Babakov, V.N.; Andreeva, L.A.; Shevchenko, V.P.; Radilov, A.S.; Myasoedov, N.F. Proteolysis of His-Phe-Arg-Trp-Pro-Gly-Pro in the blood and brain of rats in vivo. Dokl. Biochem. Biophys. 2015, 464, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Dores, R.M.; Liang, L.; Davis, P.; Thomas, A.L.; Petko, B. 60 YEARS OF POMC: Melanocortin receptors: Evolution of ligand selectivity for melanocortin peptides. J. Mol. Endocrinol. 2016, 56, T119–T133. [Google Scholar] [CrossRef] [Green Version]
- Hruby, V.J.; Wilkes, B.C.; Hadley, M.E.; Al-Obeidi, F.; Sawyer, T.K.; Staples, D.J.; Devaux, A.E.; Dym, O.; Castrucci, A.M. alpha-Melanotropin: The minimal active sequence in the frog skin bioassay. J. Med. Chem. 1987, 30, 2126–2130. [Google Scholar] [CrossRef]
- Clark, A.J.; Forfar, R.; Hussain, M.; Jerman, J.; McIver, E.; Taylor, D.; Chan, L. ACTH antagonists. Front. Endocrinol. 2016, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Todorovic, A.; Lensing, C.J.; Holder, J.R.; Scott, J.W.; Sorensen, N.B.; Haskell-Luevano, C. Discovery of Melanocortin Ligands via a Double Simultaneous Substitution Strategy Based on the Ac-His-dPhe-Arg-Trp-NH2 Template. ACS Chem. Neurosci. 2018, 9, 2753–2766. [Google Scholar] [CrossRef]
- Palmer, D.; Gonçalves, J.P.L.; Hansen, L.V.; Wu, B.; Hald, H.; Schoffelen, S.; Diness, F.; Le Quement, S.T.; Nielsen, T.E.; Meldal, M. Click-Chemistry-Mediated Synthesis of Selective Melanocortin Receptor 4 Agonists. J. Med. Chem. 2017, 60, 8716–8730. [Google Scholar] [CrossRef] [PubMed]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Myasoedov, N.F. Synacton and individual activity of synthetic and natural corticotropins. J. Mol. Recognit. 2016, 30, e2597. [Google Scholar] [CrossRef] [PubMed]
- Dergunova, L.V.; Filippenkov, I.B.; Limborska, S.A.; Myasoedov, N.F. Pharmacotranscriptomics of peptide drugs with neuroprotective properties. Med. Res. Rev. 2020, 41, 754–769. [Google Scholar] [CrossRef] [PubMed]
- An, T.; He, Z.-C.; Zhang, X.-Q.; Li, J.; Chen, A.-L.; Tan, F.; Chen, H.-D.; Lv, B.-H.; Lian, J.; Gao, S.-H.; et al. Baduanjin exerts anti-diabetic and anti-depression effects by regulating the expression of mRNA, lncRNA, and circRNA. Chin. Med. 2019, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskii, I.I.; Danchev, N.D. The optimizing action of the synthetic peptide Selank on a conditioned active avoidance reflex in rats. Neurosci. Behav. Physiol. 2003, 33, 639–643. [Google Scholar] [CrossRef]
- Kasian, A.; Kolomin, T.; Andreeva, L.; Bondarenko, E.; Myasoedov, N.; Slominsky, P.; Shadrina, M. Peptide Selank Enhances the Effect of Diazepam in Reducing Anxiety in Unpredictable Chronic Mild Stress Conditions in Rats. Behav. Neurol. 2017, 2017, 5091027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkova, A.; Shadrina, M.; Kolomin, T.; Andreeva, L.; Limborska, S.; Myasoedov, N.; Slominsky, P. Selank Administration Affects the Expression of Some Genes Involved in GABAergic Neurotransmission. Front. Pharmacol. 2016, 7, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.K.; Barbieri, E.V.; Brown, B.D.; Chen, K.-W.; Devine, D.P. Roles of the bed nucleus of stria terminalis and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides 2007, 41, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gandhi, P.R.; Standifer, K.M. Increased Nociceptive Sensitivity and Nociceptin/Orphanin FQ Levels in a Rat Model of PTSD. Mol. Pain 2012, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Yamano, Y.; Yoshioka, M.; Toda, Y.; Oshida, Y.; Chaki, S.; Hamamoto, K.; Morishima, I. Regulation of CRF, POMC and MC4R Gene Expression after Electrical Foot Shock Stress in the Rat Amygdala and Hypothalamus. J. Vet. Med. Sci. 2004, 66, 1323–1327. [Google Scholar] [CrossRef] [Green Version]
