Emerging Roles of Astrocyte Kir4.1 Channels in the Pathogenesis and Treatment of Brain Diseases
Abstract
1. Introduction
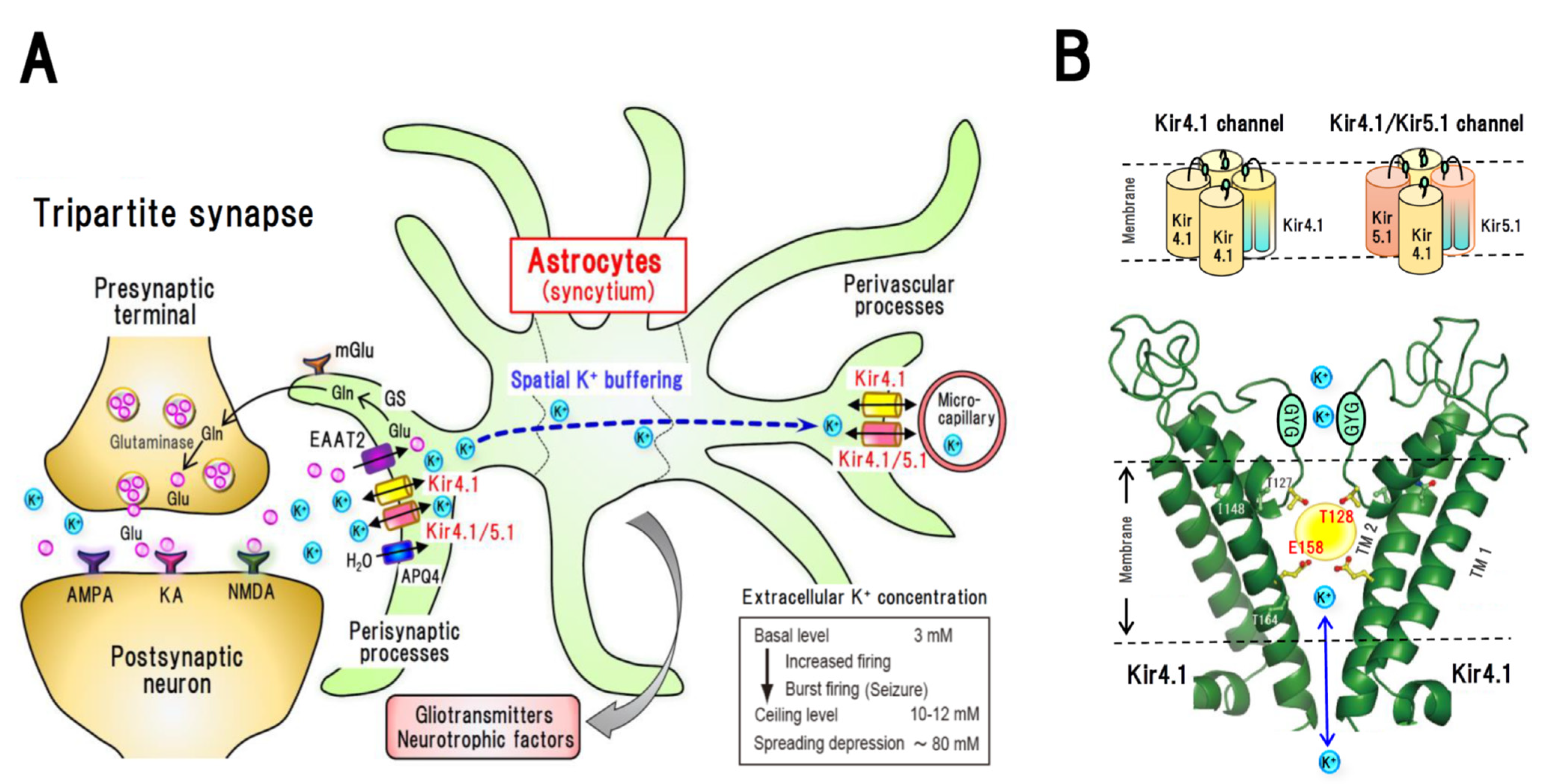
2. Astrocytic Spatial K+ Buffering and Kir4.1 Channels
3. Molecular Pharmacology of Kir4.1 Channels
4. Kir4.1 Channels and Brain Diseases
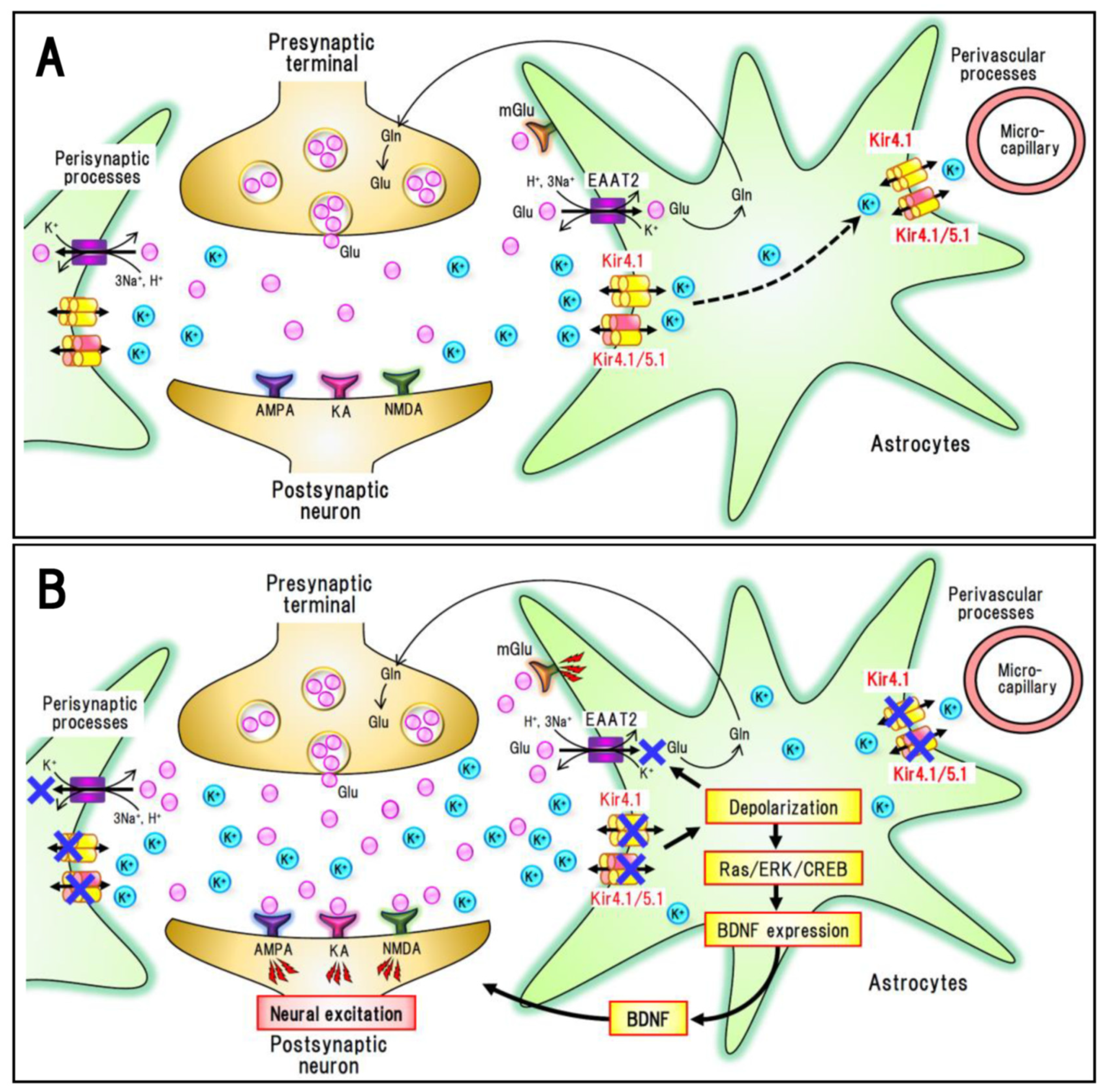
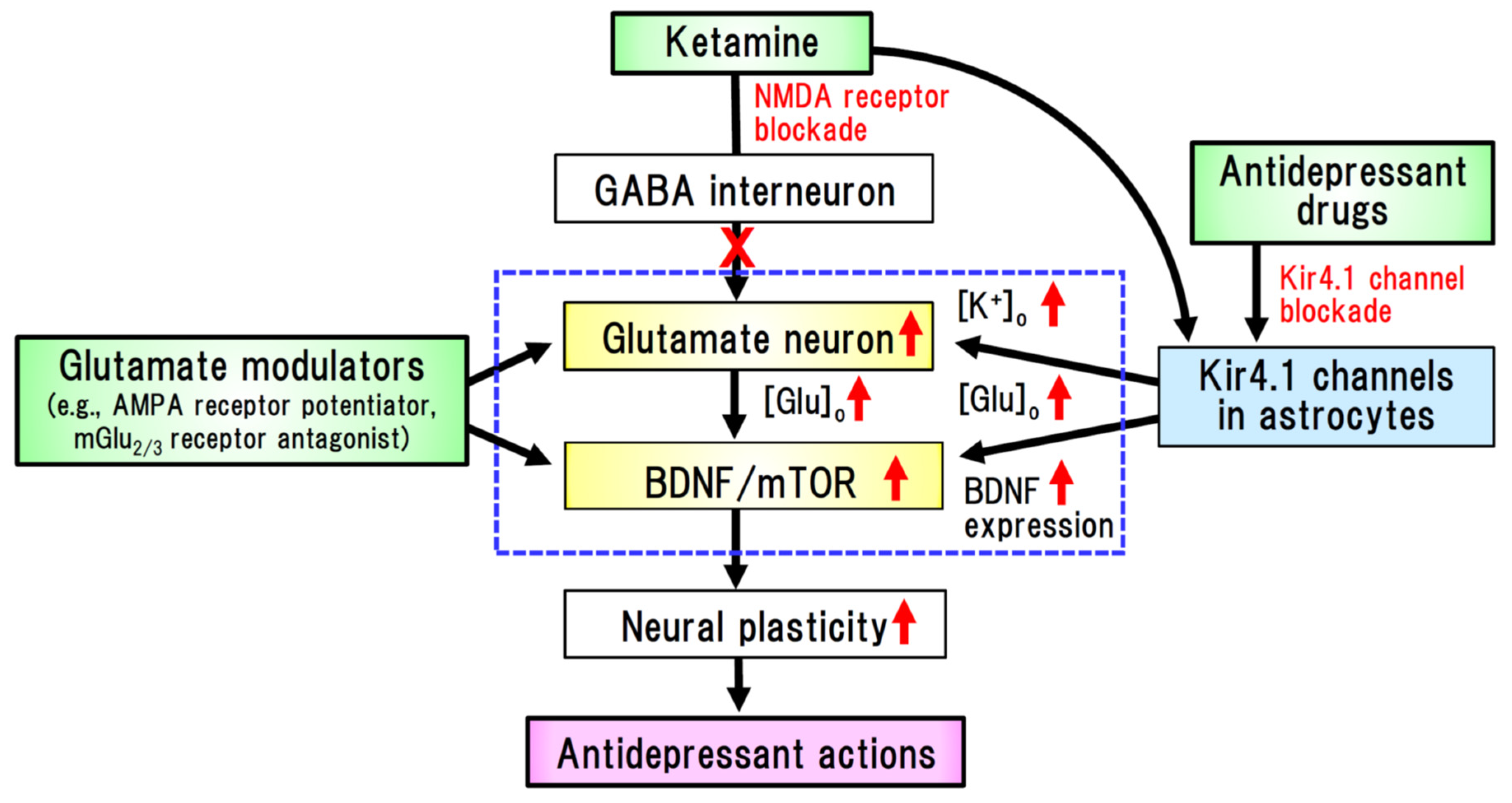
4.1. Major Depressive Disorder (MDD)
4.2. Epileptic Disorders
4.3. Other Brain Diseases
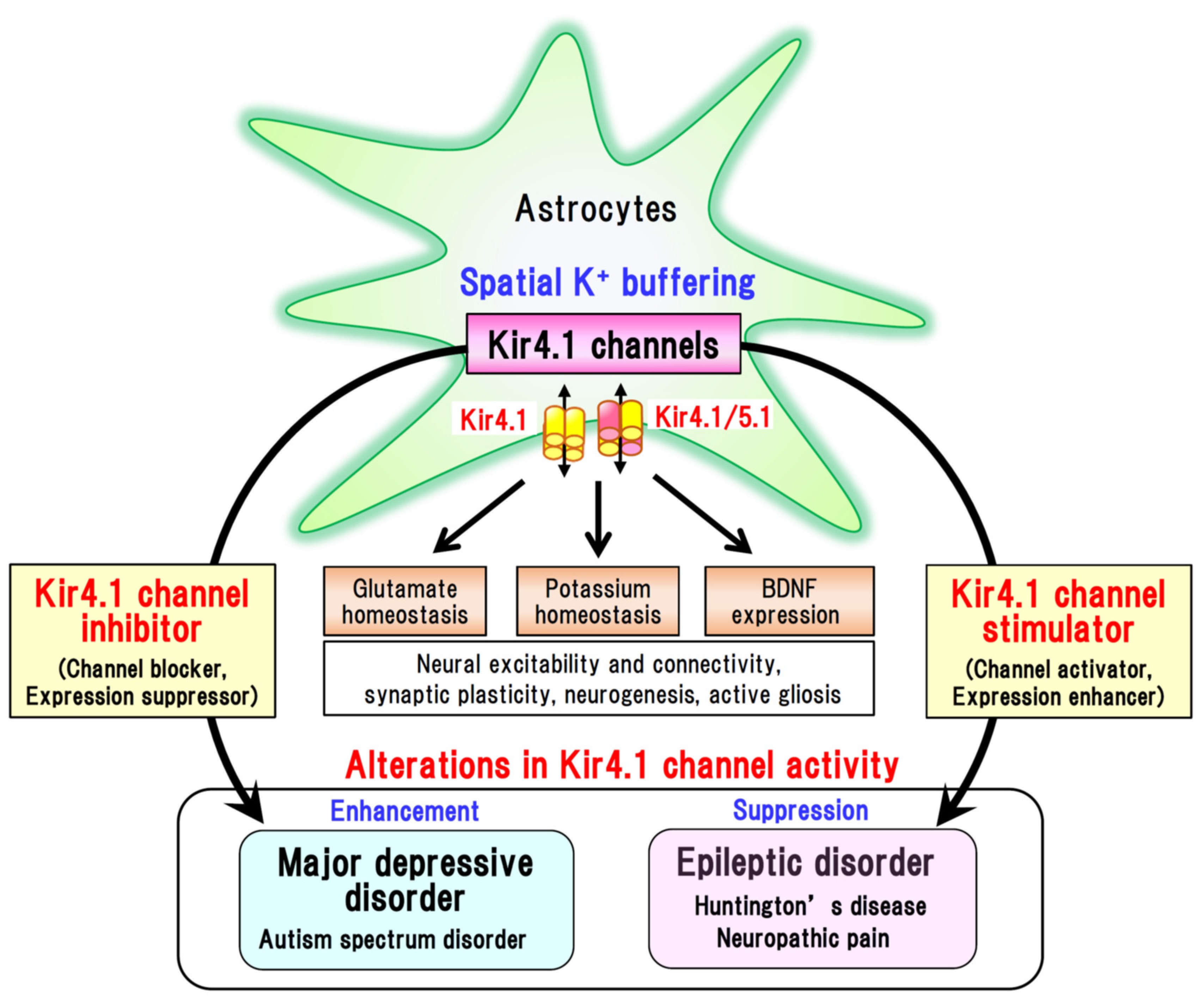
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Walz, W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 2000, 36, 291–300. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Kinboshi, M.; Shimizu, S. Inwardly Rectifying Potassium Channel Kir4.1 as a Novel Modulator of BDNF Expression in Astrocytes. Int. J. Mol. Sci. 2018, 19, 3313. [Google Scholar] [CrossRef]
- Olsen, M.L.; Sontheimer, H. Functional implications for Kir4.1 channels in glial biology: From K+ buffering to cell differentiation. J. Neurochem. 2008, 107, 589–601. [Google Scholar] [CrossRef]
- Kinboshi, M.; Mukai, T.; Nagao, Y.; Matsuba, Y.; Tsuji, Y.; Tanaka, S.; Tokudome, K.; Shimizu, S.; Ito, H.; Ikeda, A.; et al. Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front. Mol. Neurosci. 2017, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Li, J.; Chen, L.; Yu, Y.; Wu, Y. Astrocytic Kir4.1 channels and gap junctions account for spontaneous epileptic seizure. PLoS Comput. Biol. 2018, 14, e1005877. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Djukic, B.; McCarthy, K.D.; Amzica, F. Implication of Kir4.1 channel in excess potassium clearance: An in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J. Neurosci. 2010, 30, 15769–15777. [Google Scholar] [CrossRef]
- Djukic, B.; Casper, K.B.; Philpot, B.D.; Chin, L.-S.; McCarthy, K.D. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 2007, 27, 11354–11365. [Google Scholar] [CrossRef]
- Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Nichols, C.G.; Maldonado, H.M.; Baksi, K.; Reichenbach, A.; Skatchkov, S.N.; Eaton, M.J. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 2007, 55, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Mathiisen, T.M.; Ottersen, O.P. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 2004, 129, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Eulenburg, V.; Gomeza, J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res. Rev. 2010, 63, 103–112. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.M.; Sullivan, C.R.; McCullumsmith, R.E. