Probiotic Administration Mitigates Bisphenol A Reproductive Toxicity in Zebrafish
Abstract
:1. Introduction
2. Results
2.1. Fertility
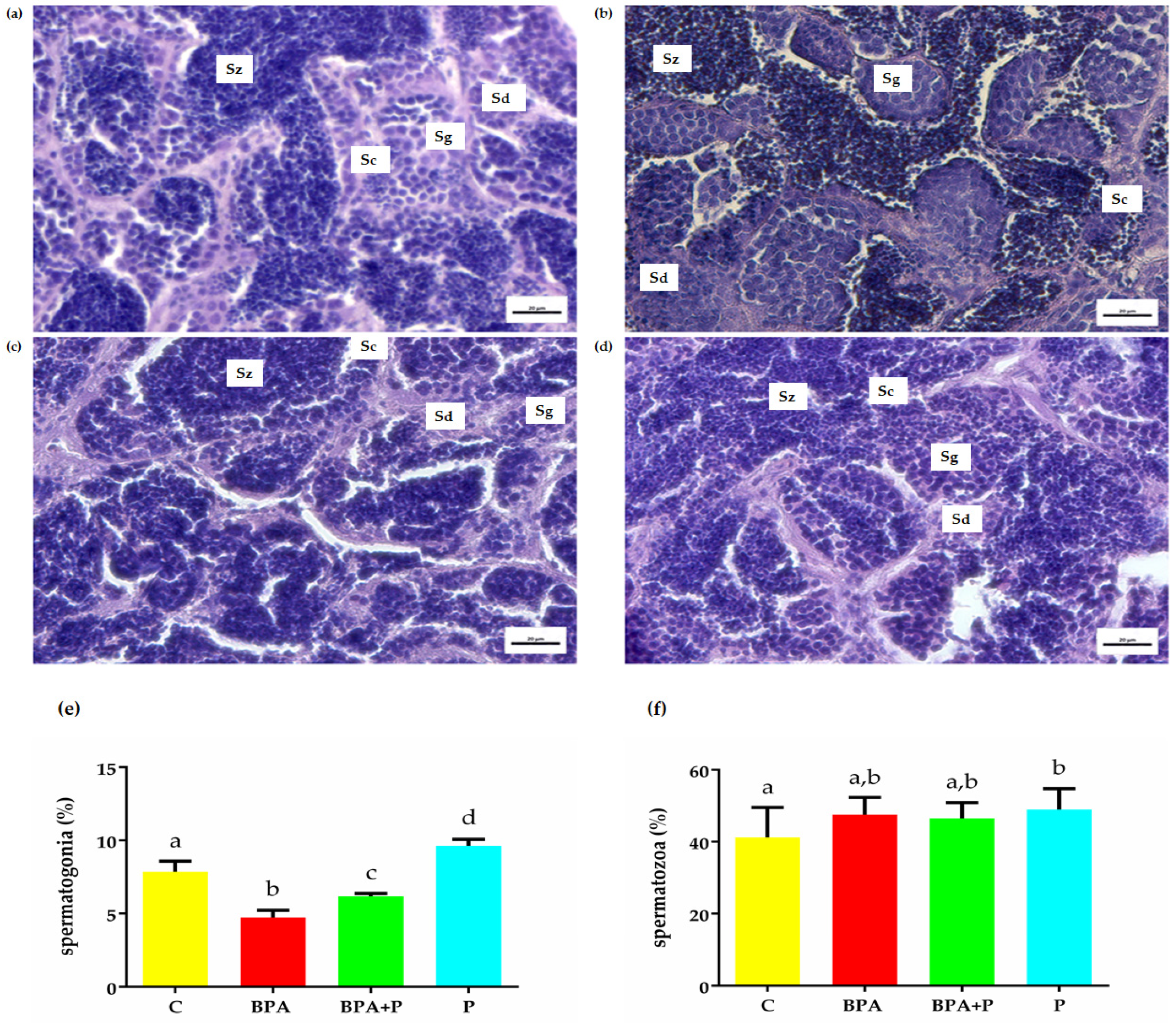
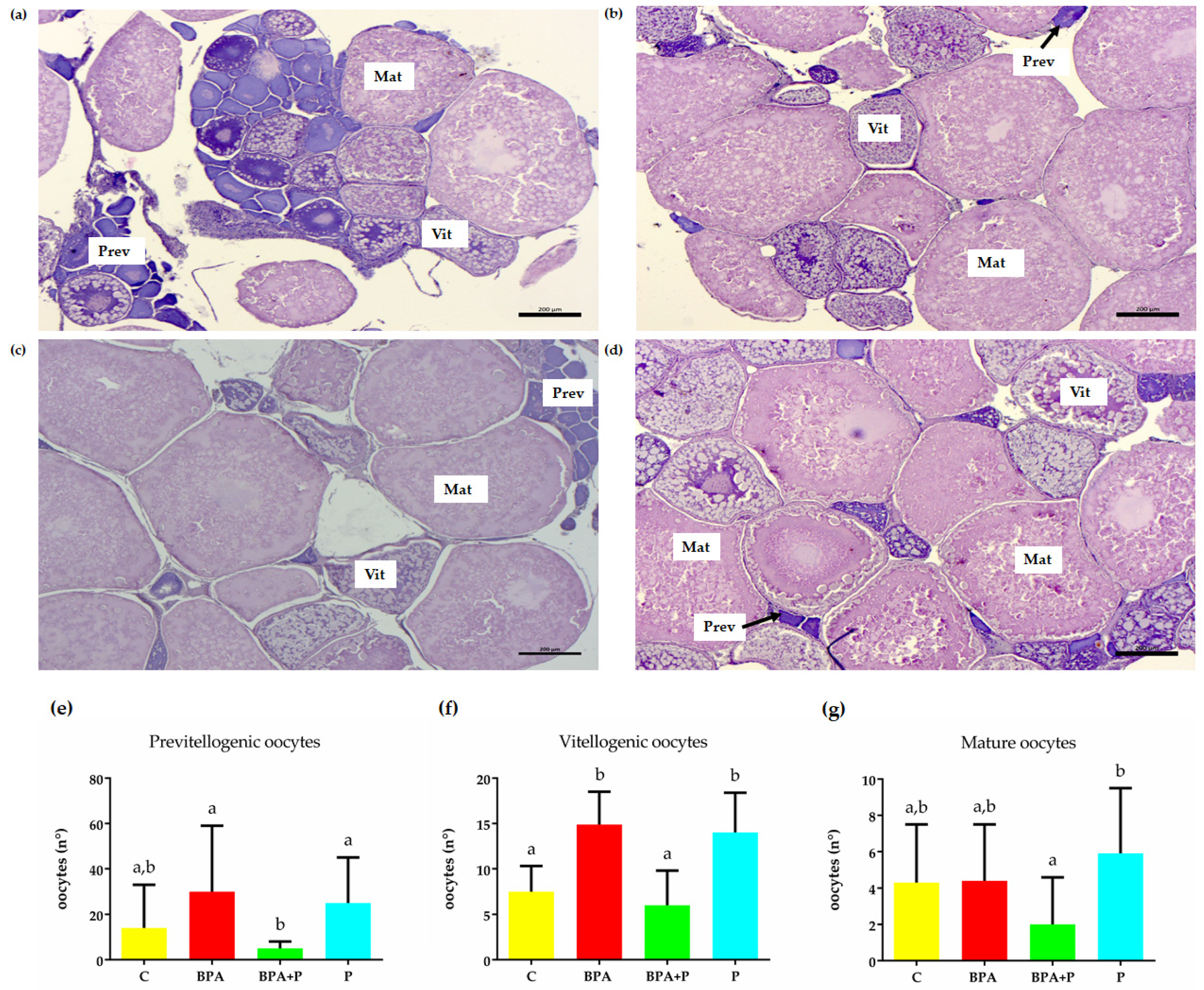
2.2. Gonadal Histological Analysis
2.3. Real Time PCR Analisys
2.3.1. Hepatic Vitellogenin (vtg) Transcription
2.3.2. Transcript of Genes Involved in Spermatogenesis
2.3.3. Transcript of Genes Involved in Follicle Growth and Maturation
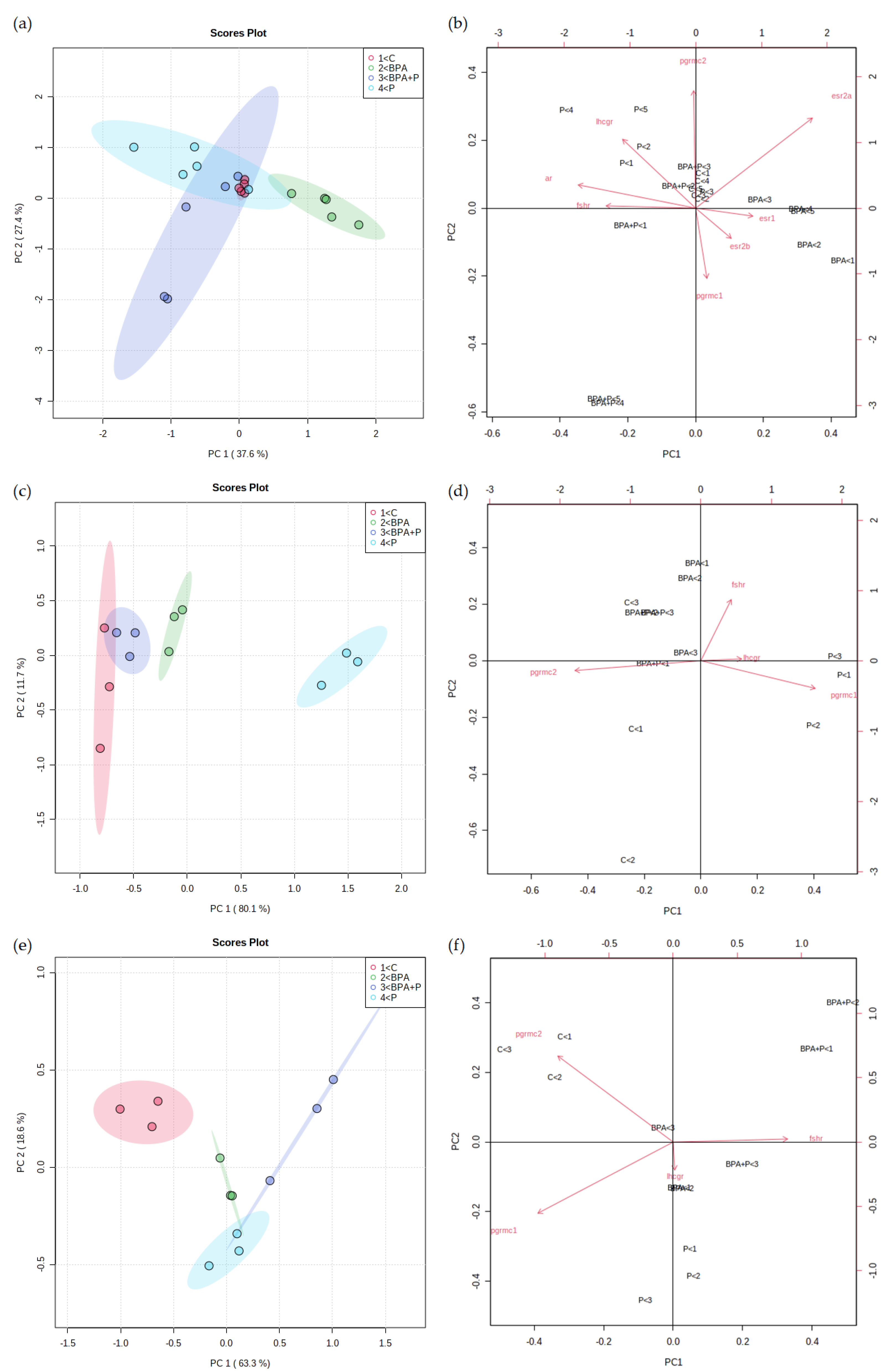
2.3.4. Multivariate Statistical Analysis
3. Discussion
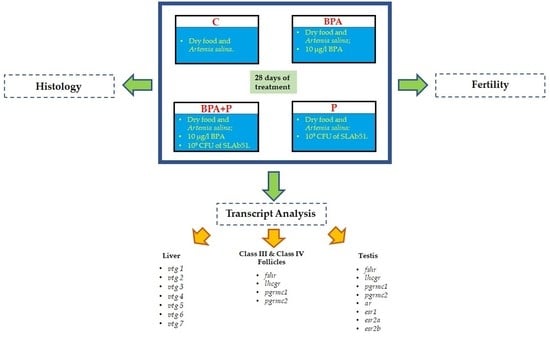
4. Materials and Methods
4.1. SLAb51® (SivoMixx®)
4.2. Animal Treatment
4.3. Fish Fertility
4.4. Gonad Histology and Image Analysis
4.5. RNA Extraction and cDNA Synthesis
4.6. Real-Time PCR
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Maradonna, F.; Carnevali, O. Lipid Metabolism Alteration by Endocrine Disruptors in Animal Models: An Overview. Front. Endocrinol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Carnevali, O.; Santangeli, S.; Forner-Piquer, I.; Basili, D.; Maradonna, F. Endocrine-disrupting chemicals in aquatic environment: What are the risks for fish gametes? Fish Physiol. Biochem. 2018, 44, 1561–1576. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Fan, X.; Dong, C.; Wang, G.; Wang, Z. Chronic exposure to Bisphenol A resulted in alterations of reproductive functions via immune defense, oxidative damage and disruption DNA/histone methylation in male rare minnow Gobiocypris rarus. Aquat. Toxicol. 2021, 236, 105849. [Google Scholar] [CrossRef]
- Kaimal, A.; Al Mansi, M.H.; Dagher, J.B.; Pope, C.; Varghese, M.G.; Rudi, T.B.; Almond, A.E.; Cagle, L.A.; Beyene, H.K.; Bradford, W.T.; et al. Prenatal exposure to bisphenols affects pregnancy outcomes and offspring development in rats. Chemosphere 2021, 276, 130118. [Google Scholar] [CrossRef]
- Lin, M.; Hua, R.; Ma, J.; Zhou, Y.; Li, P.; Xu, X.; Yu, Z.; Quan, S. Bisphenol A promotes autophagy in ovarian granulosa cells by inducing AMPK/mTOR/ULK1 signalling pathway. Environ. Int. 2021, 147, 106298. [Google Scholar] [CrossRef]
- Mathew, H.; Mahalingaiah, S. Do prenatal exposures pose a real threat to ovarian function? Bisphenol A as a case study. Reproduction 2019, 157, R143–R157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangeli, S.; Maradonna, F.; Gioacchini, G.; Cobellis, G.; Piccinetti, C.C.; Dalla Valle, L.; Carnevali, O. BPA-Induced Deregulation Of Epigenetic Patterns: Effects On Female Zebrafish Reproduction. Sci. Rep. 2016, 6, 21982. [Google Scholar] [CrossRef]
