Postharvest Treatment of Hydrogen Sulfide Delays the Softening of Chilean Strawberry Fruit by Downregulating the Expression of Key Genes Involved in Pectin Catabolism
Abstract
:1. Introduction
2. Results
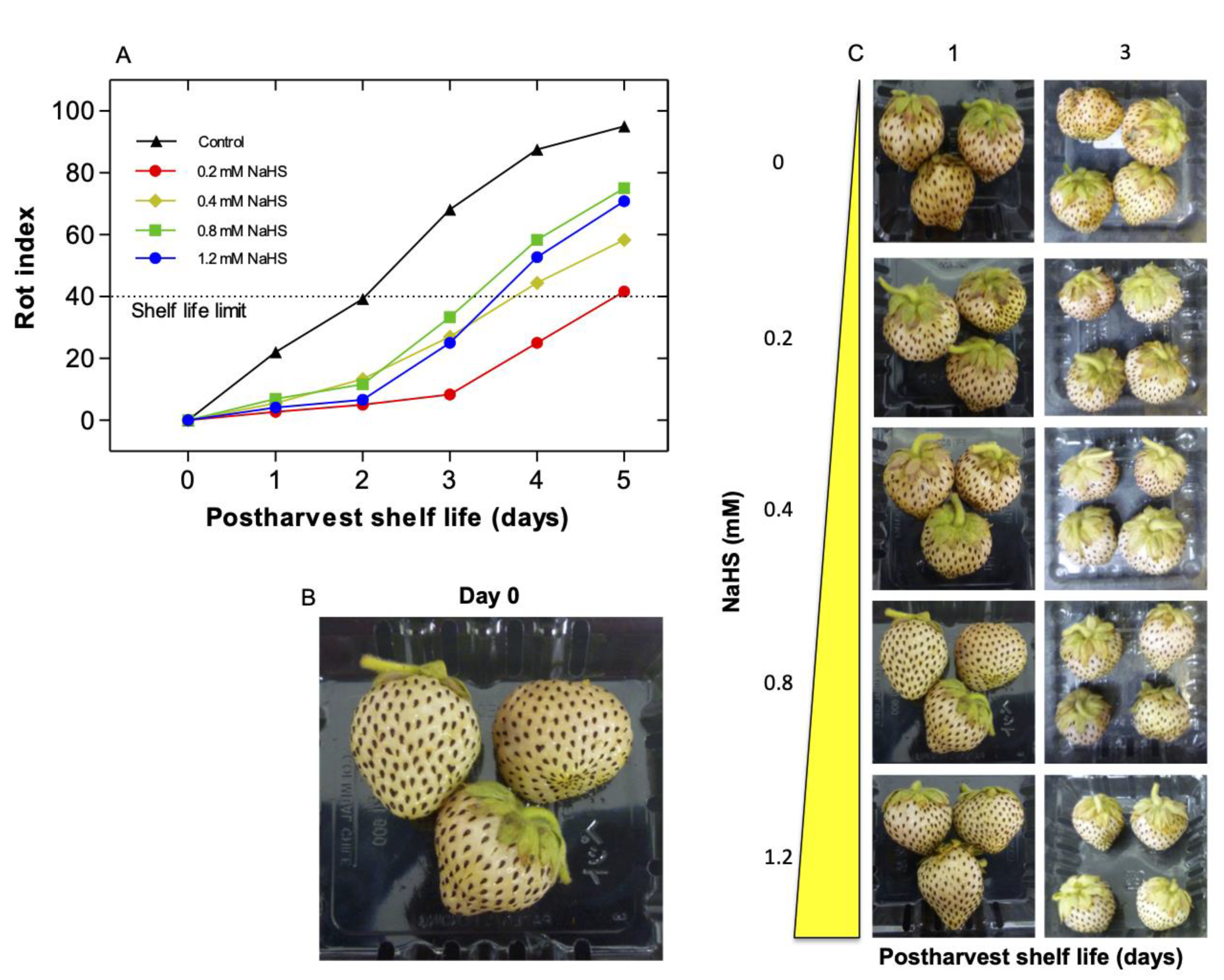
2.1. Effects of H2S Treatment on Postharvest Shelf-Life of Chilean Strawberry Fruit
2.2. Effects of H2S Treatment on Fruit Color during the Shelf-Life of Chilean Strawberry Fruit
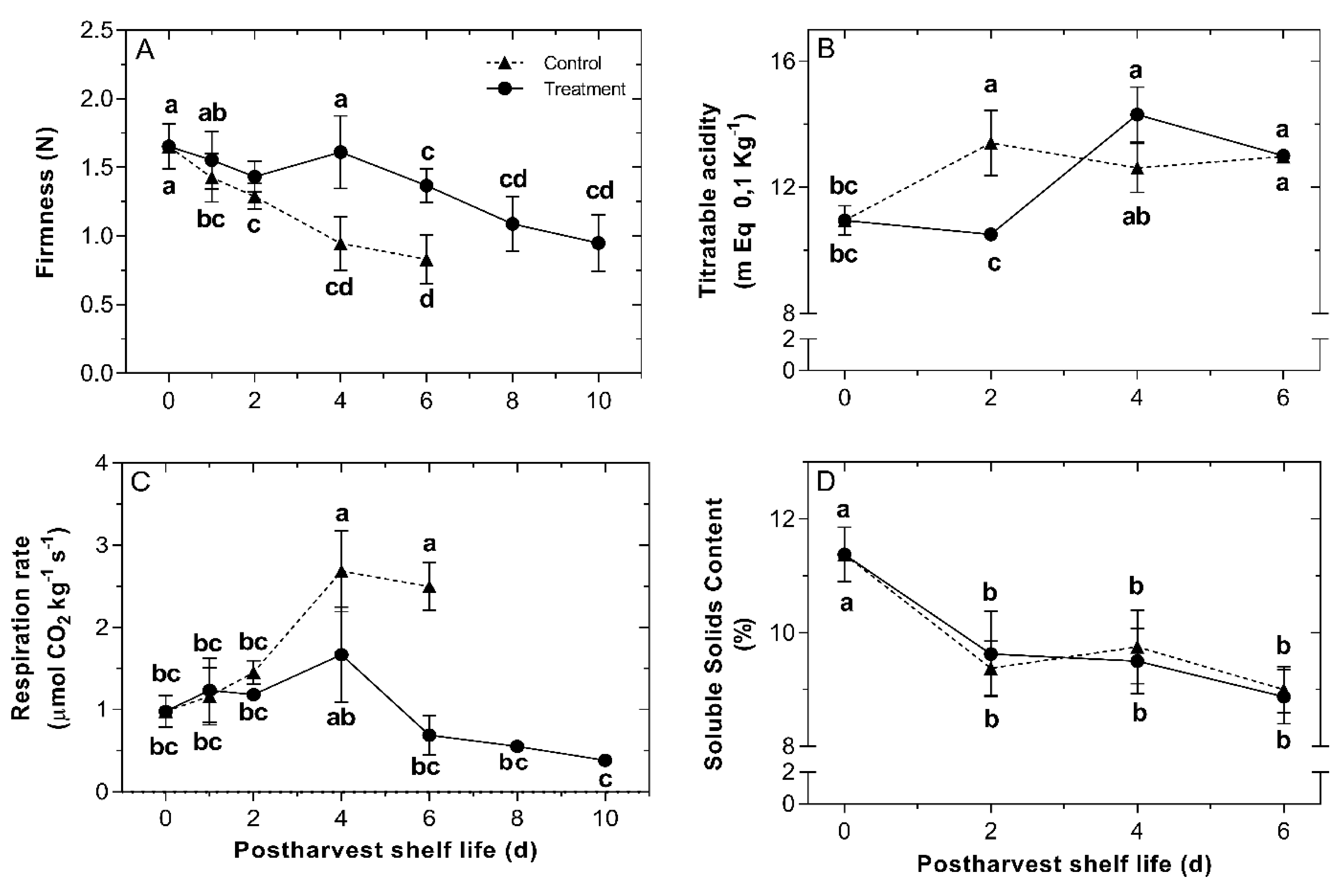
2.3. Effects of H2S Treatment on Fruit Softening and Respiration Rate of Chilean Strawberry Fruit during the Shelf-Life Period
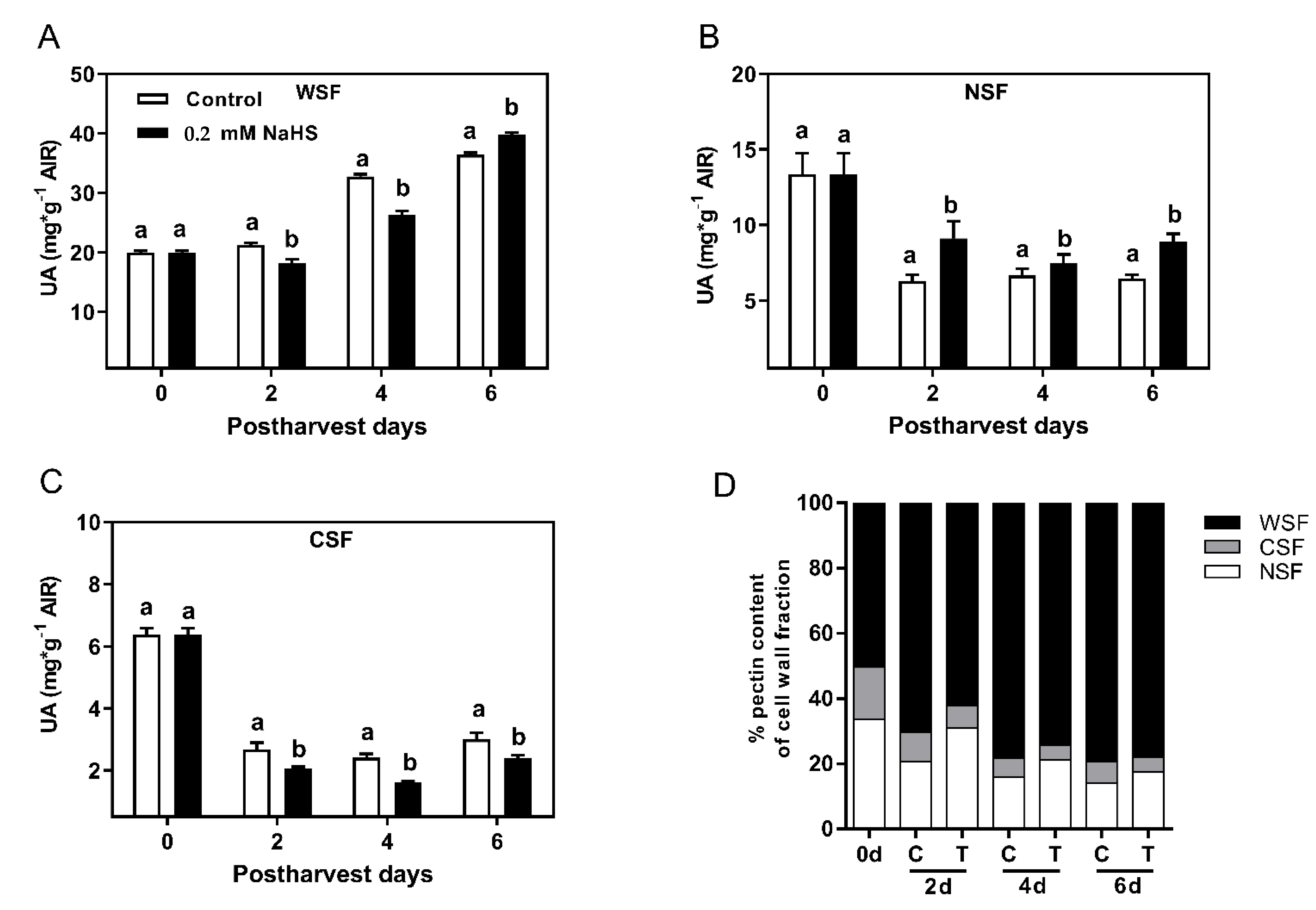
2.4. Effects of H2S Treatment on Cell Wall Polymer Solubilization during the Shelf-Life of Chilean Strawberry Fruit
2.5. Effects of H2S Treatment on the Expression of Genes Involved in Pectin Degradation during Strawberry Shelf-Life
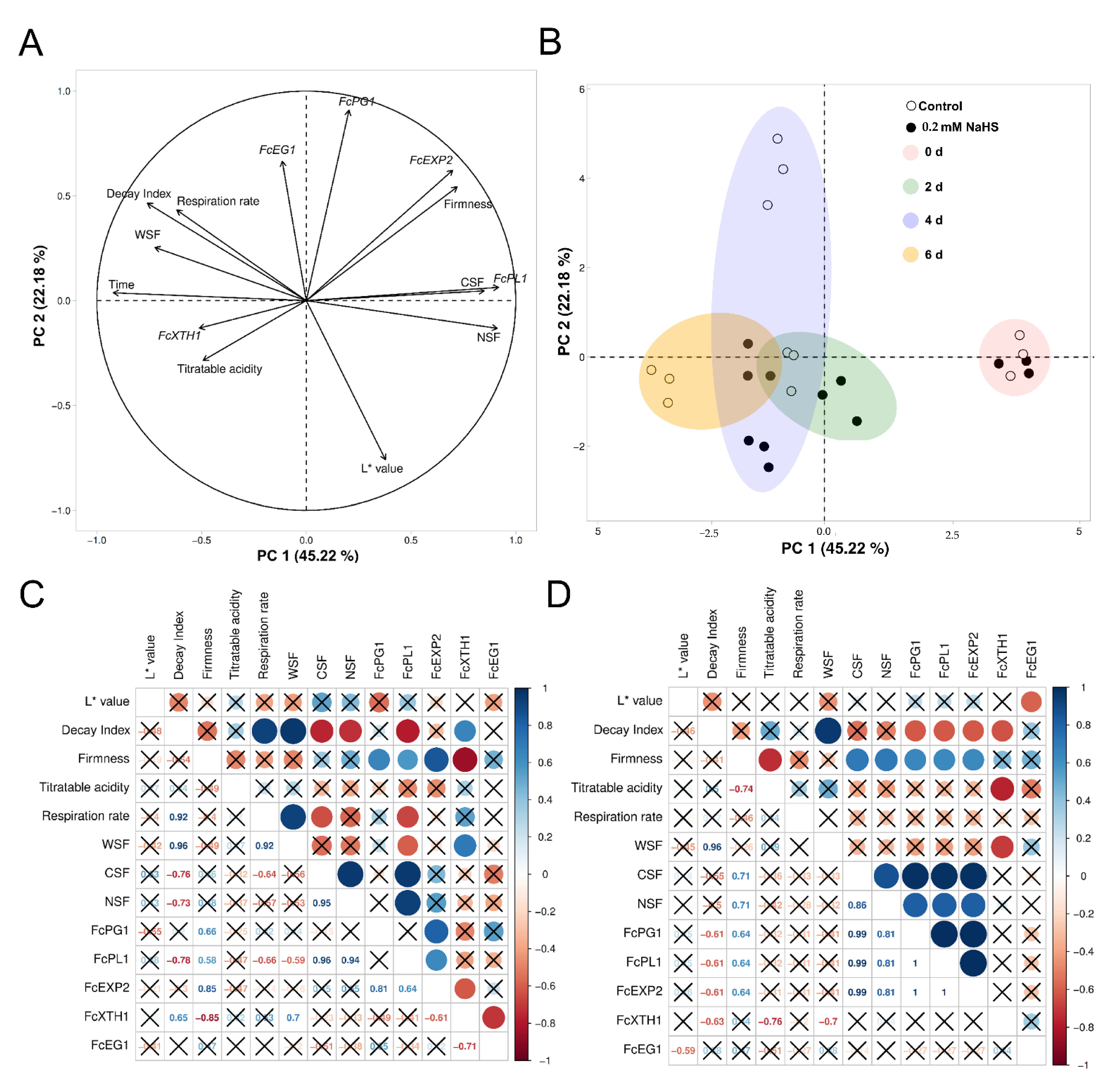
2.6. Principal Components and Correlation Analyses on Studied Parameters in H2S-Treated Strawberries
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Evaluation of Decay Index
4.3. Analysis of Fruit Chromaticity
4.4. Analysis of Fruit Respiration Rate
4.5. Fruit Firmness Measurement
4.6. Determination of Soluble Solids, pH and Titratable Acidity
4.7. Cell Wall Extraction and Cell Wall Fractionation
4.8. Analysis of Gene Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hancock, J.F. Strawberries; CABI Publishing: Wallingford, UK, 1999. [Google Scholar]
- Molinett, S.; Nuñez, F.; Moya-Leon, M.A.; Zúñiga-Hernández, J. Chilean Strawberry Consumption Protects against LPS-Induced Liver Injury by Anti-Inflammatory and Antioxidant Capability in Sprague-Dawley Rats. Evid. Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mora, F.; Zúñiga, P.E.; Figueroa, C.R. Genetic Variation and Trait Correlations for Fruit Weight, Firmness and Color Parameters in Wild Accessions of Fragaria chiloensis. Agronomy 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Letelier, L.; Gaete-Eastman, C.; Peñailillo, P.; Moya-León, M.A.; Herrera, R. Southern Species From the Biodiversity Hotspot of Central Chile: A Source of Color, Aroma, and Metabolites for Global Agriculture and Food Industry in a Scenario of Climate Change. Front. Plant Sci. 2020, 11, 1002. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M. Recent Advances in Hormonal Regulation and Cross-Talk during Non-Climacteric Fruit Development and Ripening. Horticulturae 2019, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Moya-León, M.A.; Mattus-Araya, E.; Herrera, R. Molecular Events Occurring During Softening of Strawberry Fruit. Front. Plant Sci. 2019, 10, 615. [Google Scholar] [CrossRef]
- Figueroa, N.E.; Gatica-Meléndez, C.; Figueroa, C.R. Ethylene application at the immature stage of Fragaria chiloensis fruit represses the anthocyanin biosynthesis with a concomitant accumulation of lignin. Food Chem. 2021, 358, 129913. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–339. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Dotto, M.; Martínez, G.A.; Civello, P.M. Expression of expansin genes in strawberry varieties with contrasting fruit firmness. Plant Physiol. Biochem. 2006, 44, 301–307. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Pimentel, P.; Gaete, C.; Moya, M.; Herrera, R.; Caligari, P.D.; Moya-León, M.A. Softening rate of the Chilean strawberry (Fragaria chiloensis) fruit reflects the expression of polygalacturonase and pectate lyase genes. Postharvest Biol. Technol. 2008, 49, 210–220. [Google Scholar] [CrossRef]