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr. 2017, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, M.E.; Ohno, Y. Perisynaptic astrocytes as a potential target for novel antidepressant drugs. J. Pharmacol. Sci. 2021, 145, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Reichold, M.; Zdebik, A.A.; Lieberer, E.; Rapedius, M.; Schmidt, K.; Bandulik, S.; Sterner, C.; Tegtmeier, I.; Penton, D.; Baukrowitz, D.; et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc. Natl. Acad. Sci. USA 2010, 107, 14490–14495. [Google Scholar] [CrossRef]
- Méndez-González, M.P.; Kucheryavykh, Y.V.; Zayas-Santiago, A.; Vélez-Carrasco, W.; Maldonado-Martínez, G.; Cubano, L.A.; Nichols, C.G.; Skatchkov, S.N.; Eaton, M.J. Novel KCNJ10 Gene Variations Compromise Function of Inwardly Rectifying Potassium Channel 4.1. J. Biol. Chem. 2016, 291, 7716–7726. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ohno, Y.; Lossin, C.; Hibino, H.; Inanobe, A.; Kurachi, Y. Inhibition of astroglial inwardly rectifying Kir4.1 channels by a tricyclic antidepressant, nortriptyline. J. Pharmacol. Exp. Ther. 2007, 320, 573–580. [Google Scholar] [CrossRef]
- Ohno, Y.; Hibino, H.; Lossin, C.; Inanobe, A.; Kurachi, Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007, 1178, 44–51. [Google Scholar] [CrossRef]
- Furutani, K.; Ohno, Y.; Inanobe, A.; Hibino, H.; Kurachi, Y. Mutational and in silico analyses for antidepressant block of astroglial inward-rectifier Kir4.1 channel. Mol. Pharmacol. 2009, 75, 1287–1295. [Google Scholar] [CrossRef]
- Marmoiejo-Murillo, L.G.; Aréchiga-Figueroa, I.A.; Cui, M.; Moreno-Galindo, E.G.; Navarro-Polanco, R.A.; Sánchez-Chapula, J.A.; Ferrer, T.; Rodríguez-Menchaca, A.A. Inhibition of Kir4.1 potassium channels by quinacrine. Brain Res. 2017, 1663, 87–94. [Google Scholar] [CrossRef]
- Marmoiejo-Murillo, L.G.; Aréchiga-Figueroa, I.A.; Moreno-Galindo, E.G.; Navarro-Polanco, R.A.; Rodríguez-Menchaca, A.A.; Cui, M.; Sánchez-Chapula, J.A.; Ferrer, T. Chloroquine blocks the Kir4.1 channels by an open-pore blocking mechanism. Eur. J. Pharmacol. 2017, 800, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Aréchiga-Figueroa, I.A.; Marmolejo-Murillo, L.G.; Cui, M.; Delgado-Ramírez, M.; Van Der Heyden, M.A.G.; Sánchez-Chapula, J.A.; Rodríguez-Menchaca, A.A. High-potency block of Kir4.1 channels by pentamidine: Molecular basis. Eur. J. Pharmacol. 2017, 815, 56–63. [Google Scholar] [CrossRef]