- Santangeli, S.; Maradonna, F.; Olivotto, I.; Piccinetti, C.C.; Gioacchini, G.; Carnevali, O. Effects of BPA on female reproductive function: The involvement of epigenetic mechanism. Gen. Comp. Endocrinol. 2017, 245, 122–126. [Google Scholar] [CrossRef]
- Maradonna, F.; Nozzi, V.; Dalla Valle, L.; Traversi, I.; Gioacchini, G.; Benato, F.; Colletti, E.; Gallo, P.; Di Marco Pisciottano, I.; Mita, D.G.; et al. A developmental hepatotoxicity study of dietary bisphenol A in Sparus aurata juveniles. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 166, 1–13. [Google Scholar] [CrossRef]
- Maradonna, F.; Nozzi, V.; Santangeli, S.; Traversi, I.; Gallo, P.; Fattore, E.; Mita, D.G.; Mandich, A.; Carnevali, O. Xenobiotic-contaminated diets affect hepatic lipid metabolism: Implications for liver steatosis in Sparus aurata juveniles. Aquat. Toxicol. 2015, 167, 257–264. [Google Scholar] [CrossRef]
- Carnevali, O.; Giorgini, E.; Canuti, D.; Mylonas, C.C.; Forner-Piquer, I.; Maradonna, F. Diets contaminated with Bisphenol A and Di-isononyl phtalate modify skeletal muscle composition: A new target for environmental pollutant action. Sci. Total Environ. 2019, 658, 250–259. [Google Scholar] [CrossRef]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-friendly removal methods for endocrine disrupting chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Gioacchini, G.; Maradonna, F.; Lombardo, F.; Bizzaro, D.; Olivotto, I.; Carnevali, O. Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction 2010, 140, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevali, O.; Avella, M.A.; Gioacchini, G. Effects of probiotic administration on zebrafish development and reproduction. Gen. Comp. Endocrinol. 2012, 188, 297–302. [Google Scholar] [CrossRef]
- Giorgini, E.; Conti, C.; Ferraris, P.; Sabbatini, S.; Tosi, G.; Rubini, C.; Vaccari, L.; Gioacchini, G.; Carnevali, O. Effects of Lactobacillus rhamnosus on zebrafish oocyte maturation: An FTIR imaging and biochemical analysis. Anal. Bioanal. Chem. 2010, 398, 3063–3072. [Google Scholar] [CrossRef]
- Arani, M.M.; Salati, A.P.; Keyvanshokooh, S.; Safari, O. The effect of Pediococcus acidilactici on mucosal immune responses, growth, and reproductive performance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2021, 47, 153–162. [Google Scholar] [CrossRef]
- Hu, J.; Kim, Y.H.; Kim, I.H. Effects of two bacillus strains probiotic supplement on reproduction performance, nutrient digestibility, blood profile, fecal score, excreta odor contents and fecal microflora in lactation sows, and growth performance in sucking piglets. Livest. Sci. 2021, 244, 104293. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Borrazzo, C.; Pinacchio, C.; Santinelli, L.; Innocenti, G.P.; Cavallari, E.N.; Celani, L.; Marazzato, M.; Alessandri, F.; Ruberto, F.; et al. Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study. Front. Nutr. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 1–7. [Google Scholar]
