Deep Sequencing of Small RNA Reveals the Molecular Regulatory Network of AtENO2 Regulating Seed Germination
Abstract
1. Introduction
2. Results
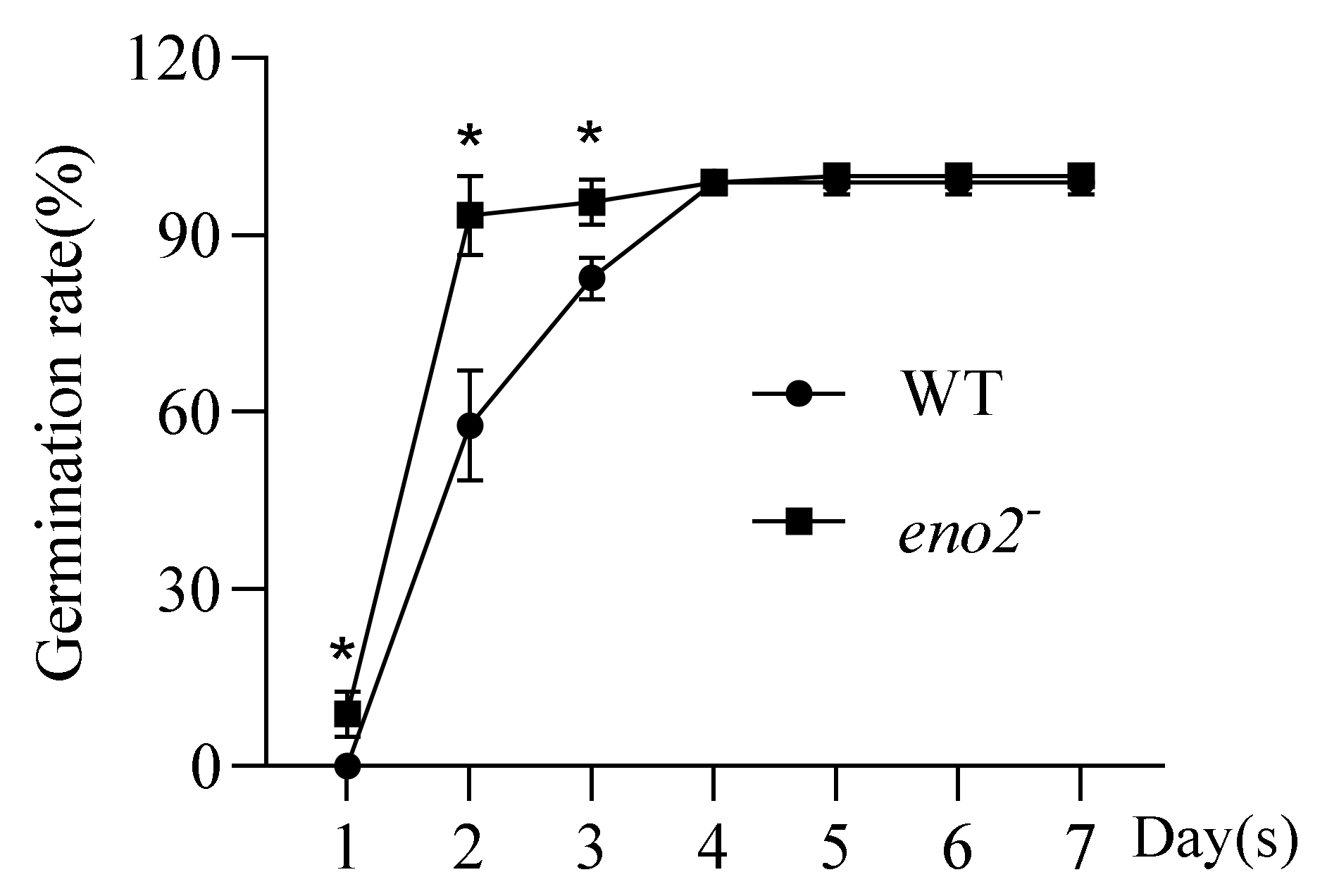
2.1. Statistical Analysis on Germination of eno2−
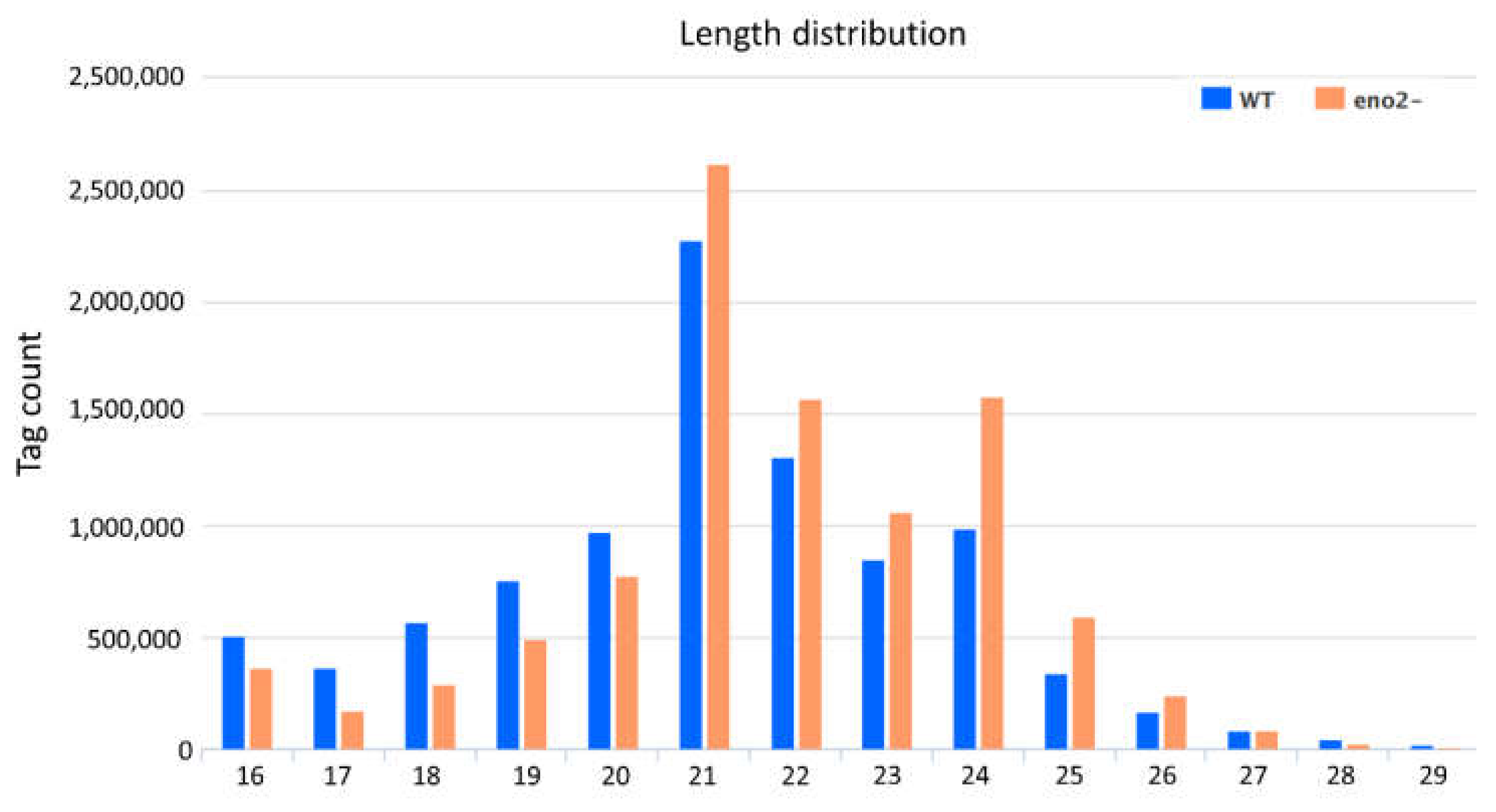
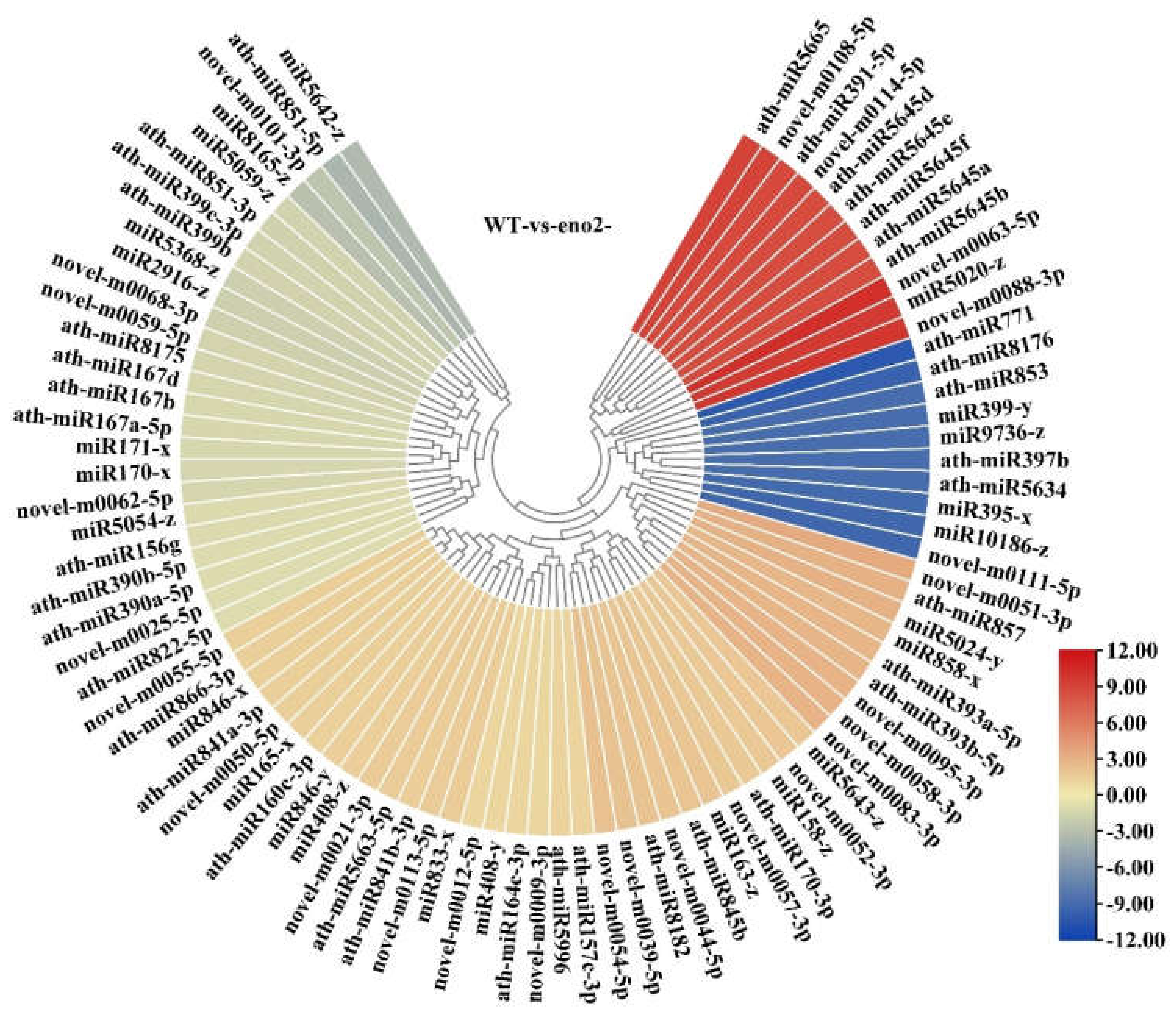
2.2. Data Mining of Small RNA Sequencing
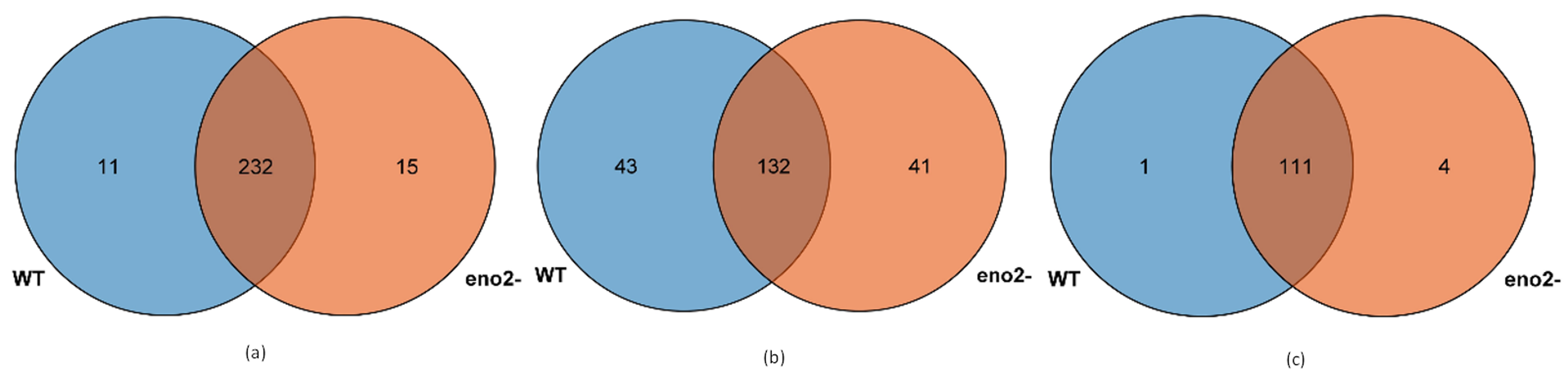
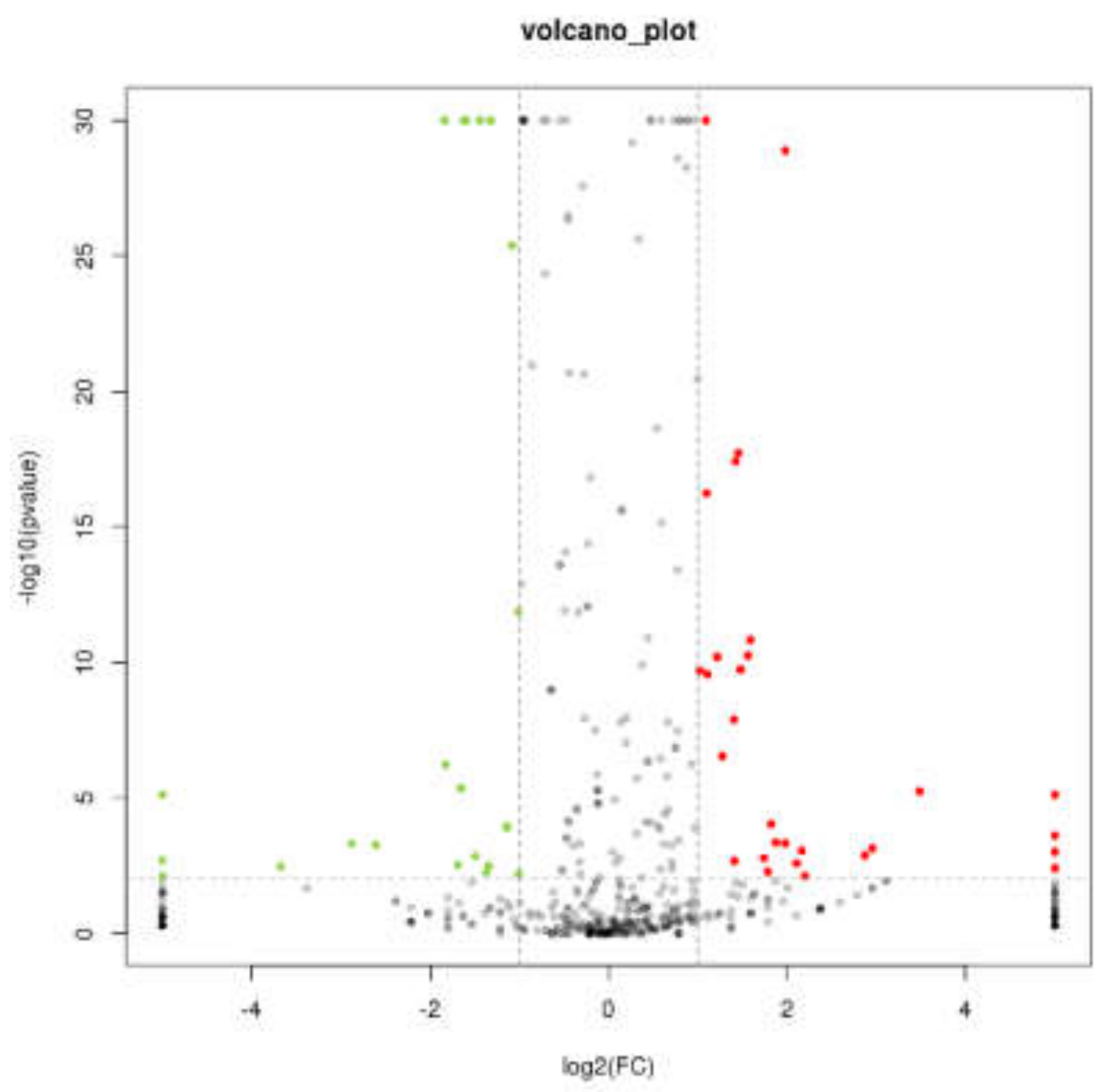
2.3. Expression Analysis of miRNA and Prediction of Key Target Genes
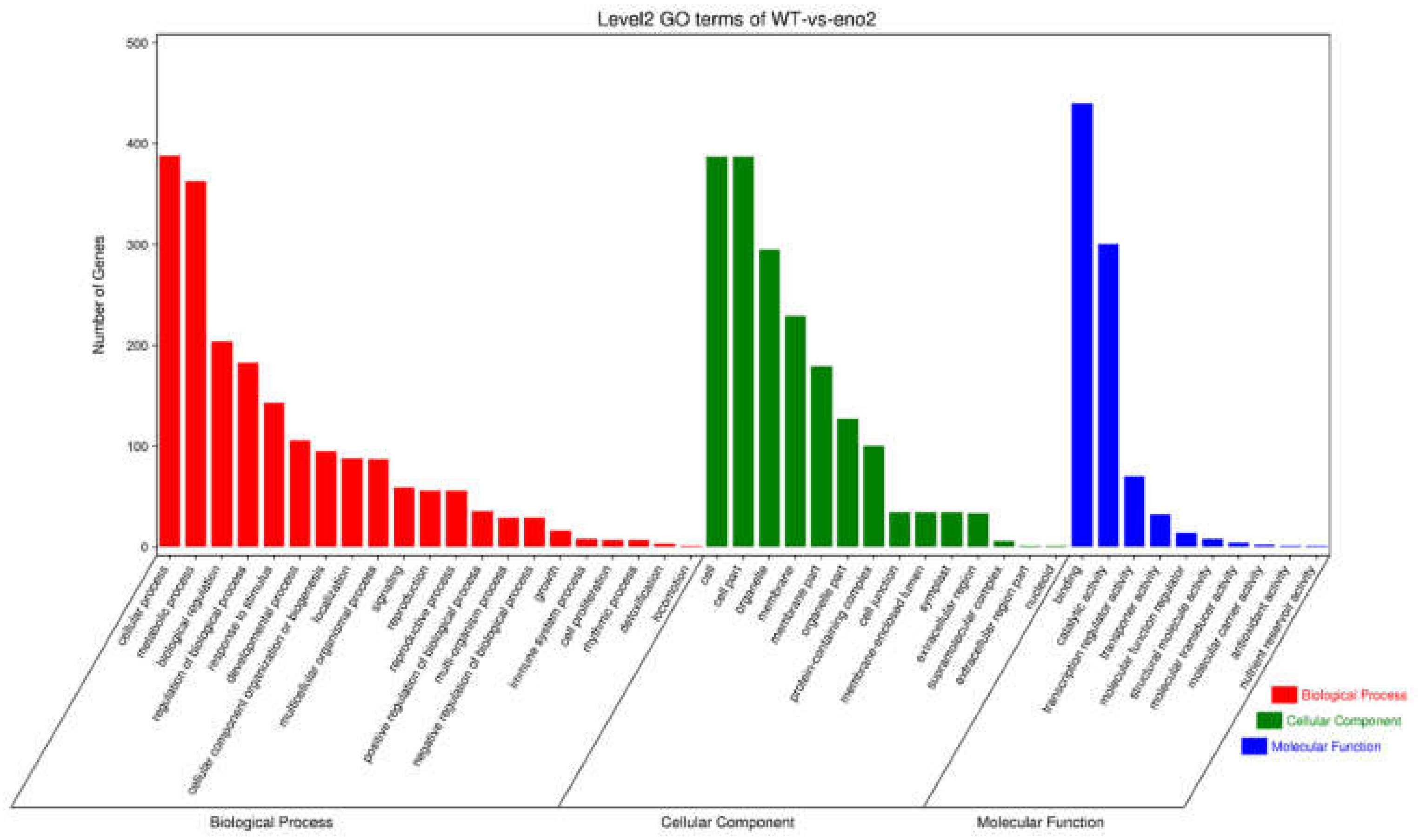
2.4. GO Analysis of Differentially Expressed Target Genes of miRNAs
2.5. KEGG Pathways Analysis of Differentially Expressed Target Genes of miRNAs
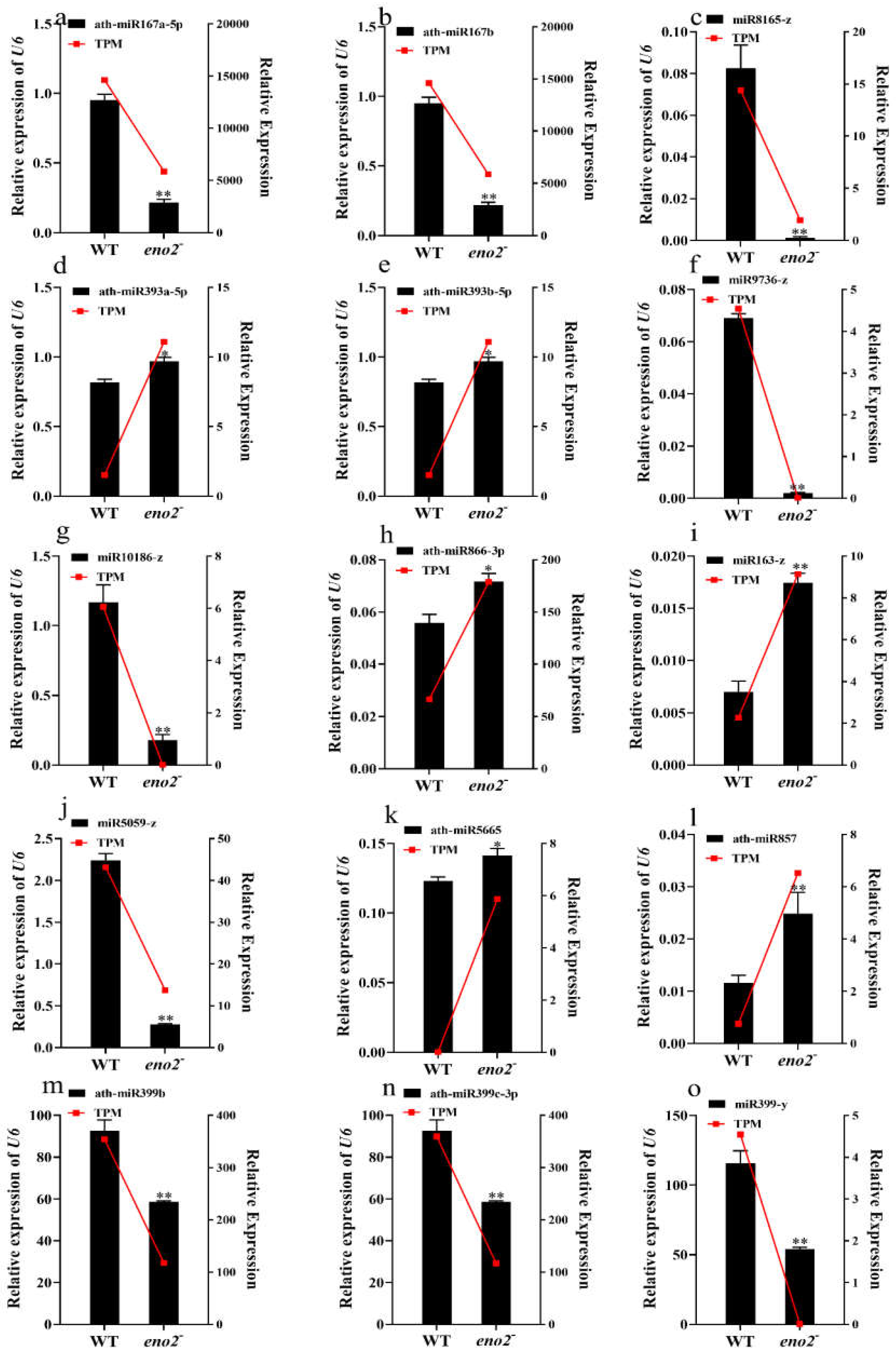
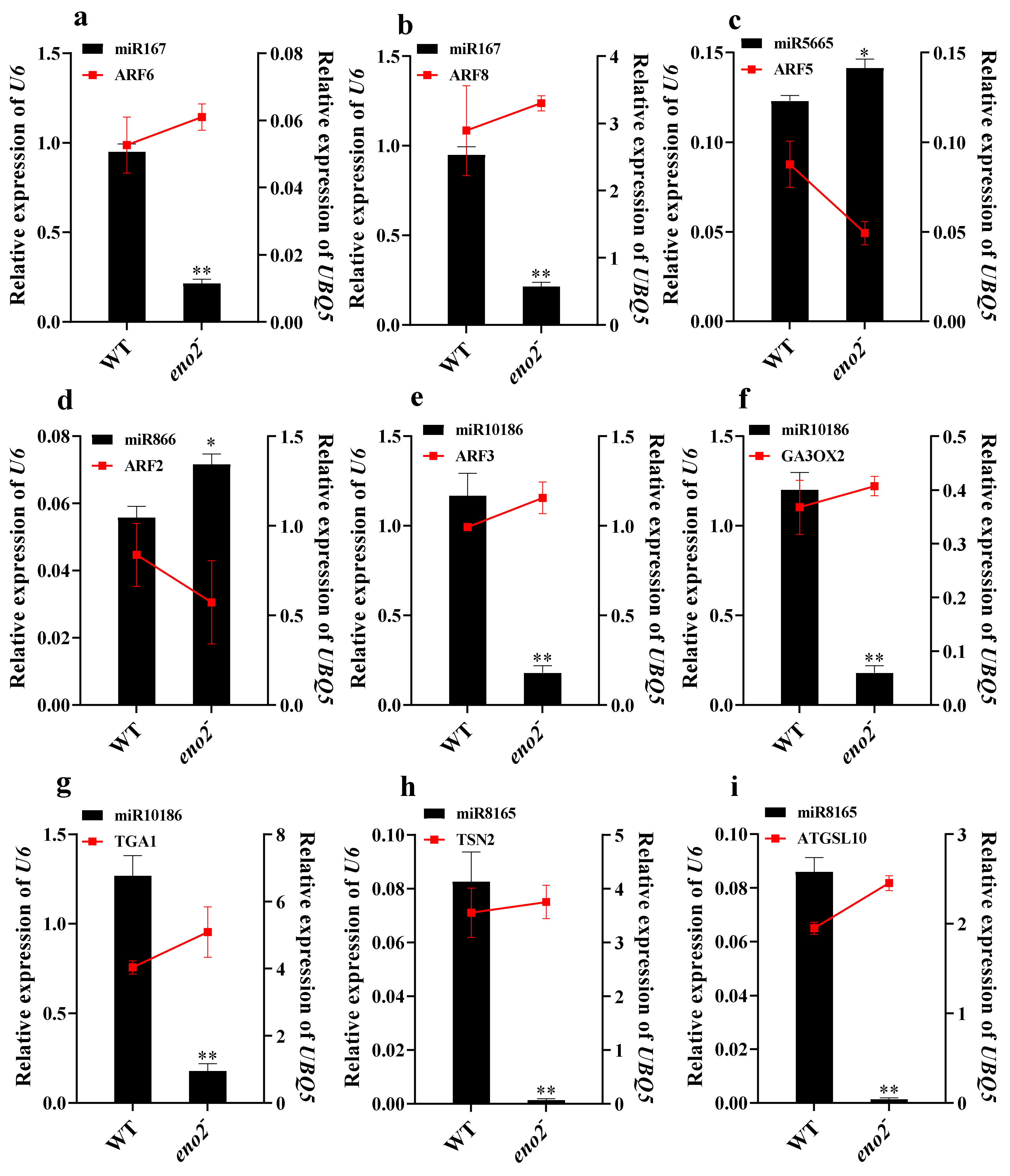
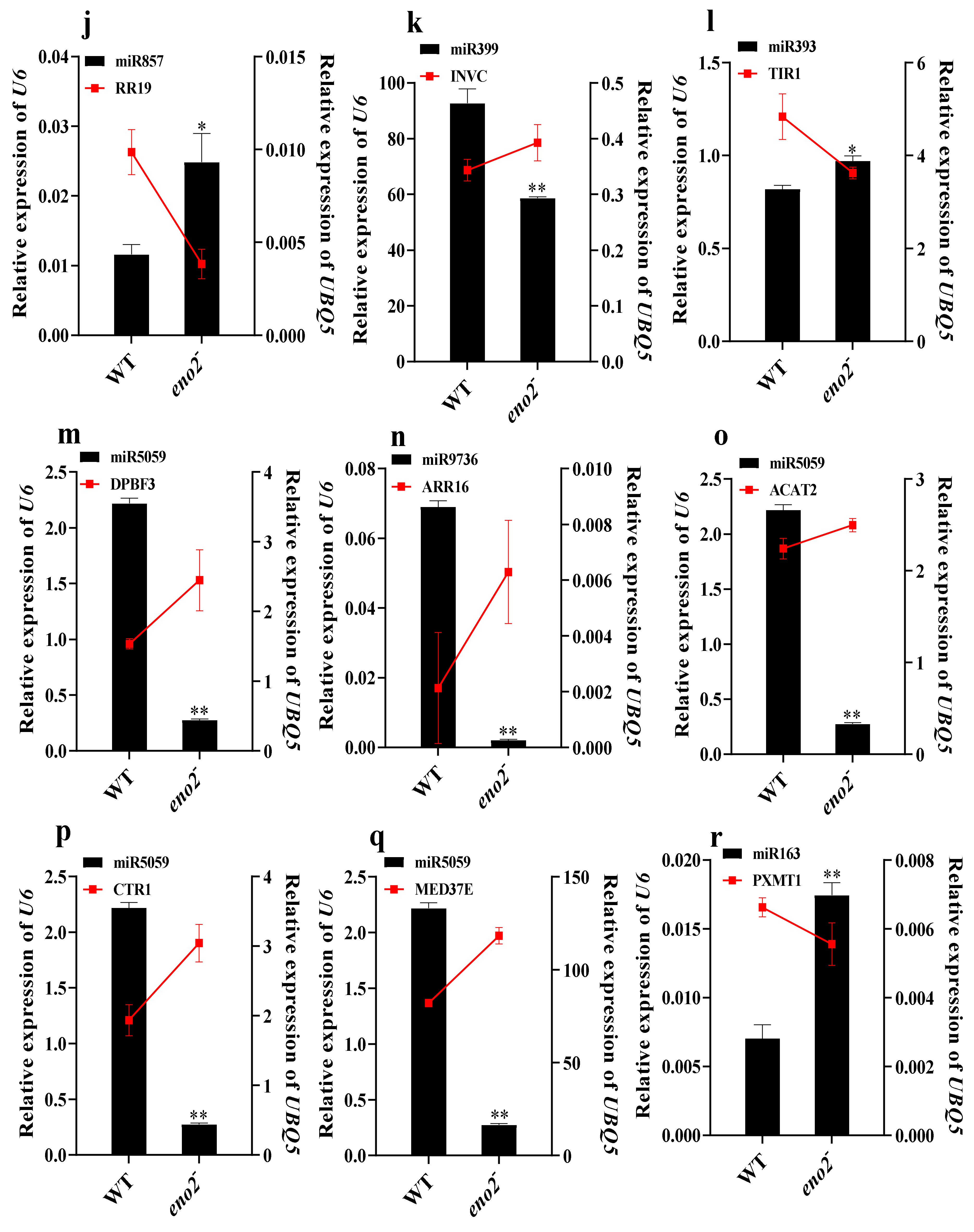
2.6. qRT-PCR Validation of miRNAs and Target Genes
3. Discussion
3.1. Gibberellin and Abscisic Acid
3.2. Auxin
3.3. Cytokinin
3.4. Salicylic Acid
3.5. Others
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Seed Germination Rate Analyses
4.3. Small RNA Library Construction and Sequencing
4.4. Bioinformatic Analysis
4.5. Validation of miRNA and Target Gene Expressions with qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nonogaki, H. Seed Dormancy and Germination—Emerging Mechanisms and New Hypotheses. Front Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef]
- Khan, S.; Zhou, J.L.; Ren, L.; Mojiri, A. Effects of Glyphosate on Germination, Photosynthesis and Chloroplast Morphology in Tomato. Chemosphere 2020, 258, 127350. [Google Scholar] [CrossRef] [PubMed]
- Shashikanthalu, S.P.; Ramireddy, L.; Radhakrishnan, M. Stimulation of the Germination and Seedling Growth of Cuminum cyminum L. Seeds by Cold Plasma. J. Appl. Res. Med. Aroma. 2020, 18, 100259. [Google Scholar] [CrossRef]
- Forte, C.T.; Nunes, U.R.; Cargnelutti, A.; Galon, L.; Chechi, L.; Roso, R.; Menegat, A.D.; Rossetto, E.D.; Franceschetti, M.B. Chemical and Environmental Factors Driving Germination of Solanum americanum Seeds. Weed Biol. Manag. 2019, 19, 113–120. [Google Scholar] [CrossRef]
- Nonogaki, H. ABA Responses During Seed Development and Germination. Adv. Bot. Res. 2019, 92, 171–217. [Google Scholar]