- Kharade, S.V.; Kurata, H.; Bender, A.M.; Blobaum, A.L.; Figueroa, E.E.; Duran, A.; Kramer, M.; Days, E.; Vinson, P.; Flores, D.; et al. Discovery, characterization, and effects on renal fluid and electrolyte excretion of the Kir4.1 potassium channel pore blocker, VU0134992. Mol. Pharmacol. 2018, 94, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Morán-Zendejas, R.; Delgado-Ramírez, M.; Xu, J.; Valdés-Abadía, B.; Aréchiga-Figueroa, I.A.; Cui, M.; Rodríguez-Menchaca, A.A. In vitro and in silico characterization of the inhibition of Kir4.1 channels by aminoglycoside antibiotics. Br. J. Pharmacol. 2020, 177, 4548–4560. [Google Scholar] [CrossRef] [PubMed]
- Vizi, E.S.; Kisfali, M.; Lőrincz, T. Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: Evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects. Brain Res. Bull. 2013, 93, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Patterson, P.H. The role of cytokines and growth factors in seizures and their sequelae. Prog. Neurobiol. 2001, 63, 125–149. [Google Scholar] [CrossRef]
- Castrén, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. Differential diagnosis of bipolar disorder and major depressive disorder. J. Affect. Disord. 2014, 169, S12–S16. [Google Scholar] [CrossRef]
- Galts, C.P.C.; Bettio, L.E.B.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef] [PubMed]
- Frazer, A. Serotonergic and noradrenergic reuptake inhibitors: Prediction of clinical effects from in vitro ptencies. J. Clin. Psychiatry 2001, 62, S16–S23. [Google Scholar]
- Ota, K.T.; Duman, R.S. Environmental and pharmacological modulations of cellular plasticity: Role in the pathophysiology and treatment of depression. Neurobiol. Dis. 2013, 57, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-F.; Peng, W.; Sweeney, J.A.; Jia, Z.-Y.; Gong, Q.-Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Hing, B.; Sathyaputri, L.; Potash, J.B. A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 143–167. [Google Scholar] [CrossRef]
- Marathe, S.V.; D’almeida, P.L.; Virmani, G.; Bathini, P.; Alberi, L. Effects of Monoamines and Antidepressants on Astrocyte Physiology: Implications for Monoamine Hypothesis of Depression. J. Exp. Neurosci. 2018, 12, 1179069518789149. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, K.; Ren, Q.; Chang, L.; Chen, J.; Hashimoto, K. Increased expression of inwardly rectifying Kir4.1 channel in the parietal cortex from patients with major depressive disorder. J. Affect. Disord. 2019, 245, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem. Pharmacol. 2020, 177, 113935. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Carvalho, I.P.; Lui, L.M.W.; Majeed, A.; Masand, P.S.; Gill, H.; Rodrigues, N.B.; Lipsitz, O.; Coles, A.C.; Lee, Y.; et al. The effect of intravenous, intranasal, and oral ketamine in mood disorders: A meta-analysis. J. Affect. Disord. 2020, 276, 576–584. [Google Scholar] [CrossRef]