- Cai, S.-S.; Zhou, Y.; Ye, B.-C. Reducing the reproductive toxicity activity of Lactiplantibacillus plantarum: A review of mechanisms and prospects. Environ. Sci. Pollut. Res. 2021, 1–15. [Google Scholar] [CrossRef]
- Taghizadeh Moghaddam, S.; Javadi, A.; Matin, A.A. Reduction of bisphenol A by Lactobacillus acidophilus and Lactobacillus plantarum in yoghurt. Int. J. Dairy Technol. 2020, 73, 737–742. [Google Scholar] [CrossRef]
- Tang, L.; Song, S.; Hu, C.; Lam, J.C.W.; Liu, M.; Zhou, B.; Lam, P.K.S.; Chen, L. Unexpected Observations: Probiotic Administration Greatly Aggravates the Reproductive Toxicity of Perfluorobutanesulfonate in Zebrafish. Chem. Res. Toxicol. 2020, 33, 1605–1608. [Google Scholar] [CrossRef]
- Rossi, G.; Pengo, G.; Galosi, L.; Berardi, S.; Tambella, A.M.; Attili, A.R.; Gavazza, A.; Cerquetella, M.; Jergens, A.E.; Guard, B.C.; et al. Effects of the Probiotic Mixture Slab51® (SivoMixx®) as Food Supplement in Healthy Dogs: Evaluation of Fecal Microbiota, Clinical Parameters and Immune Function. Front. Vet. Sci. 2020, 7, 7. [Google Scholar] [CrossRef]
- Castelli, V.; Angelo, M.; Lombardi, F.; Alfonsetti, M.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Grazia, M.; Giordano, A.; Desideri, G.; et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging 2020, 12, 4641–4659. [Google Scholar] [CrossRef]
- Forner-Piquer, I.; Beato, S.; Piscitelli, F.; Santangeli, S.; Di Marzo, V.; Habibi, H.R.; Maradonna, F.; Carnevali, O. Effects of BPA on zebrafish gonads: Focus on the endocannabinoid system. Environ. Pollut. 2020, 264, 114710. [Google Scholar] [CrossRef]
- Biran, J.; Levavi-Sivan, B. Endocrine Control of Reproduction, Fish. Encycl. Reprod. 2018, 63, 362–368. [Google Scholar]
- Yaron, Z.; Gur, G.; Melamed, P.; Rosenfeld, H.; Elizur, A.; Levavi-Sivan, B. Regulation of fish gonadotropins. Int. Rev. Cytol. 2003, 225, 131–185. [Google Scholar]
- Jin, P.; Wang, X.; Chang, F.; Bai, Y.; Li, Y.; Zhou, R.; Chen, L. Low dose bisphenol A impairs spermatogenesis by suppressing eproductive hormone production and promoting germ cell apoptosis in adult rats. J. Biomed. Res. 2013, 27, 135–144. [Google Scholar]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Turi, N.; Ullah, W.; Siddiqui, M.F.; Zakria, M.; Lodhi, K.Z.; Khan, M.M. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action. Food Chem. Toxicol. 2018, 121, 24–36. [Google Scholar] [CrossRef]