- Nikam, T.D.; Barmukh, R.B. GA(3) Enhances in Vitro Seed Germination in Santalum album. Seed Sci. Technol. 2009, 37, 276–280. [Google Scholar] [CrossRef]
- Peng, J.; Harberd, N.P. The role of GA-Mediated Signalling in the Control of Seed Germination. Curr. Opin. Plant. Biol. 2002, 5, 376–381. [Google Scholar] [CrossRef]
- Yang, L.E.; Peng, D.L.; Li, Z.M.; Huang, L.; Yang, J.; Sun, H. Cold Stratification, Temperature, Light, GA(3), and KNO3 Effects on Seed Germination of Primula beesiana from Yunnan, China. Plant Divers. 2020, 42, 168–173. [Google Scholar] [CrossRef]
- Liu, P.P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR 10 by microRNA160 is Critical for Seed Germination and Post-Germination Stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef]
- He, J.N.; Duan, Y.; Hua, D.P.; Fan, G.J.; Wang, L.; Liu, Y.; Chen, Z.Z.; Han, L.H.; Qu, L.J.; Gong, Z.Z. DEXH Box RNA Helicase-Mediated Mitochondrial Reactive Oxygen Species Production in Arabidopsis Mediates Crosstalk between Abscisic Acid and Auxin Signaling. Plant Cell 2012, 24, 1815–1833. [Google Scholar] [CrossRef]
- Beaudoin, N.; Serizet, C.; Gosti, F.; Giraudat, J. Interactions Between Abscisic Acid and Ethylene Signaling Cascades. Plant Cell 2000, 12, 1103–1115. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Gantait, S.; Mitra, M.; Yang, Y.X.; Li, X. Role of Ethylene Crosstalk in Seed Germination and Early Seedling Development: A Review. Plant. Physiol. Bioch. 2020, 151, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Steber, C.M.; McCourt, P. A Role for Brassinosteroids in Germination in Arabidopsis. Plant. Physiol. 2001, 125, 763–769. [Google Scholar] [CrossRef]
- Kim, S.Y.; Warpeha, K.M.; Huber, S.C. The Brassinosteroid Receptor Kinase, BRI1, Plays a Role in Seed Germination and the Release of Dormancy by Cold Stratification. J. Plant. Physiol. 2019, 241, 153031. [Google Scholar] [CrossRef] [PubMed]
- Cukor, J.; Rasakova, N.M.; Linda, R.; Linhart, L.; Gutsch, M.R.; Kunes, I. Effects of Brassinosteroid Application on Seed Germination of Scots Pine under Standard and Heat Stress Conditions. Balt. For. 2018, 24, 60–67. [Google Scholar]
