Amelogenin-Derived Peptides in Bone Regeneration: A Systematic Review
Abstract
1. Introduction
2. Methods
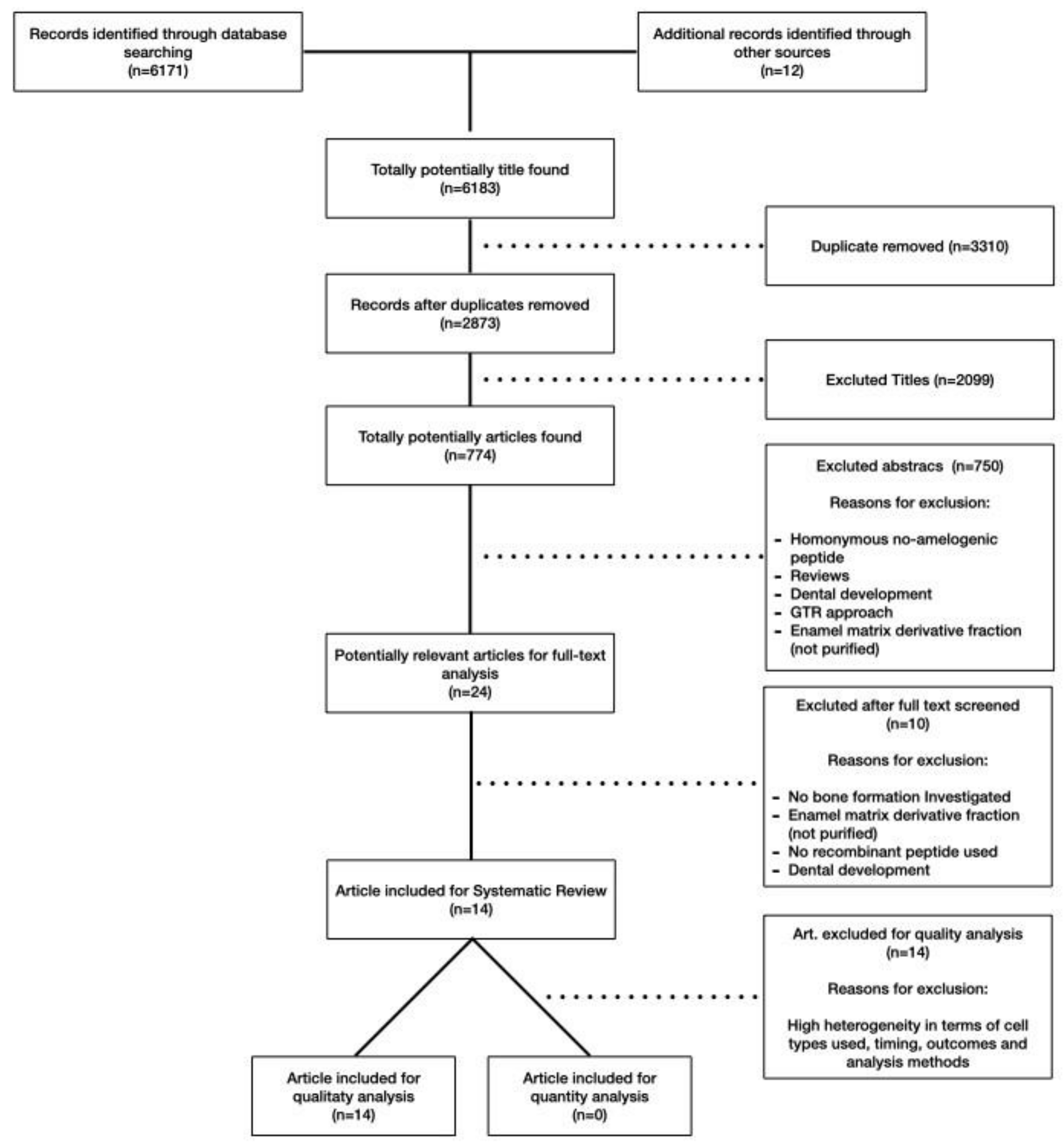
2.1. Search Strategy and Literature Screening
2.2. Exclusion Criteria
- Articles not written in English;
- Letters;
- Duplicate publications (the article with the most recent data was preferred);
- Dental development;
- Guided tissue regeneration approach;
- Lacking mineral deposition or histomorphometric analysis;
- Bone formation not investigated.
2.3. Study Selection and Data Extraction
3. Results and Discussion
3.1. Systematic Review
3.2. Amelogenins
3.2.1. Proteins and Genes
3.2.2. Biology and Translational Research
3.3. LRAP
3.3.1. LRAP as a Cell Agonist
3.3.2. LRAP Candidate Receptors
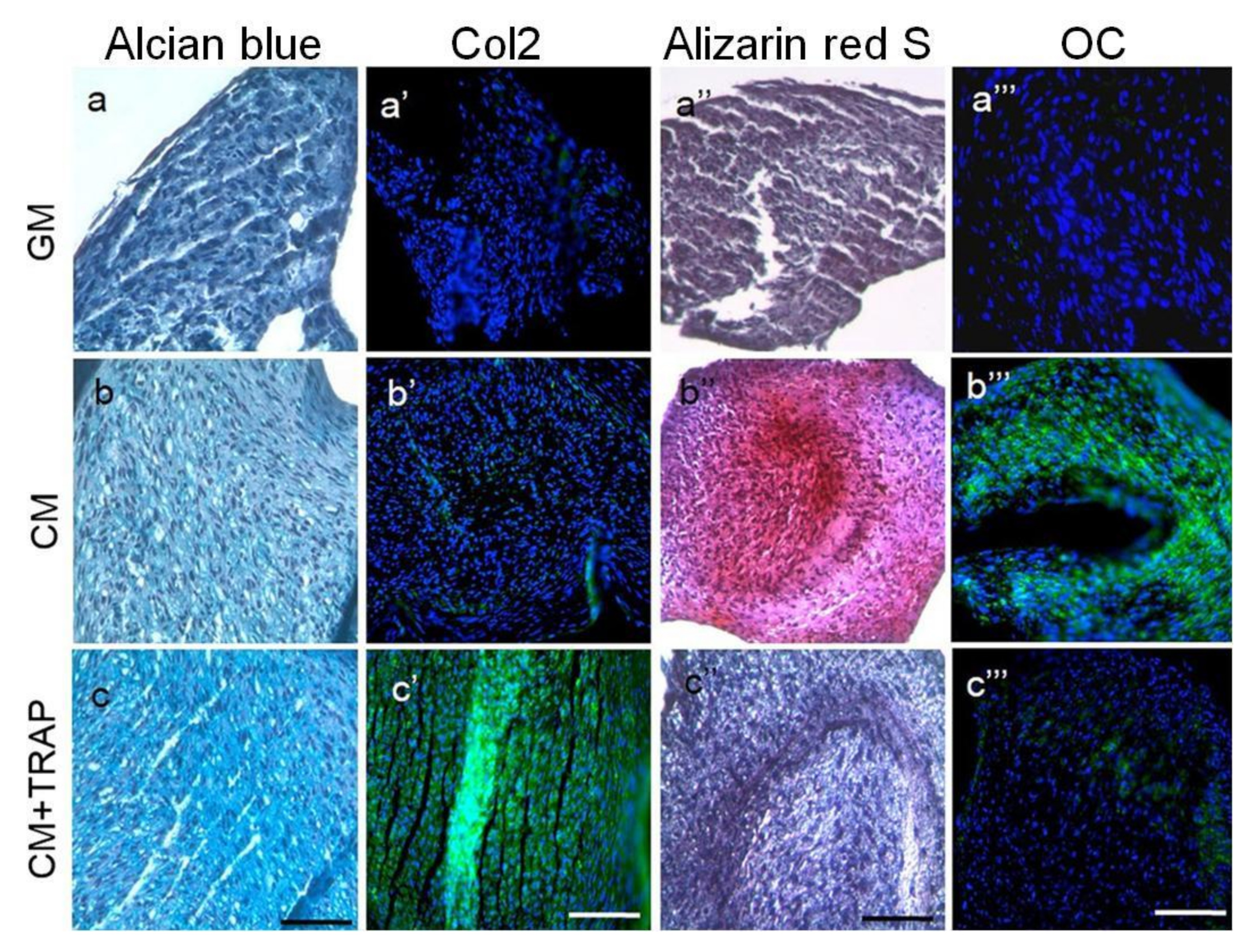
3.4. TRAP
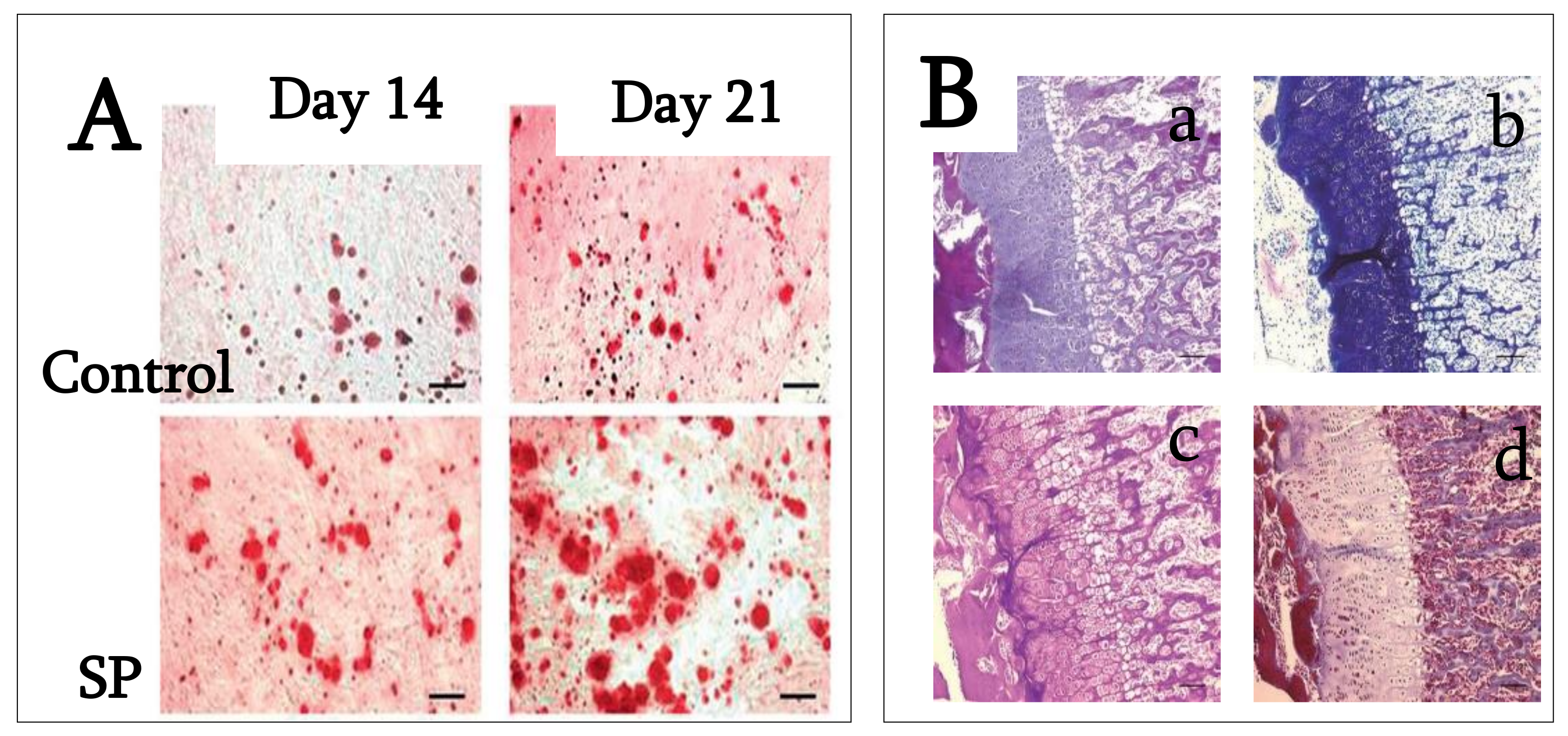
3.5. SP (Synthetic Peptide)
3.6. C11 (Amelogenin C Peptide, AMG-CP)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schliephake, H. Bone growth factors in maxillofacial skeletal reconstruction. Int. J. Oral Maxillofac. Surg. 2002, 31, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Margolis, H.C.; Beniash, E.; Fowler, C.E. Role of Macromolecular Assembly of Enamel Matrix Proteins in Enamel Formation. J. Dent. Res. 2006, 85, 775–793. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Slavkin, H.C.; Bessem, C.; Fincham, A.G.; Bringas, P.; Santos, V.; Snead, M.L.; Zeichner-David, M. Human and mouse cementum proteins immunologically related to enamel proteins. Biochim. Biophys. Acta-Gen. Subj. 1989, 991, 12–18. [Google Scholar] [CrossRef]
- Hammarström, L. The role of enamel matrix proteins in the development of cementum and periodontal tissues. Ciba Found. Symp. 1997, 205, 246–255. [Google Scholar]
- Fong, C.D.; Hammarström, L. Expression of amelin and amelogenin in epithelial root sheath remnants of fully formed rat molars. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 2000, 90, 218–223. [Google Scholar] [CrossRef]
- Sonoyama, W.; Seo, B.-M.; Yamaza, T.; Shi, S. Human Hertwig’s Epithelial Root Sheath Cells Play Crucial Roles in Cementum Formation. J. Dent. Res. 2007, 86, 594–599. [Google Scholar] [CrossRef]
- Warotayanont, R.; Frenkel, B.; Snead, M.L.; Zhou, Y. Leucine-rich amelogenin peptide induces osteogenesis by activation of the Wnt pathway. Biochem. Biophys. Res. Commun. 2009, 387, 558–563. [Google Scholar] [CrossRef]
- Haze, A.; Taylor, A.L.; Blumenfeld, A.; Rosenfeld, E.; Leiser, Y.; Dafni, L.; Shay, B.; Gruenbaum-Cohen, Y.; Fermon, E.; Haegewald, S.; et al. Amelogenin expression in long bone and cartilage cells and in bone marrow progenitor cells. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 455–460. [Google Scholar] [CrossRef]
- Deutsch, D.; Haze-Filderman, A.; Blumenfeld, A.; Dafni, L.; Leiser, Y.; Shay, B.; Gruenbaum-Cohen, Y.; Rosenfeld, E.; Fermon, E.; Zimmermann, B.; et al. Amelogenin, a major structural protein in mineralizing enamel, is also expressed in soft tissues: Brain and cells of the hematopoietic system. Eur. J. Oral Sci. 2006, 114, 183–189. [Google Scholar] [CrossRef]
- Veis, A.; Tompkins, K.; Alvares, K.; Wei, K.; Wang, L.; Wang, X.S.; Brownell, A.G.; Jengh, S.-M.; Healy, K.E. Specific Amelogenin Gene Splice Products Have Signaling Effects on Cells in Culture and in Implants in Vivo. J. Biol. Chem. 2000, 275, 41263–41272. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.; Veis, A. Polypeptides translated from alternatively spliced transcripts of the amelogenin gene, devoid of the exon 6a, b, c region, have specific effects on tooth germ development in culture. Connect. Tissue Res. 2002, 43, 224–231. [Google Scholar] [CrossRef]
- Veis, A. Amelogenin gene splice products: Potential signaling molecules. Cell. Mol. Life Sci. 2003, 60, 38–55. [Google Scholar] [CrossRef]
- Tompkins, K.; Alvares, K.; George, A.; Veis, A. Two related low molecular mass polypeptide isoforms of amelogenin have dis-tinct activities in mouse tooth germ differentiation in vitro. J. Bone Miner. Res. 2005, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone histogenesis and morphogenesis in implants of demineralized enamel and dentin. J. Oral. Surg. 1971, 29, 88–102. [Google Scholar] [PubMed]
- Yeomans, D.J.; Urist, M.R. Bone induction by decalcified dentin implanted into oral osseous and muscle tissues. Arch. Oral Biol. 1967, 12, 999–1008. [Google Scholar] [CrossRef]
- Nebgen, D.; Inoue, H.; Sabsay, B.; Wei, K.; Ho, C.-S.; Veis, A. Identification of the chondrogenic-inducing activity from bovine dentin (bCIA) as a low-molecular-mass amelogenin polypeptide. J. Dent. Res. 1999, 78, 1484–1494. [Google Scholar] [CrossRef]
- Fincham, A.; Moradianoldak, J. Amelogenin Post-translational Modifications: Carboxy-Terminal Processing and the Phosphorylation of Bovine and Porcine “TRAP” and “LRAP” Amelogenins. Biochem. Biophys. Res. Commun. 1993, 197, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Kakegawa, A.; Yamakoshi, Y.; Tsuchiya, S.; Hu, J.-C.; Gomi, K.; Arai, T.; Bartlett, J.; Simmer, J. Mmp-20 and Klk4 Cleavage Site Preferences for Amelogenin Sequences. J. Dent. Res. 2009, 88, 823–828. [Google Scholar] [CrossRef]
- Haruyama, N.; Hatakeyama, J.; Moriyama, K.; Kulkarni, A.B. Amelogenins: Multi-Functional Enamel Matrix Proteins and Their Binding Partners. J. Oral Biosci. 2011, 53, 257–266. [Google Scholar] [CrossRef]