- Klenke, U.; Constantin, S.; Wray, S. BPA directly decreases gnrh neuronal activity via noncanonical pathway. Endocrinology 2016, 157, 1980–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellini, C.; Totaro, M.; Parisi, A.; D’Andrea, S.; Lucente, L.; Cordeschi, G.; Francavilla, S.; Francavilla, F.; Barbonetti, A. Bisphenol A and Male Fertility: Myths and Realities. Front. Endocrinol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Fallah, H.P.; Rodrigues, M.S.; Zanardini, M.; Nóbrega, R.H.; Habibi, H.R. Effects of gonadotropin-inhibitory hormone on early and late stages of spermatogenesis in ex-vivo culture of zebrafish testis. Mol. Cell. Endocrinol. 2021, 520, 111087. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Yamauchi, K.; Nagahama, Y.; Takahashi, H. Induction of spermatogenesis in male japanese eel, Anguilla japonica, by a single injection of human chorionic gonadotropin. Zoolog. Sci. 1991, 8, 63–73. [Google Scholar]
- Schulz, R.; de França, L.; Lareyre, J.; Le Gac, F.; Chiarini-Garcia, H.; Nobrega, R.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Ohta, T.; Miyake, H.; Miura, C.; Kamei, H.; Aida, K.; Miura, T. Follicle-stimulating hormone induces spermatogenesis mediated by androgen production in Japanese eel, Anguilla japonica. Biol. Reprod. 2007, 77, 970–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vílchez, M.C.; Santangeli, S.; Maradonna, F.; Gioacchini, G.; Verdenelli, C.; Gallego, V.; Peñaranda, D.S.; Tveiten, H.; Pérez, L.; Carnevali, O.; et al. Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis. Theriogenology 2015, 84, 1321–1331. [Google Scholar] [CrossRef]
- Migliaccio, M.; Chioccarelli, T.; Ambrosino, C.; Suglia, A.; Manfrevola, F.; Carnevali, O.; Fasano, S.; Pierantoni, R.; Cobellis, G. Characterization of follicular atresia responsive to BPA in zebrafish by morphometric analysis of follicular stage progression. Int. J. Endocrinol. 2018, 2018, 4298195. [Google Scholar] [CrossRef] [Green Version]
- Molina, A.M.; Lora, A.J.; Blanco, A.; Monterde, J.G.; Ayala, N.; Moyano, R. Endocrine-active compound evaluation: Qualitative and quantitative histomorphological assessment of zebrafish gonads after bisphenol-A exposure. Ecotoxicol. Environ. Saf. 2013, 88, 155–162. [Google Scholar] [CrossRef]
- Molina, A.M.; Abril, N.; Morales-Prieto, N.; Monterde, J.G.; Lora, A.J.; Ayala, N.; Moyano, R. Evaluation of toxicological endpoints in female zebrafish after bisphenol A exposure. Food Chem. Toxicol. 2018, 112, 19–25. [Google Scholar] [CrossRef]