- Thibodeau, E.A.; Marquis, R.E. Ph Sensitivity of Glycolysis and Enolase Activity in Streptococci. J. Dent. Res. 1981, 60, 442. [Google Scholar]
- Andriotis, V.M.E.; Kruger, N.J.; Pike, M.J.; Smith, A.M. Plastidial Glycolysis in Developing Arabidopsis Embryos. New Phytol. 2010, 185, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Guo, Y.; Ohta, M.; Xiong, L.M.; Stevenson, B.; Zhu, J.K. LOS2, a Genetic Locus Required for Cold-Responsive Gene Transcription Encodes a Bi-Functional Enolase. EMBO J. 2002, 21, 2692–2702. [Google Scholar] [CrossRef]
- Eremina, M.; Rozhon, W.; Yang, S.Q.; Poppenberger, B. ENO2 Activity is Required for the Development and Reproductive Success of Plants, and is Feedback-Repressed by AtMBP-1. Plant J. 2015, 81, 895–906. [Google Scholar] [CrossRef]
- Kang, M.Y.; Abdelmageed, H.; Lee, S.; Reichert, A.; Mysore, K.S.; Allen, R.D. AtMBP-1, an Alternative Translation Product of LOS2, Affects Abscisic Acid Responses and is Modulated by the E3 Ubiquitin Ligase AtSAP5. Plant J. 2013, 76, 481–493. [Google Scholar] [CrossRef]
- Hu, Y.F.; Lu, Y.; Zhao, Y.; Zhou, D.X. Histone Acetylation Dynamics Integrates Metabolic Activity to Regulate Plant Response to Stress. Front. Plant Sci. 2019, 10, 1236. [Google Scholar] [CrossRef]
- Sarkar Das, S.; Yadav, S.; Singh, A.; Gautam, V.; Sarkar, A.K.; Nandi, A.K.; Karmakar, P.; Majee, M.; Sanan-Mishra, N. Expression Dynamics of miRNAs and their Targets in Seed Germination Conditions Reveals miRNA-ta-siRNA Crosstalk as Regulator of Seed Germination. Sci. Rep. 2018, 8, 1233. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Jin, J.; Qian, Q.; Huang, K.K.; Ding, Y. Small RNA and Degradome Profiling Reveals miRNA Regulation in the Seed Germination of Ancient Eudicot Nelumbo nucifera. BMC Genom. 2016, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell. 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Chen, X. Small RNAs in Development—Insights from Plants. Curr. Opin. Genet. Dev. 2012, 22, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, M.; Nath, U. Divergence in Patterns of Leaf Growth Polarity Is Associated with the Expression Divergence of miR396. Plant Cell 2015, 27, 2785–2799. [Google Scholar] [CrossRef]