- Stenovec, M.; Li, B.; Verkhratsky, A.; Zorec, R. Astrocytes in rapid ketamine antidepressant action. Neuropharmacology 2020, 173, 108158. [Google Scholar] [CrossRef]
- Athira, K.V.; Mohan, A.S.; Chakravarty, S. Rapid acting antidepressants in the mTOR pathway: Current evidence. Brain Res. Bull. 2020, 163, 170–177. [Google Scholar]
- Yang, Y.; Cui, Y.; Sang, K.; Dong, Y.; Ni, Z.; Ma, S.; Hu, H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018, 554, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hu, S.; Hu, H. Lateral Habenular Burst Firing as a Target of the Rapid Antidepressant Effects of Ketamine. Trends Neurosci. 2019, 42, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Stenovec, M.; Božić, M.; Pirnat, S.; Zorec, R. Astroglial Mechanisms of Ketamine Action Include Reduced Mobility of Kir4.1-Carrying Vesicles. Neurochem. Res. 2020, 45, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.N.; Filippi, D.; Hauser, W.A. The descriptive epidemiology of epilepsy-a review. Epilepsy Res. 2009, 85, 31–45. [Google Scholar] [CrossRef]
- Meldrum, B.S.; Rogawski, M.A. Molecular targets for antiepileptic drug development. Neurotherapeutics 2007, 4, 18–61. [Google Scholar] [CrossRef]
- Inyushin, M.; Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Nichols, C.G.; Buono, R.J.; Ferraro, T.N.; Skatchkov, S.N.; Eaton, M.J. Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia 2010, 51, 1707–1713. [Google Scholar] [CrossRef]
- Kinboshi, M.; Shimizu, S.; Mashimo, T.; Serikawa, T.; Ito, H.; Ikeda, A.; Takahashi, R.; Ohno, Y. Down-Regulation of Astrocytic Kir4.1 Channels during the Audiogenic Epileptogenesis in Leucine-Rich Glioma-Inactivated 1 (Lgi1) Mutant Rats. Int. J. Mol. Sci. 2019, 20, 1013. [Google Scholar] [CrossRef]
- Harada, Y.; Nagao, Y.; Shimizu, S.; Serikawa, T.; Terada, R.; Fujimoto, M.; Okuda, A.; Mukai, T.; Sasa, M.; Kurachi, Y.; et al. Expressional analysis of inwardly rectifying Kir4.1 channels in Noda epileptic rat (NER). Brain Res. 2013, 1517, 141–149. [Google Scholar] [CrossRef]
- Stewart, T.H.; Eastman, C.L.; Groblewski, P.A.; Fender, J.S.; Verley, D.R.; Cook, D.G.; D’Ambrosio, R. Chronic dysfunction of astrocytic inwardly rectifying K+ channels specific to the neocortical epileptic focus after fluid percussion injury in the rat. J. Neurophysiol. 2010, 104, 3345–3360. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, F.; Frasca, A.; Weissberg, I.; Parrella, S.; Friedman, A.; Vezzani, A.; Noé, F.M. Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of concomitant pathology. Epilepsia 2012, 53, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Bockenhauer, D.; Feather, S.; Stanescu, H.C.; Bandulik, S.; Zdebik, A.A.; Reichold, M.; Tobin, J.; Lieberer, E.; Sterner, C.; Landoure, G.; et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N. Engl. J. Med. 2009, 360, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Scholl, U.I.; Choi, M.; Liu, T.; Ramaekers, V.T.; Häusler, M.G.; Grimmer, J.; Tobe, S.W.; Farhi, A.; Nelson-Williams, C.; Lifton, R.P. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc. Natl. Acad. Sci. USA 2009, 106, 5842–5847. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Kucheryavykh, L.Y.; Skatchkov, S.N.; Eaton, M.J.; Nichols, C.G. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10). J. Biol. Chem. 2010, 285, 36040–36048. [Google Scholar] [CrossRef]
- Steinhäuser, C.; Seifert, G.; Bedner, P. Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia 2012, 60, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Shirozu, H.; Masuda, H.; Fukuda, M.; Fujii, Y.; Kakita, A. Pathophysiological characteristics associated with epileptogenesis in human hippocampal sclerosis. EBioMedicine 2018, 29, 38–46. [Google Scholar] [CrossRef]
- Ikeda, A.; Takeyama, H.; Bernard, C.; Nakatani, M.; Shimotake, A.; Daifu, M.; Matsuhashi, M.; Kikuchi, T.; Kunieda, T.; Matsumoto, R.; et al. Active direct current (DC) shifts and “Red slow”: Two new concepts for seizure mechanisms and identification of the epileptogenic zone. Neurosci. Res. 2020, 156, 95–101. [Google Scholar] [CrossRef]
- Zurolo, E.; De Groot, M.; Iyer, A.; Anink, J.; Van Vliet, E.A.; Heimans, J.J.; Reijneveld, J.C.; Gorter, J.A. Eleonora Aronica. Regulation of Kir4.1 expression in astrocytes and astrocytic tumors: A role for interleukin-1 β. J. Neuroinflammation 2012, 9, 280. [Google Scholar] [CrossRef]
- Hasan, S.; Balobaid, A.; Grottesi, A.; Dabbagh, O.; Cenciarini, M.; Rawashdeh, R.; AI-Sagheir, A.; Bove, C.; Macchioni, L.; Pessia, M.; et al. Lethal digenic mutations in the K+ channels Kir4.1 (KCNJ10) and SLACK (KCNT1) associated with severe-disabling seizures and neurodevelopmental delay. J. Neurophysiol. 2017, 118, 2402–2411. [Google Scholar] [CrossRef]