- Grandin, H.M.; Gemperli, A.C.; Dard, M. Enamel matrix derivative: A review of cellular effects in vitro and a model of mo-lecular arrangement and functioning. Tissue Eng. Part B. Rev. 2012, 18, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Wyganowska-Swiatkowska, M.; Urbaniak, P.; Nohawica, M.; Kotwicka, M.; Jankun, J. Enamel matrix proteins exhibit growth factor activity: A review of evidence at the cellular and molecular levels. Exp. Ther. Med. 2015, 9, 2025–2033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giannobile, W.V.; Somerman, M.J. Growth and Amelogenin-Like Factors in Periodontal Wound Healing. A Systematic Re-view. Ann. Periodontol. 2003, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Boabaid, F.; Gibson, C.W.; Kuehl, M.A.; Berry, J.E.; Snead, M.L.; Nociti, F.H.; Katchburian, E.; Somerman, M.J. Leucine-Rich Amelogenin Peptide: A Candidate Signaling Molecule During Cementogenesis. J. Periodontol. 2004, 75, 1126–1136. [Google Scholar] [CrossRef]
- Le Norcy, E.; Kwak, S.Y.; Wiedemann-Bidlack, F.B.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Margolis, H.C. Leucine-rich amelogenin peptides regulate mineralization in vitro. J. Dent. Res. 2011, 90, 1091–1097. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Philp, D.; Haruyama, N.; Shum, L.; Aragon, M.; Yuan, Z.; Gibson, C.; Sreenath, T.; Kleinman, H.; Kulkarni, A.; et al. Amelogenin-mediated Regulation of Osteoclastogenesis, and Periodontal Cell Proliferation and Migration. J. Dent. Res. 2006, 85, 144–149. [Google Scholar] [CrossRef]
- Wang, H.-J.; Tannukit, S.; Wen, X.; Shapiro, J.L.; Snead, M.L.; Paine, M.L. Using the yeast two-hybrid assay to discover protein partners for the leucine-rich amelogenin peptide and for tuftelin-interacting protein 11. Eur. J. Oral Sci. 2006, 114, 276–279. [Google Scholar] [CrossRef]
- Amin, H.D.; Olsen, I.; Knowles, J.C.; Donos, N. Differential Effect of Amelogenin Peptides on Osteogenic Differentiation In Vitro: Identification of Possible New Drugs for Bone Repair and Regeneration. Tissue Eng. Part A 2012, 18, 1193–1202. [Google Scholar] [CrossRef]
- Amin, H.D.; Olsen, I.; Knowles, J.; Dard, M.; Donos, N. A tyrosine-rich amelogenin peptide promotes neovasculogenesis in vitro and ex vi-vo. Acta Biomater. 2014, 10, 1930–1939. [Google Scholar] [CrossRef][Green Version]
- Amin, H.D.; Olsen, I.; Knowles, J.; Dard, M.; Donos, N. A procedure for identifying stem cell compartments with multi-lineage differentia-tion potential. Analyst 2011, 136, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Tominaga, K.; Tanaka, A. Analysis of eosinophilic round bodies formed after injection of enamel matrix deriva-tive into the backs of rats. J. Periodontol. 2005, 76, 1934–1941. [Google Scholar] [CrossRef]
- Ando, K.; Kunimatsu, R.; Awada, T.; Yoshimi, Y.; Tsuka, Y.; Sumi, K.; Horie, K.; Abe, T.; Nakajima, K.; Tanimoto, K. Effects of Human Full-length Amelogenin and C-terminal Amelogenin Peptide on the Proliferation of Human Mesenchymal Stem Cells Derived from Adipose Tissue. Curr. Pharm. Des. 2018, 24, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, R.; Awada, T.; Yoshimi, Y.; Ando, K.; Hirose, N.; Tanne, Y.; Sumi, K.; Tanimoto, K. The C-terminus of the amelogenin peptide influences the proliferation of hu-man bone marrow mesenchymal stem cells. J. Periodontol. 2018, 89, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Warotayanont, R.; Zhu, D.; Snead, M.L.; Zhou, Y. Leucine-rich amelogenin peptide induces osteogenesis in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 367, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, X.; Cawthorn, W.P.; MacDougald, O.A.; Stupp, S.I.; Snead, M.L.; Zhou, Y. The influence of Leucine-rich amelogenin peptide on MSC fate by induc-ing Wnt10b expression. Biomaterials 2011, 32, 6478–6486. [Google Scholar] [CrossRef]
- Newcomb, C.J.; Sur, S.; Lee, S.S.; Yu, J.M.; Zhou, Y.; Snead, M.L.; Stupp, S.I. Supramolecular Nanofibers Enhance Growth Factor Signaling by Increasing Lipid Raft Mobility. Nano Lett. 2016, 16, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Hatakeyama, Y.; Nakashima, K.; Kamogashira, N.; Hatakeyama, J.; Tamaoki, S.; Sawa, Y.; Ishikawa, H. Effects of a Chemically Synthesized Leucine-Rich Amelogenin Peptide (csLRAP) on Chondrogenic and Osteogenic Cells. J. Hard Tissue Biol. 2017, 26, 51–60. [Google Scholar] [CrossRef][Green Version]
- Amin, H.D.; Ethier, C.R. Differential effects of tyrosine-rich amelogenin peptide on chondrogenic and osteogenic differenti-ation of adult chondrocytes. Cell Tissue Res. 2016, 364, 219–224. [Google Scholar] [CrossRef]
- Kawanaka, A.; Tominaga, K.; Tanaka, A. Effect of peptide derived from Emdogain on human periodontal ligament fibro-blast. J. Osaka Dent. Univ. 2009, 43, 111–117. [Google Scholar]
- Hida, T.; Tominaga, K.; Tanaka, A. Tissue Reaction to Synthetic Oligopeptide Derived from Enamel Matrix Derivative in Rats. Oral Sci. Int. 2010, 7, 26–33. [Google Scholar] [CrossRef]
- Yasui, N.; Taguchi, Y.; Tanaka, A.; Ueda, M.; Umeda, M. Biologic effects of Emdogain Derived Oligopeptides on rat bone marrow cells. J. Oral Tissue Eng. 2012, 9, 126–135. [Google Scholar]
- Taguchi, Y.; Yasui, N.; Takahashi, S.; Tominaga, K.; Kato, H.; Komasa, S.; Shida, M.; Hayashi, H.; Tanaka, A.; Umeda, M. Hard Tissue Formation by Human Periodontal Ligament Fibroblast Cells Treated with an Emdogain-Derived Oligopeptide in vitro. J. Hard Tissue Biol. 2012, 21, 375–384. [Google Scholar] [CrossRef]
- Kato, H.; Katayama, N.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. A synthetic oligopeptide derived from enamel matrix derivative promotes the dif-ferentiation of human periodontal ligament stem cells into osteoblast-like cells with increased mineralization. J. Periodontol. 2013, 84, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Katayama, N.; Kato, H.; Taguchi, Y.; Tanaka, A.; Umeda, M. The Effects of Synthetic Oligopeptide Derived from Enamel Matrix Derivative on Cell Proliferation and Osteoblastic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 14026–14043. [Google Scholar] [CrossRef] [PubMed]