- Guo, H.S.; Xie, Q.; Fei, J.F.; Chua, N.H. MicroRNA Directs mRNA Cleavage of the Transcription Factor NAC1 to Downregulate Auxin Signals for Arabidopsis Lateral Root Development. Plant Cell 2005, 17, 1376–1386. [Google Scholar] [CrossRef]
- Wollmann, H.; Mica, E.; Todesco, M.; Long, J.A.; Weigel, D. On Reconciling the Interactions Between APETALA2, miR172 and AGAMOUS with the ABC Model of Flower Development. Development 2010, 137, 3633–3642. [Google Scholar] [CrossRef]
- Smoczynska, A.; Szweykowska-Kulinska, Z. MicroRNA-Mediated Regulation of Flower Development in Grasses. Acta Biochim. Pol. 2016, 63, 687–692. [Google Scholar] [CrossRef]
- Willmann, M.R.; Mehalick, A.J.; Packer, R.L.; Jenik, P.D. MicroRNAs Regulate the Timing of Embryo Maturation in Arabidopsis. Plant. Physiol. 2011, 155, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kang, H. Expression and Functional Analyses of microRNA417 in Arabidopsis thaliana Under Stress Conditions. Plant. Physiol. Biochem. 2007, 45, 805–811. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwak, K.J.; Jung, H.J.; Lee, H.J.; Kang, H. MicroRNA402 Affects Seed Germination of Arabidopsis thaliana Under Stress Conditions via Targeting DEMETER-LIKE Protein3 mRNA. Plant Cell Physiol. 2010, 51, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Koh, C.; Feurtado, J.A.; Tsang, E.W.T.; Cutler, A.J. MicroRNAs and their Putative Targets in Brassica napus Seed Maturation. BMC Genom. 2013, 14, 1–25. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Muller, K.; Leubner-Metzger, G. First off the Mark: Early Seed Germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Chua, N.H. ABA Induction of miR159 Controls Transcript Levels of Two MYB Factors during Arabidopsis Seed Germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Molecular Plant 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Carrera-Castano, G.; Calleja-Cabrera, J.; Pernas, M.; Gomez, L.; Onate-Sanchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant. Sci. 2018, 9, 251. [Google Scholar] [CrossRef]
- Penfield, S. Seed Dormancy and Germination. Curr. Opin. Plant Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef]