- Santiago-Doménech, N.; Jimenez-Bemudez, S.; Matas, A.J.; Rose, J.K.C.; Blanco, J.M.; Mercado, J.A.; Quesada, M.A. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J. Exp. Bot. 2008, 59, 2769–2779. [Google Scholar] [CrossRef] [Green Version]
- Ramos, P.; Parra-Palma, C.; Figueroa, C.R.; Zuñiga, P.E.; Valenzuela-Riffo, F.; Gonzalez, J.; Gaete-Eastman, C.; Morales-Quintana, L. Cell wall-related enzymatic activities and transcriptional profiles in four strawberry (Fragaria × ananassa) cultivars during fruit development and ripening. Sci. Hortic. 2018, 238, 325–332. [Google Scholar] [CrossRef]
- Rosli, H.; Civello, P.; Martínez, G. Changes in cell wall composition of three Fragaria × ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.R.; Rosli, H.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-Leon, M.A. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria × ananassa fruits. Sci. Hortic. 2010, 124, 454–462. [Google Scholar] [CrossRef]
- Koh, T.H.; Melton, L.D.; Newman, R.H. Solid-state 13C NMR characterization of cell walls of ripening strawberries. Can. J. Bot. 1997, 75, 1957–1964. [Google Scholar] [CrossRef]
- Opazo, M.C.; Figueroa, C.R.; Henríquez, J.; Herrera, R.; Bruno, C.; Valenzuela, P.D.; Moya-Leon, M.A. Characterization of two divergent cDNAs encoding xyloglucan endotransglycosylase/hydrolase (XTH) expressed in Fragaria chiloensis fruit. Plant Sci. 2010, 179, 479–488. [Google Scholar] [CrossRef]
- Koch, M.S.; Erskine, J.M. Sulfide as a phytotoxin to the tropical seagrass Thalassia testudinum: Interactions with light, salinity and temperature. J. Exp. Mar. Biol. Ecol. 2001, 266, 81–95. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [Green Version]
- Aroca, A.; Gotor, C.; Bassham, D.C.; Romero, L.C. Hydrogen Sulfide: From a Toxic Molecule to a Key Molecule of Cell Life. Antioxidants 2020, 9, 621. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.-H.; Wang, W.-H.; Zheng, C.-J.; Lin, G.-H.; Dong, X.-J.; He, J.-X.; Pei, Z.-M.; Zheng, H.-L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef] [Green Version]