- Awada, T.; Kunimatsu, R.; Yoshimi, Y.; Hirose, N.; Mitsuyoshi, T.; Sumi, K.; Tanimoto, K. Effects of C-terminal amelogenin pep-tides on the metabolism of osteoblasts. Biochem. Biophys. Res. Commun. 2017, 482, 1154–1159. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Yoshimi, Y.; Hirose, N.; Awada, T.; Miyauchi, M.; Takata, T.; Li, W.; Zhu, L.; DenBesten, P.; Tanimoto, K. The C-terminus of amelogenin enhances osteogenic differentiation of human cementoblast lineage cells. J. Periodontal Res. 2016, 52, 218–224. [Google Scholar] [CrossRef]
- Eastoe, J.E. The chemical composition of bone and teeth. Adv. Fluorine Res. Dent. Caries Prevent. 1965, 3, 5–17. [Google Scholar]
- Takagi, T.; Suzuki, M.; Baba, T.; Minegishi, K.; Sasaki, S. Complete amino acid sequence of amelogenin in developing bovine enamel. Biochem. Biophys. Res. Commun. 1984, 121, 592–597. [Google Scholar] [CrossRef]
- Lau, E.C.; Mohandas, T.K.; Shapiro, L.J.; Slavkin, H.C.; Snead, M.L. Human and mouse amelogenin gene loci are on the sex chromosomes. Genomics 1989, 4, 162–168. [Google Scholar] [CrossRef]
- Gibson, C.W.; Golub, E.E.; Abrams, W.R.; Shen, G.; Ding, W.; Rosenbloom, J. Bovine amelogenin message heterogeneity: Alternative splicing and Y-chromosomal gene transcription. Biochemistry 1992, 31, 8384–8388. [Google Scholar] [CrossRef]
- Lau, E.C.; Simmer, J.P.; Bringas, P.; Hsu, D.D.-J.; Hu, C.-C.; Zeichner-David, M.; Thiemann, F.T.; Snead, M.L.; Slavkin, H.C.; Fincham, A.G. Alternative splicing of the mouse amelogenin primary RNA transcript contributes to amelogenin heterogeneity. Biochem. Biophys. Res. Commun. 1992, 188, 1253–1260. [Google Scholar] [CrossRef]
- Bonass, W.A.; Kirkham, J.; Brookes, S.J.; Shore, R.C.; Robinson, C. Isolation and characterisation of an alternatively-spliced rat amelogenin cDNA: LRAP-a highly conserved, functional alternatively-splicedamelogenin? Biochim. Biophys. Acta Gene Struct. Expr. 1994, 1219, 690–692. [Google Scholar] [CrossRef]
- Nakahori, Y.; Takenaka, O.; Nakagome, Y. A human X-Y homologous region encodes “amelogenin. ” Genomics 1991, 9, 264–269. [Google Scholar] [CrossRef]
- Iwase, M.; Kaneko, S.; Kim, H.L.; Satta, Y.; Takahata, N. Evolutionary History of Sex-Linked Mammalian Amelogenin Genes. Cells Tissues Organs 2007, 186, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Fincham, A.; Hu, Y.; Lau, E.; Slavkin, H.; Snead, M. Amelogenin post-secretory processing during biomineralization in the postnatal mouse molar tooth. Arch. Oral Biol. 1991, 36, 305–317. [Google Scholar] [CrossRef]
- Bansal, A.K.; Shetty, D.C.; Bindal, R.; Pathak, A. Amelogenin: A novel protein with diverse applications in genetic and molecular profiling. J. Oral Maxillofac. Pathol. 2012, 16, 395–399. [Google Scholar] [CrossRef]
- Francès, F.; Portolés, O.; González, J.; Coltell, O.; Verdú, F.; Castello, A.; Corella, D. Amelogenin test: From forensics to quality control in clinical and biochemical genomics. Clin. Chim. Acta 2007, 386, 53–56. [Google Scholar] [CrossRef]
- Thangaraj, K.; Reddy, A.G.; Singh, L. Is the amelogenin gene reliable for gender identification in forensic casework and pre-natal diagnosis? Int. J. Legal Med. 2002, 116, 121–123. [Google Scholar] [CrossRef]
- Dutta, P.; Bhosale, S.; Singh, R.; Gubrellay, P.; Patil, J.; Sehdev, B.; Bhagat, S.; Bansal, T. Amelogenin Gene-The Pioneer in Gender Determination from Forensic Dental Samples. J. Clin. Diagn. Res. 2017, 11, ZC56–ZC59. [Google Scholar] [CrossRef] [PubMed]
- Van den Berge, M.; Sijen, T. A male and female RNA marker to infer sex in forensic analysis. Forensic Sci. Int. Genet. 2017, 26, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Fincham, A.; Moradian-Oldak, J.; Simmer, J. The Structural Biology of the Developing Dental Enamel Matrix. J. Struct. Biol. 1999, 126, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Toyosawa, S.; O’Huigin, C.; Figueroa, F.; Tichy, H.; Klein, J. Identification and characterization of amelogenin genes in monotremes, reptiles, and amphibians. Proc. Natl. Acad. Sci. USA 1998, 95, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.; Bonass, W.; Kirkham, J.; Robinson, C. The Human Amelogenin C-terminal Sequence is Completely Homologous to the C-terminal Sequence of Amelogenin in All Species So Far Studied. J. Dent. Res. 1994, 73, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kogure, E.; Takagi, T.; Aimoto, S.; Aobo, T. Molecular Conformation of Porcine Amelogenin in Solution: Three Folding Units at the N-terminal, Central, and C-Terminal Regions1. J. Biochem. 1993, 113, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, N.; Izumi, Y.; Aoba, T. Small-Angle X-Ray Scattering and Computer-Aided Molecular Modeling Studies of 20 kDa Fragment of Porcine Amelogenin: Does Amelogenin Adopt an Elongated Bundle Structure? J. Biochem. 1998, 123, 150–156. [Google Scholar] [CrossRef]
- Zhang, X.; Ramirez, B.E.; Liao, X.; Diekwisch, Y.G.H. Amelogenin supramolecular assembly in nanospheres defined by a complex He-lix-Coil-PPII helix 3D-Structure. PLoS ONE 2011, 6, e24952. [Google Scholar]
- Bromley, K.M.; Kiss, A.S.; Lokappa, S.B.; Ndao, M.; Evans, J.S.; Moradian-Oldak, J. Dissecting amelogenin protein nanospheres: Characterization of metastable ol-igomers. J. Biol. Chem. 2011, 286, 34643–34653. [Google Scholar] [CrossRef]