- Ravindran, P.; Kumar, P.P. Regulation of Seed Germination: The Involvement of Multiple Forces Exerted via Gibberellic Acid Signaling. Mol. Plant. 2019, 12, 1416–1417. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Chen, Z. The Pivotal Role of Abscisic Acid Signaling during Transition from Seed Maturation to Germination. Plant Cell Rep. 2017, 36, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Curaba, J.; Moritz, T.; Blervaque, R.; Parcy, F.; Raz, V.; Herzog, M.; Vachon, G. AtGA3ox2, a Key Gene Responsible for Bioactive Gibberellin Biosynthesis, is Regulated During Embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis. Plant Physiol. 2004, 136, 3660–3669. [Google Scholar] [CrossRef]
- Martin, M.L.; Lechner, L.; Zabaleta, E.J.; Salerno, G.L. A Mitochondrial Alkaline/Neutral Invertase Isoform (A/N-InvC) Functions in Developmental Energy-Demanding Processes in Arabidopsis. Planta 2013, 237, 813–822. [Google Scholar] [CrossRef]
- Liu, S.; Jia, J.; Gao, Y.; Zhang, B.; Han, Y. The AtTudor2, a Protein with SN-Tudor Domains, is Involved in Control of Seed Germination in Arabidopsis. Planta 2010, 232, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Song, Z.; Nikolau, B.J. Reverse Genetic Characterization of Two Paralogous Acetoacetyl CoA Thiolase Genes in Arabidopsis Reveals their Importance in Plant Growth and Development. Plant J. 2012, 70, 1015–1032. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.H.; Seo, D.H.; Chung, S.; Kim, S.W.; Lee, J.S.; Kim, W.T.; Lee, J.H. ABA-HYPERSENSITIVE BTB/POZ PROTEIN 1 Functions as a Negative Regulator in ABA-Mediated Inhibition of Germination in Arabidopsis. Plant Mol. Biol. 2016, 90, 303–315. [Google Scholar] [CrossRef]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Basic Leucine Zipper Transcription Factors Involved in an Abscisic Acid-Dependent Signal Transduction Pathway under Drought and High-Salinity Conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef]
- Reinhardt, D. Vascular Patterning: More than Just Auxin? Curr. Biol. 2003, 13, R485–R487. [Google Scholar] [CrossRef]
- Bohn-Courseau, I. Auxin: A Major Regulator of Organogenesis. Cr. Biol. 2010, 333, 290–296. [Google Scholar] [CrossRef]