- García-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, L.-Y.; Hu, K.-D.; He, Y.-D.; Wang, S.-H.; Luo, J.-P. Hydrogen Sulfide Promotes Wheat Seed Germination and Alleviates Oxidative Damage against Copper Stress. J. Integr. Plant Biol. 2008, 50, 1518–1529. [Google Scholar] [CrossRef]
- Zhang, P.; Luo, Q.; Wang, R.; Xu, J. Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, S.-L.; Zhang, Z.-J.; Hu, L.-Y.; Jiang, C.-X.; Wei, Z.-J.; Liu, J.; Wang, H.-L.; Jiang, S.-T. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol. Technol. 2011, 60, 251–257. [Google Scholar] [CrossRef]
- Bloem, E.; Riemenschneider, A.; Volker, J.; Papenbrock, J.; Schmidt, A.; Salac, I.; Haneklaus, S.; Schnug, E. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J. Exp. Bot. 2004, 55, 2305–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziogas, V.; Molassiotis, A.; Fotopoulos, V.; Tanou, G. Hydrogen Sulfide: A Potent Tool in Postharvest Fruit Biology and Possible Mechanism of Action. Front. Plant Sci. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, C.; Calo, L.; Romero, L.C.; Garcia, I.; Gotor, C. An O-Acetylserine(thiol)lyase Homolog with l-Cysteine Desulfhydrase Activity Regulates Cysteine Homeostasis in Arabidopsis. Plant Physiol. 2009, 152, 656–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papenbrock, J.; Riemenschneider, A.; Kamp, A.; Schulz-Vogt, H.; Schmidt, A. Characterization of Cysteine-Degrading and H2S-Releasing Enzymes of Higher Plants—From the Field to the Test Tube and Back. Plant Biol. 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Youssefian, S.; Nakamura, M.; Sano, H. Tobacco plants transformed with the O-acetylserine (thiol) lyase gene of wheat are resistant to toxic levels of hydrogen sulphide gas. Plant J. 1993, 4, 759–769. [Google Scholar] [CrossRef]