- Mukai, T.; Kinboshi, M.; Nagao, Y.; Shimizu, S.; Ono, A.; Sakagami, Y.; Okuda, A.; Fujimoto, M.; Ito, H.; Ikeda, A.; et al. Antiepileptic drugs elevate astrocytic Kir4.1 expression in the rat limbic region. Front. Pharmacol. 2018, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Brandt, C. Prevention or modification of epileptogenesis after brain insults: Experimental approaches and translational research. Pharmacol. Rev. 2010, 62, 668–700. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Caprini, M.; Nobile, M.; Rapisarda, C.; Ferroni, S. Guanosine promotes the up-regulation of inward rectifier potassium current mediated by Kir4.1 in cultured rat cortical astrocytes. J. Neurochem. 2006, 98, 430–445. [Google Scholar] [CrossRef]
- Zhao, M.; Bousquet, E.; Valamanesh, F.; Farman, N.; Jeanny, J.-C.; Jaisser, F.; Behar-Cohen, F.F. Differential regulations of AQP4 and Kir4.1 by triamcinolone acetonide and dexamethasone in the healthy and inflamed retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6340–6347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, G.; Ling, Q.; Da, C. Expression of aquaporin 4 and Kir4.1 in diabetic rat retina: Treatment with minocyclin. J. Int. Med. Res. 2011, 39, 464–479. [Google Scholar] [CrossRef]
- Saudou, F.; Humbert, S. The Biology of Huntingtin. Neuron 2016, 89, 910–926. [Google Scholar] [CrossRef]
- Khakh, B.S.; Beaumont, V.; Cachope, R.; Munoz-Sanjuan, I.; Goldman, S.A.; Grantyn, R. Unravelling and exploiting astrocyte dysfunction in Huntington’s disease. Trends Neurosci. 2017, 40, 422–437. [Google Scholar] [CrossRef]
- Proft, J.; Weiss, N. Rectifying rectifier channels in Huntington disease. Commun. Integr. Biol. 2014, 7, e29410. [Google Scholar] [CrossRef][Green Version]
- Zuccato, C.; Ciammola, A.; Rigamonti, D.; Leavitt, B.R.; Goffredo, D.; Conti, L.; MacDonald, M.E.; Friedlander, R.M.; Silani, V.; Hayden, M.R.; et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 2001, 293, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Ao, Y.; Faas, G.C.; Nwaobi, S.E.; Xu, J.; Haustein, M.D.; Anderson, M.A.; Mody, I.; Olsen, M.L.; Sofroniew, M.V.; et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014, 17, 694–703. [Google Scholar] [CrossRef]
- Jiang, R.; Diaz-Castro, B.; Looger, L.L.; Khakh, B.S. Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. J. Neurosci. 2016, 36, 3453–3470. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, Y.; Provenzano, G.; Casarosa, S. Neurobiological bases of autism-epilepsy comorbidity: A focus on excitation/inhibition imbalance. Eur. J. Neurosci. 2018, 47, 534–548. [Google Scholar] [CrossRef]
- Sicca, F.; Ambrosini, E.; Marchese, M.; Sforna, L.; Servettini, I.; Valvo, G.; Brignone, M.S.; Lanciotti, A.; Moro, F.; Grottesi, A.; et al. Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy. Sci. Rep. 2016, 6, 34325. [Google Scholar] [CrossRef] [PubMed]