- Benjamins, R.; Scheres, B. Auxin: The Looping Star in Plant Development. Annu. Rev. Plant. Biol. 2008, 59, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G.; Dole, I.; Celikel, F.G.; Harkema, H. Opening of Iris Flowers is Regulated by Endogenous Auxins. J. Plant. Physiol. 2013, 170, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Dai, X.H.; Zhao, Y.D. Auxin Biosynthesis by the YUCCA Flavin Monooxygenases Controls the Formation of Floral Organs and Vascular Tissues in Arab. Gene Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Ikezaki, M.; Fujibe, T.; Aida, M.; Tian, C.; Ueno, Y.; Yamamoto, K.T.; Machida, Y.; Nakamura, K.; Ishiguro, S. Arabidopsis AUXIN RESPONSE FACTOR6 and 8 Regulate Jasmonic Acid Biosynthesis and Floral Organ Development via Repression of Class. 1 KNOX Genes. Plant. Cell Physiol. 2010, 51, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Moller, B.K.; ten Hove, C.A.; Xiang, D.Q.; Williams, N.; Lopez, L.G.; Yoshida, S.; Smit, M.; Datla, R.; Weijers, D. Auxin Response cell-Autonomously Controls Ground Tissue Initiation in the Early Arabidopsis Embryo. Proc. Natl. Acad. Sci. USA 2017, 114, E2533–E2539. [Google Scholar] [CrossRef]
- Schruff, M.C.; Spielman, M.; Tiwari, S.; Adams, S.; Fenby, N.; Scott, R.J. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis Links Auxin Signalling, Cell Division, and the Size of Seeds and Other Organs. Development 2006, 133, 251–261. [Google Scholar] [CrossRef]
- Chen, Z.H.; Bao, M.L.; Sun, Y.Z.; Yang, Y.J.; Xu, X.H.; Wang, J.H.; Han, N.; Bian, H.W.; Zhu, M.Y. Regulation of Auxin Response by miR393-Targeted Transport Inhibitor Response Protein 1 is Involved in Normal Development in Arabidopsis. Plant Mol. Biol. 2011, 77, 619–629. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, L.; Ye, T.T.; Zhao, S.J.; Liu, Z.; Feng, Y.Q.; Wu, Y. Cytokinin Antagonizes ABA Suppression to Seed Germination of Arabidopsis by Downregulating ABI5 Expression. Plant. J. 2011, 68, 249–261. [Google Scholar] [CrossRef]