- Gioacchini, G.; Marisaldi, L.; Basili, D.; Candelma, M.; Pignalosa, P.; Aiese Cigliano, R.; Sanseverino, W.; Hardiman, G.; Carnevali, O. A de novo transcriptome assembly approach elucidates the dynamics of ovarian maturation in the swordfish (Xiphias gladius). Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Miccoli, A.; Maradonna, F.; De Felice, A.; Caputo Barucchi, V.; Estonba, A.; Genangeli, M.; Vittori, S.; Leonori, I.; Carnevali, O. Detection of endocrine disrupting chemicals and evidence of their effects on the HPG axis of the European anchovy Engraulis encrasicolus. Mar. Environ. Res. 2017, 127, 137–147. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Nguyen, T.V.; Com, E.; Lavigne, R.; Pineau, C.; Sullivan, C.V.; Bobe, J. Scrambled eggs: Proteomic portraits and novel biomarkers of egg quality in zebrafish (Danio rerio). PLoS ONE 2017, 12, e0188084. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yan, T.; Tan, J.T.T.; Gong, Z. A zebrafish vitellogenin gene (vg3) encodes a novel vitellogenin without a phosvitin domain and may represent a primitive vertebrate vitellogenin gene. Gene 2000, 256, 303–310. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Com, E.; Pineau, C.; Bobe, J. Knock out of specific maternal vitellogenins in zebrafish (Danio rerio) evokes vital changes in egg proteomic profiles that resemble the phenotype of poor quality eggs. BMC Genom. 2021, 22, 308. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Nguyen, T.; Com, E.; Pineau, C.; Bobe, J. Genome editing reveals reproductive and developmental dependencies on specific types of vitellogenin in zebrafish (Danio rerio). Mol. Reprod. Dev. 2019, 86, 1168–1188. [Google Scholar] [CrossRef] [Green Version]
- Carnevali, O.; Maradonna, F.; Gioacchini, G. Integrated control of fish metabolism, wellbeing and reproduction: The role of probiotic. Aquaculture 2017, 472, 144–155. [Google Scholar] [CrossRef]
- Faheem, M.; Lone, K.P. Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish, Ctenopharyngodon idella. Braz. J. Pharm. Sci. 2018, 53, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Chen, J.; Li, Y.; Chen, Z.; Jiang, L.; Yang, M.; Wu, M. Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2016, 130, 93–102. [Google Scholar] [CrossRef]
- Clelland, E.; Peng, C. Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 2009, 312, 42–52. [Google Scholar] [CrossRef]
- Wu, X.J.; Thomas, P.; Zhu, Y. Pgrmc1 knockout impairs oocyte maturation in zebrafish. Front. Endocrinol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, N.C.; Pru, C.A.; Yee, S.P.; Lydon, J.P.; Peluso, J.J.; Pru, J.K. Conditional ablation of progesterone receptor membrane component 2 causes female premature reproductive senescence. Endocrinology 2017, 158, 640–651. [Google Scholar] [CrossRef]