- Sicca, F.; Imbrici, P.; D’Adamo, M.C.; Moro, F.; Bonatti, F.; Brovedani, P.; Grottesi, A.; Guerrini, R.; Masi, G.; Santorelli, F.M.; et al. Autism with seizures and intellectual disability: Possible causative role of gain-of-function of the inwardly-rectifying K+ channel Kir4.1. Neurobiol. Dis. 2011, 43, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Maletzki, I.; Hülsmann, S.; Holtmann, B.; Schulz-Schaeffer, W.; Kirchhoff, F.; Bähr, M.; Neusch, C. Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2006, 99, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Bataveljić, D.; Nikolić, L.; Milosević, M.; Todorović, N.; Andjus, P.R. Changes in the astrocytic aquaporin-4 and inwardly rectifying potassium channel expression in the brain of the amyotrophic lateral sclerosis SOD1(G93A) rat model. Glia 2012, 60, 1991–2003. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef]
- Vit, J.-P.; Ohara, P.T.; Bhargava, A.; Kelley, K.; Jasmin, L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J. Neurosci. 2008, 28, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-T.; Ellison, D.H.; Wang, W.-H. Kir4.1/Kir5.1 in the DCT plays a role in the regulation of renal K + excretion. Am. J. Physiol. Ren. Physiol. 2019, 316, F582–F586. [Google Scholar] [CrossRef] [PubMed]
- Nwaobi, S.E.; Olsen, M.L. Correlating Gene-specific DNA Methylation Changes with Expression and Transcriptional Activity of Astrocytic KCNJ10 (Kir4.1). J. Vis. Exp. 2015, 103, 52406. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Kir4.1 Channel Inhibition | Binding Sites in Kir4.1 | Pharmacological Actions | Ref. | |
|---|---|---|---|---|---|
| IC50 Value (Patch Configuration) | Voltage-Dependency | ||||
| TCAs | |||||
| Nortriptyline Desipramine Imipramine | 38 μM (whole-cell) | Yes | E158, T128 | Antidepressant action | [18] |
| SSRIs | |||||
| Sertraline Fluoxetine | 7.2 μM 15.2 μM (whole-cell) | No | E158, T128 | Antidepressant action | [19] |
| Quinacrine | 1.8 μM (inside-out) | Yes | E158, T128 | Antimalarial action | [21] |
| Chloroquine | ca. 0.5 μM (inside-out) ca. 7 μM (whole-cell) | Yes | E158, T128 | Antimalarial action | [22] |
| Pentamidine | 0.097 μM (inside-out) | Yes | E158, T127, T128 | Antiprotozoal action | [23] |
| VU0134992 | 0.97 μM (whole-cell) | Yes | E158, Ile 159 | Diuretic action | [24] |
| Aminoglycosides | |||||
| Gentamycin Neomycin Kanamycin | 6.2 μM 63.8 μM 76.8 μM (inside-out) | Yes | E158, T128 | Antibiotic action | [25] |
| Kir4.1-Related Disorders | Changes in Kir4.1 Channels | Clinical Phenotype | Ref. |
|---|---|---|---|
| Animal models | |||