- Huang, X.Z.; Zhang, X.Y.; Gong, Z.Z.; Yang, S.H.; Shi, Y.T. ABI4 Represses the Expression of type-A ARRs to Inhibit Seed Germination in Arab. Plant J. 2017, 89, 354–365. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kravets, V.; Ruelland, E. Salicylic Acid Binding Proteins (SABPs): The Hidden Forefront of Salicylic Acid Signalling. Int. J. Mol. Sci. 2019, 20, 4377. [Google Scholar] [CrossRef]
- Ali, M.; Hayat, S.; Ahmad, H.; Ghani, M.I.; Amin, B.; Atif, M.J.; Cheng, Z. Priming of Solanum melongena L. Seeds Enhances Germination, Alters Antioxidant Enzymes, Modulates ROS, and Improves Early Seedling Growth: Indicating Aqueous Garlic Extract as Seed-Priming Bio-Stimulant for Eggplant Production. Appl. Sci. 2019, 9, 2203. [Google Scholar] [CrossRef]
- Li, Y.-F.; Huang, L.-L.; Liu, X.-L.; Li, X.-S.; Tan, H.-H. Exogenous Salicylic Acid Alleviates Halosulfuron-Methyl Toxicity by Coordinating the Antioxidant System and Improving Photosynthesis in Soybean (Glycine max Merr.). Acta Physiol. Plant 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.T.; Cai, S.Y.; Ruan, X.L.; Chen, S.Y.; Mei, G.F.; Ruan, G.H.; Cao, D.D. Salicylic Acid Enhances Sunflower Seed Germination under Zn2+ Stress via Involvement in Zn2+ Metabolic Balance and Phytohormone Interactions. Sci. Hortic Amst. 2021, 275, 109702. [Google Scholar] [CrossRef]
- Budimir, J.; Treffon, K.; Nair, A.; Thurow, C.; Gatz, C. Redox-active Cysteines in TGACG-BINDING FACTOR 1 (TGA1) do not Play a Role in Salicylic Acid or Pathogen-Induced Expression of TGA1-Regulated Target Genes in Arabidopsis thaliana. New Phytol. 2020. [Google Scholar] [CrossRef]
- Chung, P.J.; Park, B.S.; Wang, H.; Liu, J.; Jang, I.C.; Chua, N.H. Light-Inducible MiR163 Targets PXMT1 Transcripts to Promote Seed Germination and Primary Root Elongation in Arab. Plant Physiol. 2016, 170, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, X.; Hong, Z. Precocious Pollen Germination in Arabidopsis Plants with Altered Callose Deposition during Microsporogenesis. Planta 2010, 231, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef]