- Buchko, G.W.; Tarasevich, B.J.; Bekhazi, J.; Snead, M.L.; Shaw, W.J. A solution NMR investigation into the early events of amelogenin nano-sphere self-assembly initiated with sodium chloride or calcium chloride. Biochemistry 2008, 47, 13215–13222. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; Wen, H.B.; Fincham, A.G.; Iijima, M. Amelogenin nanospheres modulate crystal habit of octacalcium phos-phate and hydroxyapatite crystals in in vitro model systems. MRS Online Proc. Libr. 2000, 620, 471. [Google Scholar]
- Fang, P.-A.; Margolis, H.C.; Conway, J.F.; Simmer, J.P.; Dickinson, G.H.; Beniash, E. Cryogenic Transmission Electron Microscopy Study of Amelogenin Self-Assembly at Different pH. Cells Tissues Organs 2011, 194, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L.; Heijl, L.; Gestrelius, S. Periodontal regeneration in a buccal dehiscence model in monkeys after applica-tion of enamel matrix proteins. J. Clin. Periodontol. 1997, 24, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain) for periodontal tissue regenera-tion in intrabony defects. A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 247–266. [Google Scholar] [PubMed]
- Amin, H.; Olsen, I.; Knowles, J.; Dard, M.; Donos, N. Interaction of enamel matrix proteins with human periodontal ligament cells. Clin. Oral Investig. 2016, 20, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Carnes, D.; Steffensen, B.; Cochran, D.L. Cellular Effects of Enamel Matrix Derivative Are Associated With Different Molecular Weight Fractions Following Separation by Size-Exclusion Chromatography. J. Periodontol. 2009, 80, 648–656. [Google Scholar] [CrossRef]
- Suzuki, S.; Nagano, T.; Yamakoshi, Y.; Gomi, K.; Arai, T.; Fukae, M.; Katagiri, T.; Oida, S. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-{beta}. J. Dent. Res. 2005, 84, 510–514. [Google Scholar] [CrossRef]
- Maycock, J.; Wood, S.R.; Brookes, S.J.; Shore, R.C.; Robinson, C.; Kirkham, J. Characterization of a porcine amelogenin preparation, EMDOGAIN, a biological treatment for periodontal disease. Connect. Tissue Res. 2002, 43, 472–476. [Google Scholar] [CrossRef]
- Mumulidu, A.; Hildebrand, B.; Fabi, B.; Hammarström, L.; Cochran, D.L.; Dard, M.; LeMoult, S. Purification and analysis of a 5kDa component of enamel matrix derivative. J. Chromatogr. B 2007, 857, 210–218. [Google Scholar] [CrossRef]
- Carinci, F.; Piattelli, A.; Guida, L.; Perrotti, V.; Laino, G.; Oliva, A.; Annunziata, M.; Palmieri, A.; Pezzetti, F. Effects of Emdogain on osteoblast gene expression. Oral Dis. 2006, 12, 329–342. [Google Scholar] [CrossRef]
- Gestrelius, S.; Lyngstadaas, S.P.; Hammarström, L. Emdogain--periodontal regeneration based on biomimicry. Clin. Oral Investig. 2000, 4, 120–125. [Google Scholar] [CrossRef]
- Zeichner-David, M.; Chen, L.-S.; Hsu, Z.; Reyna, J.; Catón, J.; Bringas, P. Amelogenin and ameloblastin show growth-factor like activity in periodontal ligament cells. Eur. J. Oral Sci. 2006, 114, 244–253. [Google Scholar] [CrossRef]
- Hu, C.-C.; Fukae, M.; Uchida, T.; Qian, Q.; Zhang, C.; Ryu, O.; Tanabe, T.; Yamakoshi, Y.; Murakami, C.; Dohi, N.; et al. Sheathlin: Cloning, cDNA/Polypeptide Sequences, and Immunolocalization of Porcine Enamel Sheath Proteins. J. Dent. Res. 1997, 76, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.; Fukae, M.; Uchida, T.; Qian, Q.; Zhang, C.; Ryu, O.; Tanabe, T.; Yamakoshi, Y.; Murakami, C.; Dohi, N.; et al. Cloning and Characterization of Porcine Enamelin mRNAs. J. Dent. Res. 1997, 76, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Fukae, M.; Tanabe, T.; Uchida, T.; Lee, S.-K.; Ryu, O.-H.; Murakami, C.; Wakida, K.; Simmer, J.; Yamada, Y.; Bartlett, J. Enamelysin (matrix metalloproteinase-20): Localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. J. Dent. Res. 1998, 77, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Stout, B.M.; Alent, B.J.; Pedalino, P.; Holbrook, R.; Gluhak-Heinrich, J.; Cui, Y.; Harris, M.A.; Gemperli, A.C.; Cochran, D.L.; Deas, D.E.; et al. Enamel Matrix Derivative: Protein Components and Osteoinductive Properties. J. Periodontol. 2014, 85, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Leme, A.F.P.; Kantovitz, K.R.; Martins, E.N.L.; Sallum, E.A.; Casati, M.Z.; Nociti, F.H.; Junior, F.H.N. Leucine-Rich Amelogenin Peptide (LRAP) Uptake by Cementoblast Requires Flotillin-1 Mediated Endocytosis. J. Cell. Physiol. 2016, 232, 556–565. [Google Scholar] [CrossRef]
- Schwartz, Z.; Carnes, D.L., Jr.; Pulliam, R.; Lohmann, C.H.; Sylvia, V.L.; Liu, Y.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Porcine fetal enamel matrix derivative stimulates proliferation but not differenti-ation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J. Periodontol. 2000, 71, 1287–1296. [Google Scholar]
- Van der Pauw, M.T.; Van den Bos, T.; Everts, V.; Beertsen, W. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor beta1 release of perio-dontal ligament and gingival fibroblasts. J. Periodontol. 2000, 71, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, S.R.; Carnes, D.L.; Cochran, D.L. In Vitro Effects of Enamel Matrix Derivative on Microvascular Cells. J. Periodontol. 2007, 78, 141–151. [Google Scholar] [CrossRef]
- Saito, K.; Konishi, I.; Nishiguchi, M.; Hoshino, T.; Fujiwara, T. Amelogenin binds to both heparan sulfate and bone morphogenetic protein 2 and pharmacologically suppresses the effect of noggin. Bone 2008, 43, 371–376. [Google Scholar] [CrossRef]
- Reseland, J.E.; Reppe, S.; Larsen, A.M.; Berner, H.S.; Reinholt, F.P.; Gautvik, K.M.; Slaby, I.; Lyngstadaas, S.P. The effect of enamel matrix derivative on gene expression in osteoblasts. Eur. J. Oral Sci. 2006, 114, 205–211. [Google Scholar] [CrossRef]