| Kir4.1 conditional knockout mice | Kir4.1 channel dysfunction Reduced uptake of extracellular K+ and glutamate into astrocytes | Body tremor, Ataxia, High susceptibility to GTCSs, Premature death | [9,11] |
| Noda epileptic rat | Reduced expression of Kir4.1 in the amygdala (presynaptic processes) | Spontaneous GTCSs | [52] |
| Lgi1 mutant rats | Reduced expression of Kir4.1 in the temporal lobe | High susceptibility to audiogenic kindling | [51] |
| Seizure susceptible DBA/2 mice | T262S mutation in Kir4.1 gene Dysfunction of Kir4.1 channel Reduced uptake of glutamate | High susceptibility to GTCSs, | [50] |
| Trauma-induced epilepsy rat | Chronic loss of Kir4.1 in the cerebral cortex (presynaptic processes) | Spontaneous partial seizures of cortical origin | [53] |
| Human disorders | |||
| EAST/SeSaME syndrome | Loss-of-function mutations in Kir4.1 gene Kir4.1 channel dysfunction | GTCSs, Ataxia, Deafness, Mental retardation, Increased K+ excretion | [16,55,56,57] |
| Mesial temporal lobe epilepsy | Reduced Kir4.1 expression in the temporal lobe | Refractory partial seizures | [58] |
| Temporal lobe epilepsy with hippocampal sclerosis | Reduced Kir4.1 expression in the hippocampus | Refractory partial seizures | [59] |
| Focal cortical dysplasia type1 | Reduced Kir4.1 expression in seizure foci | Refractory partial seizures | [60] |
| Glioma with epilepsy | Reduced Kir4.1 expression in the glioma site | GTCSs | [61] |
| Lethal epileptic disorder | Double mutations in Kir4.1 and SLACK genes Kir4.1 channel dysfunction | Severe-disabling seizures Developmental delay Early death | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohno, Y.; Kunisawa, N.; Shimizu, S. Emerging Roles of Astrocyte Kir4.1 Channels in the Pathogenesis and Treatment of Brain Diseases. Int. J. Mol. Sci. 2021, 22, 10236. https://doi.org/10.3390/ijms221910236
Ohno Y, Kunisawa N, Shimizu S. Emerging Roles of Astrocyte Kir4.1 Channels in the Pathogenesis and Treatment of Brain Diseases. International Journal of Molecular Sciences. 2021; 22(19):10236. https://doi.org/10.3390/ijms221910236
Chicago/Turabian StyleOhno, Yukihiro, Naofumi Kunisawa, and Saki Shimizu. 2021. "Emerging Roles of Astrocyte Kir4.1 Channels in the Pathogenesis and Treatment of Brain Diseases" International Journal of Molecular Sciences 22, no. 19: 10236. https://doi.org/10.3390/ijms221910236
APA StyleOhno, Y., Kunisawa, N., & Shimizu, S. (2021). Emerging Roles of Astrocyte Kir4.1 Channels in the Pathogenesis and Treatment of Brain Diseases. International Journal of Molecular Sciences, 22(19), 10236. https://doi.org/10.3390/ijms221910236