- Ge, Y.; Hu, K.-D.; Wang, S.-S.; Hu, L.-Y.; Chen, X.-Y.; Li, Y.-H.; Yang, Y.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 2017, 12, e0180113. [Google Scholar] [CrossRef]
- Yao, G.-F.; Li, C.; Sun, K.-K.; Tang, J.; Huang, Z.-Q.; Yang, F.; Huang, G.-G.; Hu, L.-Y.; Jin, P.; Hu, K.-D.; et al. Hydrogen Sulfide Maintained the Good Appearance and Nutrition in Post-harvest Tomato Fruits by Antagonizing the Effect of Ethylene. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-J.; Hu, K.-D.; Song, C.-B.; Ma, R.-H.; Li, Z.-R.; Zheng, J.-L.; Fu, L.-H.; Wei, Z.-J.; Zhang, H. Hydrogen Sulfide Alleviates Postharvest Senescence of Grape by Modulating the Antioxidant Defenses. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.-Y.; Hu, S.-L.; Wu, J.; Li, Y.-H.; Zheng, J.-L.; Wei, Z.-J.; Liu, J.; Wang, H.-L.; Liu, Y.-S.; Zhang, H. Hydrogen Sulfide Prolongs Postharvest Shelf Life of Strawberry and Plays an Antioxidative Role in Fruits. J. Agric. Food Chem. 2012, 60, 8684–8693. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, J.Y.; Zhu, L.P.; Li, C.L.; Wang, Q.G. Cooperative effects of hydrogen sulfide and nitric oxide on delaying sof-tening and decay of strawberry. Int. J. Agric. Biol. Eng. 2014, 7, 114–122. [Google Scholar] [CrossRef]
- Zheng, J.L.; Hu, L.Y.; Hu, K.D.; Wu, J.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates senescence of fresh-cut apple by reg-ulating antioxidant defense system and senescence-related gene expression. Hortscience 2016, 51, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.-D.; Wang, Q.; Hu, L.-Y.; Gao, S.-P.; Wu, J.; Li, Y.-H.; Zheng, J.-L.; Han, Y.; Liu, Y.