- Markov, D.D.; Yatsenko, K.A.; Inozemtseva, L.S.; Grivennikov, I.A.; Myasoedov, N.F.; Dolotov, O.V. Systemic N-terminal fragments of adrenocorticotropin reduce inflammation- and stress-induced anhedonia in rats. Psychoneuroendocrinology 2017, 82, 173–186. [Google Scholar] [CrossRef]
- Asmarin, I.P.; Nezavibat’ko, V.N.; Miasoedov, N.F.; Kamenskiĭ, A.A.; Grivennikov, I.A.; Ponomareva-Stepnaia, M.A.; Andreeva, L.A.; Kaplan, A.I.; Koshelev, V.B.; Riasina, T. V [A nootropic adrenocorticotropin analog 4-10-semax (l5 years experience in its design and study)]. Zhurnal Vyss. Nervn. Deiatelnosti Im. I P Pavlov. 1997, 47, 420–430. [Google Scholar]
- Kaplan, A.Y.; Kochetova, A.G.; Nezavibathko, V.N.; Rjasina, T.V.; Ashmarin, I.P. Synthetic ACTH analogue semax displays nootropic-like activity in humans. Neurosci. Res. Commun. 1996, 19, 115–123. [Google Scholar] [CrossRef]
- Manchenko, D.M.; Glazova, N.Y.; Levitskaya, N.; Andreeva, L.A.; Kamenskii, A.A.; Myasoedov, N.F. The Nootropic and Analgesic Effects of Semax Given via Different Routes. Neurosci. Behav. Physiol. 2012, 42, 264–270. [Google Scholar] [CrossRef]
- D’Adamo, P.; Wolfer, D.P.; Kopp, C.; Tobler, I.; Toniolo, D.; Lipp, H.-P. Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. Eur. J. Neurosci. 2004, 19, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Cancela, L.; Bregonzio, C.; Molina, V. Anxiolytic-like effect induced by chronic stress is reversed by naloxone pretreatment. Brain Res. Bull. 1995, 36, 209–213. [Google Scholar] [CrossRef]
- Glazova, N.Y.; Atanov, M.S.; Pyzgareva, A.V.; Andreeva, L.A.; Manchenko, D.M.; Markov, D.D.; Inozemtseva, L.S.; Dolotov, O.V.; Levitskaya, N.; Kamensky, A.A.; et al. Neurotropic activity of ACTH7–10PGP, an analog of an ACTH fragment. Dokl. Biol. Sci. 2011, 440, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Sazma, M.A.; Shields, G.; Yonelinas, A.P. The effects of post-encoding stress and glucocorticoids on episodic memory in humans and rodents. Brain Cogn. 2018, 133, 12–23. [Google Scholar] [CrossRef]
- Haider, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Sadir, S.; Liaquat, L.; Naqvi, F.; Zuberi, N.A.; Shakeel, H.; Perveen, T. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res. Bull. 2015, 115, 1–8. [Google Scholar] [CrossRef]
- Padovan, C.; Guimarães, F. Restraint-induced hypoactivity in an elevated plus-maze. Braz. J. Med. Biol. Res. 2000, 33, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Amin, S.N.; Hassan, S.S.; Khashaba, A.S.; Youakim, M.F.; Latif, N.S.A.; Rashed, L.A.; Yassa, H.D. Hippocampal and Cerebellar Changes in Acute Restraint Stress and the Impact of Pretreatment with Ceftriaxone. Brain Sci. 2020, 10, 193. [Google Scholar] [CrossRef] [Green Version]
- Busnardo, C.; Crestani, C.C.; Scopinho, A.A.; Packard, B.A.; Resstel, L.B.; Correa, F.M.; Herman, J.P. Nitrergic neurotransmission in the paraventricular nucleus of the hypothalamus modulates autonomic, neuroendocrine and behavioral responses to acute restraint stress in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 90, 16–27. [Google Scholar] [CrossRef]