- He, J.; Jiang, J.; Safavi, K.E.; Spångberg, L.S.; Zhu, Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteopro-tegerin expression. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2004, 97, 239–245. [Google Scholar] [CrossRef]
- He, J.; King, Y.; Jiang, J.; Safavi, K.E.; Spångberg, L.S.; Zhu, Q. Enamel matrix derivative inhibits TNF-α–induced apoptosis in osteoblastic MC3T3-E1 cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 761–767. [Google Scholar] [CrossRef]
- Lee, A.Z.; Jiang, J.; He, J.; Safavi, K.E.; Spångberg, L.S.; Zhu, Q. Stimulation of cytokines in osteoblasts cultured on enamel matrix derivative. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 133–138. [Google Scholar] [CrossRef]
- Miron, R.J.; Oates, C.J.; Molenberg, A.; Dard, M.; Hamilton, D.W. The effect of enamel matrix proteins on the spreading, proliferation and differen-tiation of osteoblasts cultured on titanium surfaces. Biomaterials 2010, 31, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M.; Suzuki, N.; Takayama, T.; Shoji, T.; Otsuka, K.; Ito, K. Enamel matrix derivative stimulates chondrogenic differentiation of ATDC5 cells. J. Periodontal Res. 2006, 42, 131–137. [Google Scholar] [CrossRef]
- Narukawa, M.; Suzuki, N.; Takayama, T.; Shoji, T.; Otsuka, K.; Ito, K. Enamel matrix derivative stimulates osteogenesis- and chondrogene-sis-related transcription factors in C3H10T1/2 cells. Acta Biochim. Biophys. Sin. 2007, 39, 1–7. [Google Scholar] [CrossRef]
- Takayama, T.; Suzuki, N.; Narukawa, M.; Tokunaga, T.; Otsuka, K.; Ito, K. Enamel Matrix Derivative Stimulates Core Binding Factor α1/Runt-Related Transcription Factor-2 Expression via Activation of Smad1 in C2C12 Cells. J. Periodontol. 2005, 76, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Itoh, D.; Kuroda, S.; Kondo, H.; Umezawa, A.; Ohya, K.; Ohyama, T.; Kasugai, S. The effects of enamel matrix derivative (EMD) on osteoblastic cells in culture and bone regeneration in a rat skull defect. J. Periodontal Res. 2003, 38, 333–342. [Google Scholar] [CrossRef]
- Weißhaupt, P.; Bernimoulin, J.-P.; Trackman, P.; Hägewald, S. Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Rincon, J.C.; Xiao, Y.; Young, W.G.; Bartold, P.M. Production of osteopontin by cultured porcine epithelial cell rests of Malassez. J. Periodontal Res. 2005, 40, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Zhang, Y.; Sculean, A.; Pippenger, B.; Shirakata, Y.; Kandalam, U.; Hernandez, M. Oste-ogain® loaded onto an absorbable collagen sponge induces attachment and osteoblast differentiation of ST2 cells in vitro. Clin Oral Investig. 2017, 21, 2265–2272. [Google Scholar] [CrossRef][Green Version]
- Itoh, N.; Kasai, H.; Ariyoshi, W.; Harada, E.; Yokota, M.; Nishihara, T. Mechanisms involved in the enhancement of osteoclast formation by enamel matrix derivative. J. Periodontal Res. 2006, 41, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Kasai, H.; Yamaguchi, K.; Nishihara, T. Enamel matrix derivative promotes osteoclast cell formation by RANKL production in mouse marrow cultures. J. Dent. 2005, 33, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Bosshardt, D.; Lang, N.P.; Graziani, F.; Tonetti, M.; Karring, T.; Kostopoulos, L. Bone formation by enamel matrix proteins and xenografts: An experimental study in the rat ramus. Clin. Oral Implant. Res. 2004, 16, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Intini, G.; Andreana, S.; Buhite, R.J.; Bobek, L.A. A Comparative Analysis of Bone Formation Induced by Human Demineralized Freeze-Dried Bone and Enamel Matrix Derivative in Rat Calvaria Critical-Size Bone Defects. J. Periodontol. 2008, 79, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Potijanyakul, P.; Sattayasansakul, W.; Pongpanich, S.; Leepong, N.; Kintarak, S. Effects of Enamel Matrix Derivative on Bioactive Glass in Rat Calvarium Defects. J. Oral Implant. 2010, 36, 195–204. [Google Scholar] [CrossRef]
- Shahriari, S.; Houshmand, B.; Razavian, H.; Khazaei, S.; Abbas, F.M. Effect of the combination of enamel matrix derivatives and deproteinized bovine bone materials on bone formation in rabbits’ calvarial defects. Dent. Res. J. 2012, 9, 422–426. [Google Scholar]
- Sawae, Y.; Sahara, T.; Kawana, F.; Sasaki, T. Effects of enamel matrix derivative on mineralized tissue formation during bone wound healing in rat parietal bone defects. Qjm: Int. J. Med. 2002, 51, 413–423. [Google Scholar] [CrossRef]
- Kawana, F.; Sawae, Y.; Sahara, T.; Tanaka, S.; Debari, K.; Shimizu, M.; Sasaki, T. Porcine enamel matrix derivative enhances trabecular bone regeneration during wound healing of injured rat femur. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2001, 264, 438–446. [Google Scholar] [CrossRef]
- Heijl, L.; Heden, G.; Svärdström, G.; Ostgren, A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodon-tal defects. J. Clin. Periodontol. 1997, 24, 705–714. [Google Scholar] [CrossRef]
- Cornelini, R.; Scarano, A.; Piattelli, M.; Andreana, S.; Covani, U.; Quaranta, A.; Piattelli, A. Effect of Enamel Matrix Derivative (Emdogain) on Bone Defects in Rabbit Tibias. J. Oral Implant. 2004, 30, 69–73. [Google Scholar] [CrossRef]
- Wang, H.L.; Boyapati, L. "pASS" principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Wei, L.; Bosshardt, D.D.; Buser, D.; Sculean, A.; Zhang, Y. Effects of enamel matrix proteins in combination with a bovine-derived natural bone mineral for the repair of bone defects. Clin. Oral Investig. 2013, 18, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Macaluso, G.M.; Guizzardi, S.; Vescovini, R.; Passeri, M.; Passeri, G. Osteoprotegerin and receptor activator of nuclear factor-kappa B ligand mod-ulation by enamel matrix derivative in human alveolar osteoblasts. J. Periodontol. 2006, 77, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Morotome, Y.; Tanabe, T.; Fukae, M.; Ishikawa, I.; Oida, S. Noggin blocks osteoinductive activity of porcine enamel extracts. J. Dent. Res. 2002, 81, 387–391. [Google Scholar] [CrossRef]