- Burge, S.W.; Daub, J.; Eberhardt, R.; Tate, J.; Barquist, L.; Nawrocki, E.P.; Eddy, S.R.; Gardner, P.P.; Bateman, A. Rfam 11.0: 10 Years of RNA Families. Nucleic Acids Res. 2013, 41, D226–D232. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, J.M.; Liu, G.; Zhang, J.B.; Wang, J.Y.; Liu, C.K.; Fang, M.Y. A Comprehensive microRNA Expression Profile of the Backfat Tissue from Castrated and Intact Full-Sib Pair Male Pigs. BMC Genom. 2014, 15, 47. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Unique (WT) | Abundance (WT) | Unique (eno2−) | Abundance (eno2−) | |
|---|---|---|---|---|
| rRNA | 66,175 (6.28%) | 2,068,165 (21.41%) | 58,311 (5.73%) | 2,196,583 (21.81%) |
| scRNA | 741 (0.07%) | 5638 (0.06%) | 784 (0.08%) | 8955 (0.09%) |
| snRNA | 20,029 (1.90%) | 1,533,088 (15.87%) | 19,108 (1.88%) | 1,306,885 (12.98%) |
| snoRNA | 2916 (0.28%) | 18,664 (0.19%) | 3127 (0.31%) | 24,790 (0.25%) |
| tRNA | 4756 (0.45%) | 241,070 (2.50%) | 4960 (0.49%) | 350,910 (3.48%) |
| exon_sense | 73,972 (7.02%) | 587,871 (6.08%) | 61,192 (6.02%) | 528,694 (5.25%) |
| miRNA | 5407 (0.51%) | 1,320,827 (13.68%) | 5629 (0.56%) | 1,532,594 (15.22%) |
| unann | 714,821 (67.84%) | 2,733,234 (28.29%) | 662,205 (65.11%) | 2,632,778 (26.14%) |
| total | 1,053,660 | 9,661,299 | 1,017,006 | 10,071,178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zheng, L.; Bing, J.; Liu, H.; Zhang, G. Deep Sequencing of Small RNA Reveals the Molecular Regulatory Network of AtENO2 Regulating Seed Germination. Int. J. Mol. Sci. 2021, 22, 5088. https://doi.org/10.3390/ijms22105088
Wu Y, Zheng L, Bing J, Liu H, Zhang G. Deep Sequencing of Small RNA Reveals the Molecular Regulatory Network of AtENO2 Regulating Seed Germination. International Journal of Molecular Sciences. 2021; 22(10):5088. https://doi.org/10.3390/ijms22105088
Chicago/Turabian StyleWu, Yu, Lamei Zheng, Jie Bing, Huimin Liu, and Genfa Zhang. 2021. "Deep Sequencing of Small RNA Reveals the Molecular Regulatory Network of AtENO2 Regulating Seed Germination" International Journal of Molecular Sciences 22, no. 10: 5088. https://doi.org/10.3390/ijms22105088
APA StyleWu, Y., Zheng, L., Bing, J., Liu, H., & Zhang, G. (2021). Deep Sequencing of Small RNA Reveals the Molecular Regulatory Network of AtENO2 Regulating Seed Germination. International Journal of Molecular Sciences, 22(10), 5088. https://doi.org/10.3390/ijms22105088