-S.; Zhang, H. Hydrogen Sulfide Prolongs Postharvest Storage of Fresh-Cut Pears (Pyrus pyrifolia) by Alleviation of Oxidative Damage and Inhibition of Fungal Growth. PLoS ONE 2014, 9, e85524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Wang, W.; Shi, J.; Zhang, W.; Shen, Y.; Du, H.; Wu, S. Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. J. Sci. Food Agric. 2014, 94, 2699–2704. [Google Scholar] [CrossRef]
- Hu, H.; Shen, W.; Li, P. Effects of hydrogen sulphide on quality and antioxidant capacity of mulberry fruit. Int. J. Food Sci. Technol. 2013, 49, 399–409. [Google Scholar] [CrossRef]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.R.; Opazo, M.C.; Vera, P.; Arriagada, O.; Díaz, M.; Moya-León, M.A. Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chem. 2012, 132, 2014–2022. [Google Scholar] [CrossRef]
- Luo, Z.; Li, D.; Du, R.; Mou, W. Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci. Hortic. 2015, 183, 144–151. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Du, R.; Luo, Z. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 2016, 208, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Yonggen, S.; Wei, W.; Weil, Z.; Liqin, Z. Hydrogen sulfide facilitating enhancement of antioxidant ability and maintenance of fruit quality of kiwifruits during low temperature storage. Trans. Chin. Soc. Agric. Eng. 2015, 31, 367–372. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, X.; Ma, P.; Yin, W.; Wang, Y.; Chen, Y.; Chen, S.; Jia, H. Hydrogen sulfide promotes hypocotyl elongation via increasing cellulose content and changing the arrangement of cellulose fibrils in alfalfa. J. Exp. Bot. 2020, 71, 5852–5864. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Pimentel, P.; Dotto, M.C.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Expression of five expansin genes during softening of Fragaria chiloensis fruit: Effect of auxin treatment. Postharvest Biol. Technol. 2009, 53, 51–57. [Google Scholar] [CrossRef]