- Hoang, A.; Klebe, R.; Steffensen, B.; Ryu, O.; Simmer, J.; Cochran, D. Amelogenin is a cell adhesion protein. J. Dent. Res. 2002, 81, 497–500. [Google Scholar] [CrossRef]
- Du, C.; Schneider, G.; Zaharias, R.; Abbott, C.; Seabold, D.; Stanford, C.; Moradian-Oldak, J. Apatite/Amelogenin Coating on Titanium Promotes Osteogenic Gene Expression. J. Dent. Res. 2005, 84, 1070–1074. [Google Scholar] [CrossRef]
- Svensson, J.; Andersson, C.; Reseland, J.E.; Lyngstadaas, P.; Bülow, L. Histidine tag fusion increases expression levels of active recombinant ame-logenin in Escherichia coli. Protein Expr. Purif. 2006, 48, 134–141. [Google Scholar] [CrossRef]
- Terada, C.; Komasa, S.; Kusumoto, T.; Kawazoe, T.; Okazaki, J. Effect of Amelogenin Coating of a Nano-Modified Titanium Surface on Bioactivity. Int. J. Mol. Sci. 2018, 19, 1274. [Google Scholar] [CrossRef]
- Fukuda, T.; Sanui, T.; Toyoda, K.; Tanaka, U.; Taketomi, T.; Uchiumi, T.; Nishimura, F. Identification of Novel Amelogenin-Binding Proteins by Proteomics Analysis. PLoS ONE 2013, 8, e78129. [Google Scholar] [CrossRef]
- Zhang, H.; Tompkins, K.; Garrigues, J.; Snead, M.L.; Gibson, C.W.; Somerman, M.J. Full length amelogenin binds to cell surface LAMP-1 on tooth root/periodontium associated cells. Arch. Oral Biol. 2010, 55, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Fukuda, T.; Sanui, T.; Tanaka, U.; Yamamichi, K.; Atomura, R.; Maeda, H.; Tomokiyo, A.; Taketomi, T.; Uchiumi, T.; et al. Grp78 Is Critical for Amelogenin-Induced Cell Migration in a Multipotent Clonal Human Periodontal Ligament Cell Line. J. Cell. Physiol. 2016, 231, 414–427. [Google Scholar] [CrossRef]
- Shapiro, J.L.; Wen, X.; Okamoto, C.T.; Wang, H.J.; Lyngstadaas, S.P.; Goldberg, M.; Snead, M.L.; Paine, M.L. Cellular uptake of amelogenin, and its localization to CD63, and Lamp1-positive vesicles. Cell. Mol. Life Sci. 2006, 64, 244–256. [Google Scholar] [CrossRef]
- Tanimoto, K.; Kunimatsu, R.; Tanne, Y.; Huang, Y.-C.; Michida, M.; Yoshimi, Y.; Miyauchi, M.; Takata, T.; Tanne, K. Differential Effects of Amelogenin on Mineralization of Cementoblasts and Periodontal Ligament Cells. J. Periodontol. 2012, 83, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, H.L.; Berry, J.E.; Foster, B.L.; Gibson, C.W.; Li, Y.; Kulkarni, A.B.; Snead, M.L.; Somerman, M.J. Amelogenin: A Potential Regulator of Cementum-Associated Genes. J. Periodontol. 2003, 74, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.C.; Fong, H.K.; Foster, B.L.; Paine, M.L.; Gibson, C.W.; Snead, M.L.; Somerman, M.J. Amelogenins regulate expression of genes associated with cementoblasts in vitro. Eur. J. Oral Sci. 2006, 114, 239–243. [Google Scholar] [CrossRef]
- Yuan, Z.; Collier, P.; Rosenbloom, J.; Gibson, C. Analysis of amelogenin mRNA during bovine tooth development. Arch. Oral Biol. 1996, 41, 205–213. [Google Scholar] [CrossRef]
- Fincham, A.G.; Belcourt, A.B.; Termine, J.D.; Butler, W.T.; Cothran, W.C. Dental enamel matrix: Sequences of two amelogenin polypeptides. Biosci. Rep. 1981, 1, 771–778. [Google Scholar] [CrossRef]
- Masica, D.L.; Gray, J.J.; Shaw, W.J. Partial High-Resolution Structure of Phosphorylated and Non-phosphorylated Leucine-Rich Amelogenin Protein Adsorbed to Hydroxyapatite. J. Phys. Chem. C 2011, 115, 13775–13785. [Google Scholar] [CrossRef][Green Version]
- Lu, J.X.; Xu, Y.S.; Buchko, G.W.; Shaw, W.J. Mineral association changes the secondary structure and dynamics of murine amelog-enin. J. Dent. Res. 2013, 92, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Tarasevich, B.J.; Perez-Salas, U.; Masica, D.L.; Philo, J.; Kienzle, P.; Krueger, S.; Majkrzak, C.F.; Gray, J.L.; Shaw, W.J. Neutron reflectometry studies of the adsorbed structure of the amelogen-in, LRAP. J. Phys. Chem. B 2013, 117, 3098–3109. [Google Scholar] [CrossRef]
- Yamazaki, H.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Margolis, H.C. Protein Phosphorylation and Mineral Binding Affect the Secondary Structure of the Leucine-Rich Amelogenin Peptide. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Ma, C.-W.; Zhang, J.; Dong, X.-Q.; Lu, J.-X. Amyloid structure of high-order assembly of Leucine-rich amelogenin revealed by solid-state NMR. J. Struct. Biol. 2019, 206, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Le, T.Q.; Zhang, Y.; Li, W.; Denbesten, P.K. The effect of LRAP on enamel organ epithelial celldifferentiation. J. Dent Res. 2007, 86, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ren, A.; Pugach, M.K. Truncated amelogenin and LRAP transgenes improve Amelx null mouse enamel. Matrix Biol. 2016, 52–54, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Habelitz, S.; DenBesten, P.K.; Marshall, S.J.; Marshall, G.W.; Li, W. Self-assembly and effect on crystal growth of the leucine-rich amelogenin peptide. Eur. J. Oral Sci. 2006, 114, 315–319. [Google Scholar] [CrossRef]
- Tarasevich, B.J.; Lea, S.; Shaw, W.J. The leucine rich amelogenin protein (LRAP) adsorbs as monomers or dimers onto sur-faces. J. Struct. Biol. 2010, 169, 266–276. [Google Scholar] [CrossRef][Green Version]
- Shaw, W.J.; Campbell, A.A.; Paine, M.L.; Snead, M.L. The COOH terminus of the amelogenin, LRAP, is oriented next to the hydrax-yapatite surface. J. Biol. Chem. 2004, 279, 40263–40266. [Google Scholar] [CrossRef]