- Wu, X.J.; Zhu, Y. Downregulation of nuclear progestin receptor (Pgr) and subfertility in double knockouts of progestin receptor membrane component 1 (pgrmc1) and pgrmc2 in zebrafish. Gen. Comp. Endocrinol. 2020, 285, 113275. [Google Scholar] [CrossRef]
- Hu, C.; Liu, M.; Tang, L.; Sun, B.; Huang, Z.; Chen, L. Probiotic Lactobacillus rhamnosus modulates the impacts of perfluorobutanesulfonate on oocyte developmental rhythm of zebrafish. Sci. Total Environ. 2021, 776, 1–9. [Google Scholar] [CrossRef]
- Hu, C.; Liu, M.; Tang, L.; Liu, H.; Sun, B.; Chen, L. Probiotic intervention mitigates the metabolic disturbances of perfluorobutanesulfonate along the gut-liver axis of zebrafish. Chemosphere 2021, 284, 131374. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.C.W.; Tang, L.; Tang, L.; Hu, C.; Liu, M.; Liu, M.; Lam, P.K.S.; Zhou, B. Probiotic Modulation of Lipid Metabolism Disorders Caused by Perfluorobutanesulfonate Pollution in Zebrafish. Environ. Sci. Technol. 2020, 54, 7494–7503. [Google Scholar] [CrossRef]
- Liu, M.; Tang, L.; Hu, C.; Sun, B.; Huang, Z.; Chen, L. Interaction between probiotic additive and perfluorobutanesulfonate pollutant on offspring growth and health after parental exposure using zebrafish. Ecotoxicol. Environ. Saf. 2021, 214, 112107. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Mi, Z.; Huo, R.; Zhou, T.; Hai, H.; Kwok, L.Y.; Sun, Z.; Chen, Y.; Zhang, H. Screening for Lactobacillus plantarum strains that possess organophosphorus pesticide-degrading activity and metabolomic analysis of phorate degradation. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A review on gut remediation of selected environmental contaminants: Possible roles of probiotics and gut microbiota. Nutrients 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; Ma, Y.; Huang, W.; Ling, Y.; Sun, L.; Wang, X.; Zeng, A.; Dahlgren, R.A.; Wang, C.; Wang, H. Dietary Lactobacillus plantarum ST-III alleviates the toxic effects of triclosan on zebrafish (Danio rerio) via gut microbiota modulation. Fish Shellfish Immunol. 2019, 84, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Maradonna, F.; Gioacchini, G.; Notarstefano, V.; Fontana, C.M.; Citton, F.; Valle, L.D.; Giorgini, E.; Carnevali, O. Knockout of the glucocorticoid receptor impairs reproduction in female zebrafish. Int. J. Mol. Sci. 2020, 21, 9073. [Google Scholar] [CrossRef]
- Selman, K.; Wallace, R.A.; Sarka, A.; Qi, X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J. Morphol. 1993, 218, 203–224. [Google Scholar] [CrossRef]