- Tu, B.-X.; Wang, L.-F.; Zhong, X.-L.; Hu, Z.-L.; Cao, W.-Y.; Cui, Y.-H.; Li, S.-J.; Zou, G.-J.; Liu, Y.; Zhou, S.-F.; et al. Acute restraint stress alters food-foraging behavior in rats: Taking the easier Way while suffered. Brain Res. Bull. 2019, 149, 184–193. [Google Scholar] [CrossRef]
- Hata, T.; Nishikawa, H.; Itoh, E.; Funakami, Y. Anxiety-Like Behavior in Elevated Plus-Maze Tests in Repeatedly Cold-Stressed Mice. Jpn. J. Pharmacol. 2001, 85, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Kukharsky, M.S.; Ninkina, N.N.; An, H.; Telezhkin, V.; Wei, W.; De Meritens, C.R.; Cooper-Knock, J.; Nakagawa, S.; Hirose, T.; Buchman, V.L.; et al. Long non-coding RNA Neat1 regulates adaptive behavioural response to stress in mice. Transl. Psychiatry 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Parmigiani, S.; Ferrari, P.; Palanza, P.; Rodgers, R. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol. Behav. 2000, 71, 509–516. [Google Scholar] [CrossRef]
- Costa-Nunes, J.P.; Zubareva, O.; Araújo-Correia, M.; Valença, A.; Schroeter, C.A.; Pawluski, J.; Vignisse, J.; Steinbusch, H.; Hermes, D.; Phillipines, M.; et al. Altered emotionality, hippocampus-dependent performance and expression of NMDA receptor subunit mRNAs in chronically stressed mice. Stress 2013, 17, 108–116. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, W.-J.; Liao, J.-M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opmeer, E.M.; Kortekaas, R.; Aleman, A. Depression and the role of genes involved in dopamine metabolism and signalling. Prog. Neurobiol. 2010, 92, 112–133. [Google Scholar] [CrossRef]
- Whitton, A.; Treadway, M.T.; Pizzagalli, D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry 2015, 28, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Guo, L.; Xu, A.; Cui, S.; Wang, J.-H. Molecular Mechanism for Stress-Induced Depression Assessed by Sequencing miRNA and mRNA in Medial Prefrontal Cortex. PLoS ONE 2016, 11, e0159093. [Google Scholar] [CrossRef] [Green Version]
- Davies, S.; Noor, S.; Carpentier, E.; Deviche, P. Innate immunity and testosterone rapidly respond to acute stress, but is corticosterone at the helm? J. Comp. Physiol. B 2016, 186, 907–918. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; McEwen, B.S. Acute Stress Enhances while Chronic Stress Suppresses Cell-Mediated Immunity in Vivo: A Potential Role for Leukocyte Trafficking. Brain Behav. Immun. 1997, 11, 286–306. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Gulati, K.; Rai, N. Stress, anxiety, and immunomodulation: A pharmacological analysis. Vitam. Horm. 2017, 103, 1–25. [Google Scholar]
- Johnson, J.D.; Barnard, D.F.; Kulp, A.C.; Mehta, D. Neuroendocrine Regulation of Brain Cytokines After Psychological Stress. J. Endocr. Soc. 2019, 3, 1302–1320. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; De Andrade, N.Q.; Morris, G.; Fernandes, B.