- Shaw, W.J.; Ferris, K.; Tarasevich, B.; Larson, J.L. The Structure and Orientation of the C-Terminus of LRAP. Biophys. J. 2008, 94, 3247–3257. [Google Scholar] [CrossRef]
- Le, T.Q.; Gochin, M.; Featherstone, J.D.B.; Li, W.; DenBesten, P.K. Comparative calcium binding of leucine-rich amelogenin pep-tide and full-length amelogenin. Eur. J. Oral Sci. 2006, 114, 320–326. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials-biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Veis, A. Identification of the functional activity of the [A-4] amelogenin gene splice product in newborn mouse ameloblasts. Bone 2008, 42, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Villalobo, A. Nitric oxide and cell proliferation. FEBS J. 2006, 273, 2329–2344. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Ischiropoulos, H.; Leboy, P.S.; Adams, S.L.; Shapiro, I.M. Nitric oxide–nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone 2005, 37, 37–45. [Google Scholar] [CrossRef]
- Tiribuzi, R.; Crispoltoni, L.; Tartacca, F.; Orlacchio, A.; Martino, S.; Palmerini, C.A.; Orlacchio, A. Nitric oxide depletion alters hematopoietic stem cell commitment toward im-munogenic dendritic cells. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Speziali, A.; Delcogliano, M.; Tei, M.M.; Placella, G.; Chillemi, M.; Tiribuzi, R.; Cerulli, G. Chondropenia: Current concept review. Musculoskelet. Surg. 2015, 99, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; van Oers, R.F.M.; Bakker, A.D.; Bacabac, R.G. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos. Int. 2013, 25, 1427–1437. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, N.; Shi, H.; Liu, J.; Shi, L.; Zhang, B.; Wang, H.; Ji, J.; Chu, P.K. Upregulation of BMSCs Osteogenesis by Positively-Charged Tertiary Amines on Polymeric Implants via Charge/iNOS Signaling Pathway. Sci. Rep. 2015, 5, 9369. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Kunimatsu, R.; Michida, M.; Yoshioka, M.; Yoshimi, Y.; Kato, Y.; Tanne, K. Effects of human full-length amelogenin on the proliferation of human mesen-chymal stem cells derived from bone marrow. Cell Tissue Res. 2010, 342, 205–212. [Google Scholar] [CrossRef]
- Zhang, C. Molecular mechanisms of osteoblast-specific transcription factor Osterix effect on bone formation. Beijing Da Xue Xue Bao. Yi Xue Ban J. Peking Univ. Heal. Sci. 2012, 44, 659–665. [Google Scholar]
- Tang, W.; Yang, F.; Li, Y.; de Crombrugghe, B.; Jiao, H.; Xiao, G.; Zhang, C. Transcriptional regulation of Vascular Endothelial Growth Factor (VEGF) by osteo-blast-specific transcription factor Osterix (Osx) in osteoblasts. J. Biol. Chem. 2012, 287, 1671–1678. [Google Scholar] [CrossRef]
- Villa, O.; Wohlfahrt, J.C.; Mdla, I.; Petzold, C.; Reseland, J.E.; Snead, M.L.; Lyngstadaas, S.P. Proline-Rich Peptide Mimics Effects of Enamel Matrix Derivative on Rat Oral Muco-sa Incisional Wound Healing. J. Periodontol. 2015, 86, 1386–1395. [Google Scholar] [CrossRef]
- Zhao, M.; Harris, S.E.; Horn, D.; Geng, Z.; Nishimura, R.; Mundy, G.R.; Chen, D. Bone morphogenetic protein receptor signaling is necessary for normal murine post-natal bone formation. J. Cell Biol. 2002, 157, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Ganss, B.; Goldberg, M.; Moradian-Oldak, J.; Paine, M.L.; Snead, M.L.; Wen, X.; White, S.N.; Zhou, Y.L. Protein–Protein Interactions of the Developing Enamel Matrix. Current Topics Dev. Biol. 2006, 74, 57–115. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J. Membrane Organization and Lipid Rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef]
- Banning, A.; Tomasovic, A.; Tikkanen, R. Functional aspects of membrane association of reggie/flotillin proteins. Curr. Protein Pept. Sci. 2011, 12, 725–735. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Liu, Y.-S.; Li, L.; He, Y.-L. Research advances on flotillins. Virol. J. 2011, 8, 479. [Google Scholar] [CrossRef]
- Liu, F.; Kohlmeier, S.; Wang, C.-Y. Wnt signaling and skeletal development. Cell. Signal. 2008, 20, 999–1009. [Google Scholar] [CrossRef]
- Amin, H.D.; Olsen, I.; Knowles, J.C.; Dard, M.; Donos, N. Effects of enamel matrix proteins on multi-lineage differentiation of periodontal ligament cells in vitro. Acta Biomater. 2013, 9, 4796–4805. [Google Scholar] [CrossRef]
- Jonke, E.; Gemperli, A.C.; Zhang, T.; Özdemir, B.; Dard, M.; Rausch-Fan, X.; Andrukhov, O. Effect of tyrosine-rich amelogenin peptide on behavior and differentiation of en-dothelial cells. Clin. Oral Investig. 2016, 20, 2275–2284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aspriello, S.D.; Zizzi, A.; Spazzafumo, L.; Rubini, C.; Lorenzi, T.; Marzioni, D.; Bullon, P.; Piemontese, M. Effects of Enamel Matrix Derivative on Vascular Endothelial Growth Factor Expression and Microvessel Density in Gingival Tissues of Periodontal Pocket: A Comparative Study. J. Periodontol. 2011, 82, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Villar, C.C.; Carnes, D.L.; Dard, M.; Chun, Y.-H.P.; Cochran, D.L. Angiogenic activity of an enamel matrix derivative (EMD) and EMD-derived proteins: An experimental study in mice. J. Clin. Periodontol. 2010, 38, 253–260. [Google Scholar] [CrossRef]