- Forner-Piquer, I.; Maradonna, F.; Gioacchini, G.; Santangeli, S.; Allarà, M.; Piscitelli, F.; Habibi, H.R.; Di Marzo, V.; Carnevali, O. Dose-Specific Effects of Di-Isononyl Phthalate on the Endocannabinoid System and on Liver of Female Zebrafish. Endocrinology 2017, 158, 3462–3476. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. Validities of mRNA quantification using recombinant RNA and recombinant. Biotechnol. Lett. 2001, 23, 275–282. [Google Scholar] [CrossRef]
| Female Liver | C | BPA | BPA+P | P |
|---|---|---|---|---|
| vtg1 | 7.89 ± 1.05 (a) | 3.25 ± 1.20 (b) | 1.82 ± 0.73 (c) | 5.36 ± 0.87 (d) |
| vtg2 | 1.69 ± 0.20 (a) | 2.00 ± 0.82 (a) | 1.51 ± 0.49 (a) | 2.52 ± 0.35 (a) |
| vtg3 | 4.52 ± 0.37 (a,b) | 3.69 ± 0.91 (a) | 5.03 ± 0.59 (b) | 6.49± 0.49 (c) |
| vtg4 | 3.51 ± 1.20 (a) | 3.26 ± 0.52 (a) | 2.78 ± 0.87 (a) | 8.66 ± 0.50 (b) |
| vtg5 | 2.10 ± 0.63 (a) | 2.37 ± 0.35 (a) | 4.41 ± 0.65 (b) | 5.08 ± 0.59 (b) |
| vtg6 | 8.39 ± 0.32 (a) | 3.29 ± 0.96 (b) | 3.46 ± 0.68 (b) | 9.39 ± 0.89 (a) |
| vtg7 | 2.59 ± 0.44 (a) | 11.42 ± 0.84 (b) | 6.08 ± 0.60 (c) | 23.02 ± 0.79 (d) |
| Testis | C | BPA | BPA+P | P |
|---|---|---|---|---|
| fshr | 10.77 ± 2.22 (a) | 2.82 ± 0.27 (b) | 7.3 ± 1.82 (a) | 9.72 ± 4.67 (a) |
| lhcgr | 6.9 ± 0.69 (a) | 3.92 ± 0.94 (b) | 3.45 ± 1.45 (b) | 5.6 ± 2.14 (a,b) |
| ar | 5.15 ± 0.76 (a) | 1.88 ± 0.69 (b) | 3.57 ± 2.10 (a,b) | 2.81 ± 1.64 (a,b) |
| esr1 | 8.41 ± 0.61 (a) | 8.57 ± 1.20 (a) | 3.4 ± 1.12 (b) | 3.03 ± 1.10 (b) |
| esr2a | 5.31 ± 0.84 (a) | 8.11 ± 1.52 (b) | 2.05 ± 0.70 (c) | 2.12 ± 1.41 (c) |
| esr2b | 6.17± 1.23 (a) | 4.03 ± 1.2 (a,b) | 2.05 ± 0.41 (b,c) | 1.1 ± 0.32 (c) |
| pgrmc1 | 5.60 ± 1.37 (a,b) | 7.96 ± 2.06 (a) | 5.52 ± 1.89 (a,b) | 2.63 ± 0.93 (b) |
| pgrmc2 | 7.34 ± 2.06 (a) | 3.99 ± 1.50 (b) | 5.64 ± 1.14 (a,b) | 3.46 ± 1.17 (b) |
| (a) | ||||
| Class III Follicles | C | BPA | BPA+P | P |
| fshr | 1.85 ± 0.80 (a) | 5.15 ± 0.85 (b) | 4.03 ± 0.12 (b) | 1.18 ± 0.25 (a) |
| lhcgr | 3.39 ± 0.82 (a) | 5.63 ± 0.19 (b) | 3.32 ± 0.4 (a) | 1.41 ± 0.57 (c) |
| pgrmc1 | 4.95 ± 0.07 (a) | 6.91 ± 0.20 (b) | 6.33 ± 1.19 (a,b) | 11.70 ± 0.02 (c) |
| pgrmc2 | 40.04 ± 5.60 (a) | 19.85 ± 1.54 (b) | 38.75 ± 1.90 (a) | 1.25 ± 0.36 (c) |
| (b) | ||||
| Class IV Follicles | C | BPA | BPA+P | P |
| fshr | 1.72 ± 0.68 (a) | 4.43 ± 0.77 (b) | 4.05 ± 0.24 (b,c) | 3.02 ± 0.49 (a,c) |
| lhcgr | 4.30 ± 0.27 (a) | 6.65 ± 0.36 (b) | 4.44 ± 0.29 (a) | 6.01 ± 0.47 (b) |
| pgrmc1 | 3.41 ± 0.77 (a) | 4.22 ± 0.69 (a) | 1.65 ± 0.92 (b) | 4.11 ± 0.84 (a) |
| pgrmc2 | 33.21 ± 1.82 (a) | 24.79 ± 4.68 (b) | 15.37 ± 0.66 (c) | 16.45 ± 0.35 (c) |
| Gene Name | Symbol | Forward | Reverse | Source |