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2017, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, J.; Barak, Y.; Chengappa, K.; Rapoport, A.; Rebey, M.; Barak, V. Cerebrospinal Cytokine Levels in Patients with Acute Depression. Neuropsychobiology 1999, 40, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Meza, J.M.; Jarillo-Luna, R.A.; Rivera-Aguilar, V.; Miliar-García, A.; Campos-Rodríguez, R. Cytokine profile of NALT during acute stress and its possible effect on IgA secretion. Immunol. Lett. 2017, 188, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Dygalo, N.N.; Bannova, A.V.; Sukhareva, E.V.; Shishkina, G.T.; Ayriyants, K.A.; Kalinina, T. Effects of short-term exposure to lithium on antiapoptotic Bcl-xL protein expression in cortex and hippocampus of rats after acute stress. Biochemistry 2017, 82, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborska, S.A.; Skvortsova, V.I.; Myasoedov, N.F.; Dergunova, L.V. The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: Genome-wide transcriptional analysis. BMC Genom. 2014, 15, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavchanskiĭ, V.V.; Tvorogova, T.V.; Botsina, A.I.; Skvortsova, V.I.; Limborskaia, S.A.; Miasoedov, N.F.; Dergunova, L. V [The effect of semax and its C-end peptide PGP on expression of the neurotrophins and their receptors in the rat brain during incomplete global ischemia]. Mol. Biol. 2011, 45, 1026–1035. [Google Scholar]
- Stavchanskiǐ, V.V.; Tvorogova, T.V.; Botsina, A.I.; Limborskaia, S.A.; Skvortsova, V.I.; Miasoedov, N.F.; Dergunova, L.V. The effect of semax and its C-end peptide PGP on Vegfa gene expression in the rat brain during incomplete global ischemia. Mol. Biol. 2013, 47, 461–466. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Dmitrieva, V.G.; Limborska, S.A.; Myasoedov, N.F.; Dergunova, L.V. Semax, an analog of ACTH(4−7), regulates expression of immune response genes during ischemic brain injury in rats. Mol. Genet. Genom. 2017, 292, 635–653. [Google Scholar] [CrossRef]
- Filippenkov, I.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’Kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Mozerov, S.A.; Gubsky, L.V.; Limborska, S.A. Genome-wide transcriptome analysis using RNA-Seq reveals a large number of differentially expressed genes in a transient MCAO rat model. BMC Genom. 2018, 19, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyunova, T.V.; Andreeva, L.; Shevchenko, K.; Myasoedov, N. Peptide-based Anxiolytics: The Molecular Aspects of Heptapeptide Selank Biological Activity. Protein Pept. Lett. 2018, 25, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hay, D.; Pioszak, A.A. Calcitonin and Amylin Receptor Peptide Interaction Mechanisms. J. Biol. Chem. 2016, 291, 8686–8700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlavey, C.J. Introduction to the Hypothalamic-Pituitary-Adrenal Axis: Healthy and Dysregulated Stress Responses, Developmental Stress and Neurodegeneration. J. Undergrad. Neurosci. Educ. 2018, 16, R59–R60. [Google Scholar]
- Ciccocioppo, R.; de Guglielmo, G.; Hansson, A.C.; Ubaldi, M.; Kallupi, M.; Cruz, M.T.; Oleata, C.S.; Heilig, M.; Roberto, M. Restraint Stress Alters Nociceptin/Orphanin FQ and CRF Systems in the Rat Central Amygdala: Significance for Anxiety-Like Behaviors. J. Neurosci. 2014, 34, 363–372. [Google Scholar] [CrossRef]
- Spitzer, M.; Wildenhain, J.; Rappsilber, J.; Tyers, M. BoxPlotR: A web tool for generation of box plots. Nat. Methods 2014, 11, 121–122. [Google Scholar] [CrossRef]
- Chomczynski, P. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/sra/PRJNA633551 (accessed on 17 May 2020).