- Zavala, J.F.A.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasic, K.; Hernandez, A.; Korban, S.S. RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol. Biol. Rep. 2004, 22, 437–438. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinett, S.A.; Alfaro, J.F.; Sáez, F.A.; Elgueta, S.; Moya-León, M.A.; Figueroa, C.R. Postharvest Treatment of Hydrogen Sulfide Delays the Softening of Chilean Strawberry Fruit by Downregulating the Expression of Key Genes Involved in Pectin Catabolism. Int. J. Mol. Sci. 2021, 22, 10008. https://doi.org/10.3390/ijms221810008
Molinett SA, Alfaro JF, Sáez FA, Elgueta S, Moya-León MA, Figueroa CR. Postharvest Treatment of Hydrogen Sulfide Delays the Softening of Chilean Strawberry Fruit by Downregulating the Expression of Key Genes Involved in Pectin Catabolism. International Journal of Molecular Sciences. 2021; 22(18):10008. https://doi.org/10.3390/ijms221810008
Chicago/Turabian StyleMolinett, Sebastian A., Juan F. Alfaro, Felipe A. Sáez, Sebastian Elgueta, María A. Moya-León, and Carlos R. Figueroa. 2021. "Postharvest Treatment of Hydrogen Sulfide Delays the Softening of Chilean Strawberry Fruit by Downregulating the Expression of Key Genes Involved in Pectin Catabolism" International Journal of Molecular Sciences 22, no. 18: 10008. https://doi.org/10.3390/ijms221810008
APA StyleMolinett, S. A., Alfaro, J. F., Sáez, F. A., Elgueta, S., Moya-León, M. A., & Figueroa, C. R. (2021). Postharvest Treatment of Hydrogen Sulfide Delays the Softening of Chilean Strawberry Fruit by Downregulating the Expression of Key Genes Involved in Pectin Catabolism. International Journal of Molecular Sciences, 22(18), 10008. https://doi.org/10.3390/ijms221810008