|---|---|---|---|---|
| Vitellogenin 1 | vtg 1 | GATTAAGCGTACACTGAGACCA | AGCCACTTCTTGTCCAAATACT | [61] |
| Vitellogenin 2 | vtg 2 | TGCCGCATGAAACTTGAATCT | GTTCTTACTGGTGCACAGCC | [61] |
| Vitellogenin 3 | vtg 3 | GGGAAAGGATTCAAGATGTTCAGA | ATTTGCTGATTTCAACTGGGAGAC | [61] |
| Vitellogenin 4 | vtg 4 | TCCAGACGGTACTTTCACCA | CTGACAGTTCTGCATCAACACA | [61] |
| Vitellogenin 5 | vtg 5 | ATTGCCAAGAAAGAGCCCAA | TTCAGCCTCAAACAGCACAA | [61] |
| Vitellogenin 6 | vtg 6 | TTTGGTGTGAGAACTGGAGG | CCAGTTTGTGAGTGCTTTCAG | [61] |
| Vitellogenin 7 | vtg 7 | TTGGTGTGAGAACTGGAGGA | TTGCAAGTGCCTTCAGTGTA | [61] |
| Luteinizing hormone receptor | lhcgr | GGCGAAGGCTAGATGGCACAT | TCGCAATCTGGTTCATCAATA | [8] |
| Follicle stimulating hormone | fshr | GGATTCTTCACCGTCTTCTCC | TGTAGCTGCTCAACTCAAACA | [8] |
| Estrogen recptor 1 | esr1 | GGTCCAGTGTGGTGTCCTCT | AGAAAGCTTTGCATCCCTCA | [8] |
| Estrogen receptor 2a | esr2a | TTGTGTTCTCCAGCATGAGC | CCACATATGGGGAAGGAATG | [8] |
| Estrogen receptor 2b | esr2b | TAGTGGGACTTGGACCGAAC | TTCACACGACCACACTCCAT | [8] |
| Membrane-associated progesterone receptor component 1 | pgrmc1 | CGGTTGTGATGGAGCAGATT | AGTAGCGCCAGTTCTGGTCA | [8] |
| Membrane-associated progesterone receptor component 2 | pgrmc2 | ACAACGAGCTGCTGAATGTG | ATGGGCCAGTTCAGAGTGAG | [8] |
| Androgen receptor | ar | ACTGGGACCGAATAAAGCCC | ATGTAATCGCAGCCGGAGAC | [8] |
| Ribosomal protein 13 | rpl13 | TCTGGAGACTGTAAGAGGTATGC | AGACGCACAATCTTGAGAGCAG | [8] |
| Ribosomal protein large, P0 | rplp0 | CTGAACATCTCGCCCTTCTC | TAGCCGATCTGCAGACACAC | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giommi, C.; Habibi, H.R.; Candelma, M.; Carnevali, O.; Maradonna, F. Probiotic Administration Mitigates Bisphenol A Reproductive Toxicity in Zebrafish. Int. J. Mol. Sci. 2021, 22, 9314. https://doi.org/10.3390/ijms22179314
Giommi C, Habibi HR, Candelma M, Carnevali O, Maradonna F. Probiotic Administration Mitigates Bisphenol A Reproductive Toxicity in Zebrafish. International Journal of Molecular Sciences. 2021; 22(17):9314. https://doi.org/10.3390/ijms22179314
Chicago/Turabian StyleGiommi, Christian, Hamid R. Habibi, Michela Candelma, Oliana Carnevali, and Francesca Maradonna. 2021. "Probiotic Administration Mitigates Bisphenol A Reproductive Toxicity in Zebrafish" International Journal of Molecular Sciences 22, no. 17: 9314. https://doi.org/10.3390/ijms22179314
APA StyleGiommi, C., Habibi, H. R., Candelma, M., Carnevali, O., & Maradonna, F. (2021). Probiotic Administration Mitigates Bisphenol A Reproductive Toxicity in Zebrafish. International Journal of Molecular Sciences, 22(17), 9314. https://doi.org/10.3390/ijms22179314