| Gene Symbol | Primer (5’-3’) | RefSeq ID | Amplicon Size (bp) | Effectiveness ± Standard Error |
|---|---|---|---|---|
| Tnnt2 | F: AACAGGAGGAAGGCTGAAGAT | NM_012676 | 278 | 1.88 ± 0.03 |
| R: TATTTCTGCTGCTTGAACTT | ||||
| Pla2g3 | F: CAAGTTCCACCTGCTCAACA | NM_001106015 | 207 | 2.02 ± 0 |
| R: GTGCCTTTATCCCAGAAATG | ||||
| Lrg1 | F: ACTTGGACCTGGCGGAGAA | NM_001009717.1 | 108 | 1.9 ± 0.05 |
| R: GCAGCGACTCAAGCAGGTT | ||||
| Rt1-Ba | F: TGTGGAGGTCAAGACGACATT | NM_001008831 | 344 | 1.92 ± 0.03 |
| R: AAAGCAGATGAGGGTGTT | ||||
| Drd1 | F: CATAGAGACGGTGAGCATTA | NM_012546 | 251 | 1.96 ± 0 |
| R: TGTGTGTGACAGGTTGGAT | ||||
| Irf7 | F: ATACTCCCATCTTTGACTTC | NM_001033691 | 230 | 1.98 ± 0.04 |
| R: ACTGCTGCTGTCCAGAGA | ||||
| Dcn | F: AATGGCAGTCTGGCTAATGT | NM_024129 | 250 | 2.02 ± 0.03 |
| R: TGAAGGTGTGTGGGTGAAT | ||||
| Gapdh | F: ACTCTACCCACGGCAAGTTCAACG | NM_017008.4 | 148 | 2.01 ± 0.03 |
| R: GTAGACTCCACGACATACTCAGCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippenkov, I.B.; Stavchansky, V.V.; Glazova, N.Y.; Sebentsova, E.A.; Remizova, J.A.; Valieva, L.V.; Levitskaya, N.G.; Myasoedov, N.F.; Limborska, S.A.; Dergunova, L.V. Antistress Action of Melanocortin Derivatives Associated with Correction of Gene Expression Patterns in the Hippocampus of Male Rats Following Acute Stress. Int. J. Mol. Sci. 2021, 22, 10054. https://doi.org/10.3390/ijms221810054
Filippenkov IB, Stavchansky VV, Glazova NY, Sebentsova EA, Remizova JA, Valieva LV, Levitskaya NG, Myasoedov NF, Limborska SA, Dergunova LV. Antistress Action of Melanocortin Derivatives Associated with Correction of Gene Expression Patterns in the Hippocampus of Male Rats Following Acute Stress. International Journal of Molecular Sciences. 2021; 22(18):10054. https://doi.org/10.3390/ijms221810054
Chicago/Turabian StyleFilippenkov, Ivan B., Vasily V. Stavchansky, Natalya Yu. Glazova, Elena A. Sebentsova, Julia A. Remizova, Liya V. Valieva, Natalia G. Levitskaya, Nikolai F. Myasoedov, Svetlana A. Limborska, and Lyudmila V. Dergunova. 2021. "Antistress Action of Melanocortin Derivatives Associated with Correction of Gene Expression Patterns in the Hippocampus of Male Rats Following Acute Stress" International Journal of Molecular Sciences 22, no. 18: 10054. https://doi.org/10.3390/ijms221810054
APA StyleFilippenkov, I. B., Stavchansky, V. V., Glazova, N. Y., Sebentsova, E. A., Remizova, J. A., Valieva, L. V., Levitskaya, N. G., Myasoedov, N. F., Limborska, S. A., & Dergunova, L. V. (2021). Antistress Action of Melanocortin Derivatives Associated with Correction of Gene Expression Patterns in the Hippocampus of Male Rats Following Acute Stress. International Journal of Molecular Sciences, 22(18), 10054. https://doi.org/10.3390/ijms221810054