- Kasaj, A.; Meister, J.; Lehmann, K.; Stratul, S.-I.; Schlee, M.; Stein, J.M.; Willershausen, B.; Schmidt, M. The influence of enamel matrix derivative on the angiogenic activity of primary endothelial cells. J. Periodontal Res. 2011, 47, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; An, N.; Bruckmann, C.; Dard, M.; Andrukhov, O.; Matejka, M.; Rausch-Fan, X. Effects of Enamel Matrix Derivative on Proliferation/Viability, Migration, and Ex-pression of Angiogenic Factor and Adhesion Molecules in Endothelial Cells In Vitro. J. Periodontol. 2009, 80, 1622–1630. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, C.-L.; Lin, M.T. Enamel matrix derivative exhibits angiogenic effect in vitro and in a murine model. J. Clin. Periodontol. 2003, 30, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound Healing--Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Albelda, S.M.; Smith, C.W.; Ward, P.A. Adhesion molecules and inflammatory injury. FASEB J. 1994, 8, 504–512. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Peters, K.G.; De Vries, C.; Williams, L.T. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc. Natl. Acad. Sci. USA 1993, 90, 8915–8919. [Google Scholar] [CrossRef]
- Jones, N.; Iljin, K.; Dumont, D.J.; Alitalo, K. Tie receptors: New modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2001, 2, 257–267. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; Bouropoulos, N.; Wang, L.; Gharakhanian, N. Analysis of self-assembly and apatite binding properties of amelogenin proteins lacking the hydrophilic C-terminal. Matrix Biol. 2002, 21, 197–205. [Google Scholar] [CrossRef]
- Zhu, L.; Tanimoto, K.; Le, T.; DenBesten, P.K.; Li, W. Functional Roles of Prolines at Amelogenin C Terminal during Tooth Enamel Formation. Cells Tissues Organs 2008, 189, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ryu, O.; Fincham, A.; Hu, C.-C.; Zhang, C.; Qian, Q.; Bartlett, J.; Simmer, J. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J. Dent. Res. 1999, 78, 743–750. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Kunimatsu, R.; Hirose, N.; Awada, T.; Miyauchi, M.; Takata, T.; Li, W.; Zhu, L.; Denbesten, P.; Tanne, K.; et al. Effects of C-Terminal Amelogenin Peptide on Proliferation of Human Cemento-blast Lineage Cells. J. Periodontol. 2016, 87, 820–827. [Google Scholar] [CrossRef] [PubMed]

| First Author | Peptide | Main Exclusion Criteria | Ref. |
|---|---|---|---|
| Boabaid et al. (2004) | LRAP | Osteoblast differentiation not investigated | [24] |
| Le Norcy et al. (2011) | LRAP | Bone formation not investigated | [25] |
| Hatakeyama et al. (2006) | LRAP | Bone formation not investigated | [26] |
| Wang et al. (2006) | LRAP | Bone formation not investigated | [27] |
| Amin et al. (2012) | TRAP and LRAP | No recombinant peptides | [28] |
| Amin et al. (2014) | TRAP | No recombinant peptides | [29] |
| Amin et al. (2011) | Enamel matrix derivative peptides | Bone formation not investigated | [30] |
| Kim et al. (2005) | Enamel matrix derivative peptides | No recombinant peptides | [31] |
| Ando et al. (2018) | C11 | No recombinant peptides | [32] |
| Kunimatsu et al. (2018) | C11 | Osteoblast differentiation not investigated | [33] |
| First Author (Year) | Peptide | Cell/Cell Line | Concentration | Time Point | Main Results | Ref. |
|---|---|---|---|---|---|---|
| Warotayanont et al. (2008) | LRAP | RW4 and AMEL-/- ESCs | 10 ng/mL | 10 and 20 d | The addition of exogenous LRAP significantly increases the mineral deposition and the expression of BSP and Osx. | [34] |
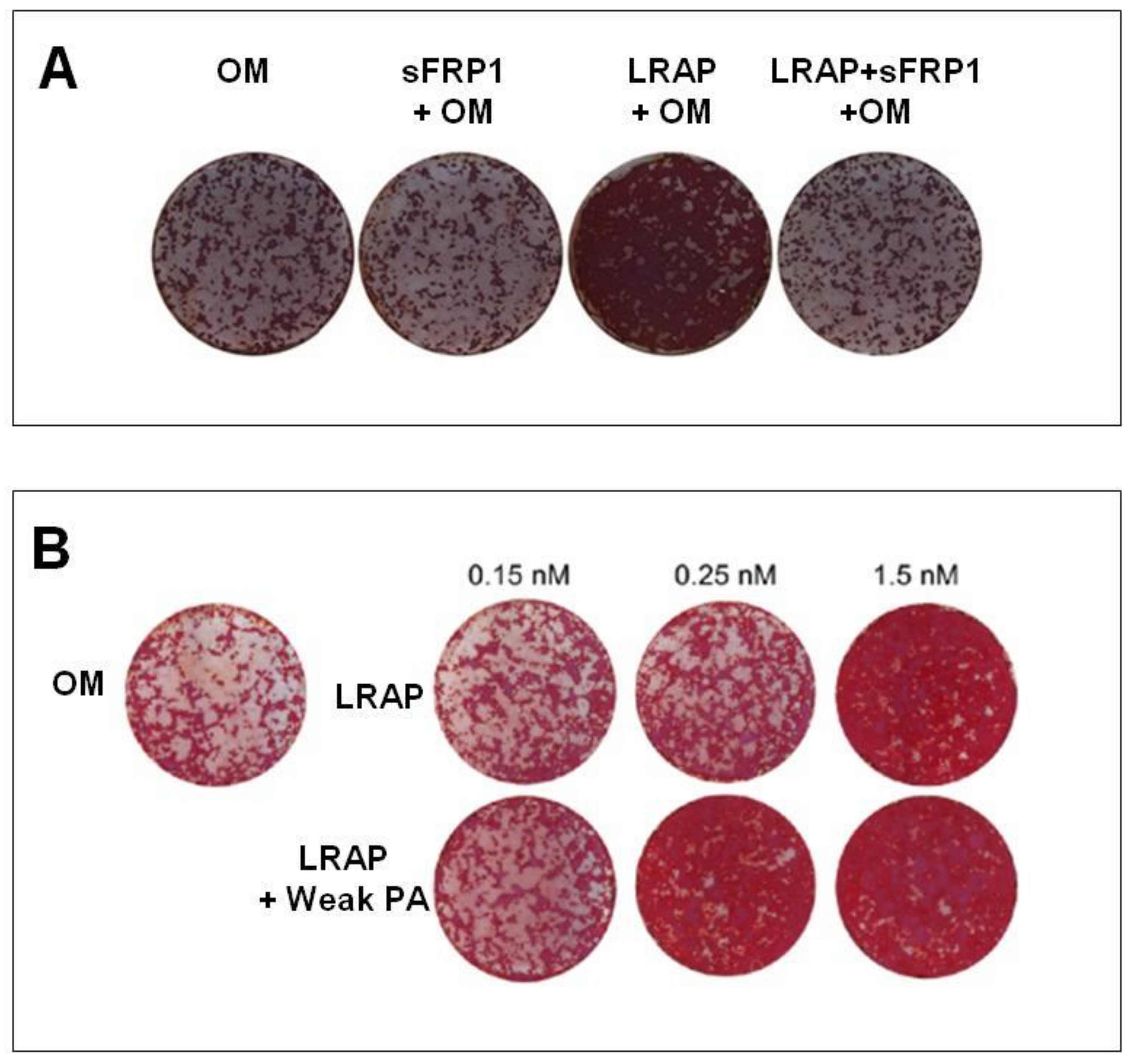
| Warotayanont et al. (2009) | LRAP | RW4 and MC3T-E1 | 10 ng/mL | 4, 6 hand 20 d | LRAP increases the level of Wnt agonist(s) and induced an up-regulation of Osx and BSP of EB cells. The Wnt antagonist sFRP-1 blocks LRAP-mediated osteogenesis. | [8] |
| Wen et al. (2011) | LRAP | ST2 and MC3T3 cells | 10 ng/mL | 14 d | LRAP treatment elevates the Wnt10b expression level and promotes osteogenesis of mesenchymal stem cells. | [35] |
| Newcomb et al. (2016) | LRAP | ST2 | 0.15 nM, 0.25 nM and 1.5 nM | 14 d | Gene expression was similar between LRAP and BMP-2 treatment. LRAP enhanced osteo-differentiation through the activation of the canonical Wnt/β-catenin signaling pathway. | [36] |
| Matsuda et al. (2017) | LRAP | MC3T3-E1 and ATDC5 | 10 ng/mL | 7, 14, 28 d | LRAP could promote “in vitro” osteo-chondrogenic differentiation. LAMP-1 may be involved in the differentiation and proliferation of these cells. | [37] |
| Amin et al. (2016) | TRAP | HACs | 1, 10, 50 and 100 μg/mL | 21 d | TRAP suppresses hypertrophic mineralization and concomitantly promotes chondrogenic differentiation of HACs. | [38] |
| Kawanaka et al. (2009) | SP | HPdLF | 1, 10 and 100 ng/mL | 7 d | The mRNA content of BMPR1A was increased in HPdL F cultured with synthetic peptide. SP might convert HPdLF to bone-forming cells. | [39] |
| Hida et al. (2010) | SP | In Vivo study (rats) | 0.3, 3, 7.5,15 and 30 mg/mL | 1, 3, 5, 7, 14 d | The synthetic peptide combined with an extended-release scaffold seems to produce hard tissues, such as cartilage and bone. | [40] |
| Yasui et al. (2012) | SP | RBMCs | 20, 100, 500 and 1000 ng/mL | 7, 14 d | SP facilitates cell proliferation and induces differentation into osteoblast. | [41] |
| Taguchi et al. (2012) | SP | HPdLF | 5, 20, 100, 200 or 500 ng/mL. | 28 d | SP accelerated calcification, increases ALP activity and OCN production. | [42] |
| Kato et al. (2013) | SP | PDLSC | 100 ng/mL | 2, 3, 5, 7, 21 d | SP enhances the formation of calcified nodules and osteocalcin production. | [43] |
| Katayama et al. (2014) | SP | MSCs | 0. 1, 10, 100 and1000 ng/mL | 7 and 14 d | SP promotes cell proliferation, osteoblast differentiation, and mineralization in human MSCs. | [44] |
| Awada et al. (2017) | C11 | MC3T3-E1 | 0, 100, or1000ng/mL | 7, 14, 21 d | Enhanced cell proliferation, but no difference with control group in terms of osteogenic differentiation and expression of ALP and BSP was observed. | [45] |
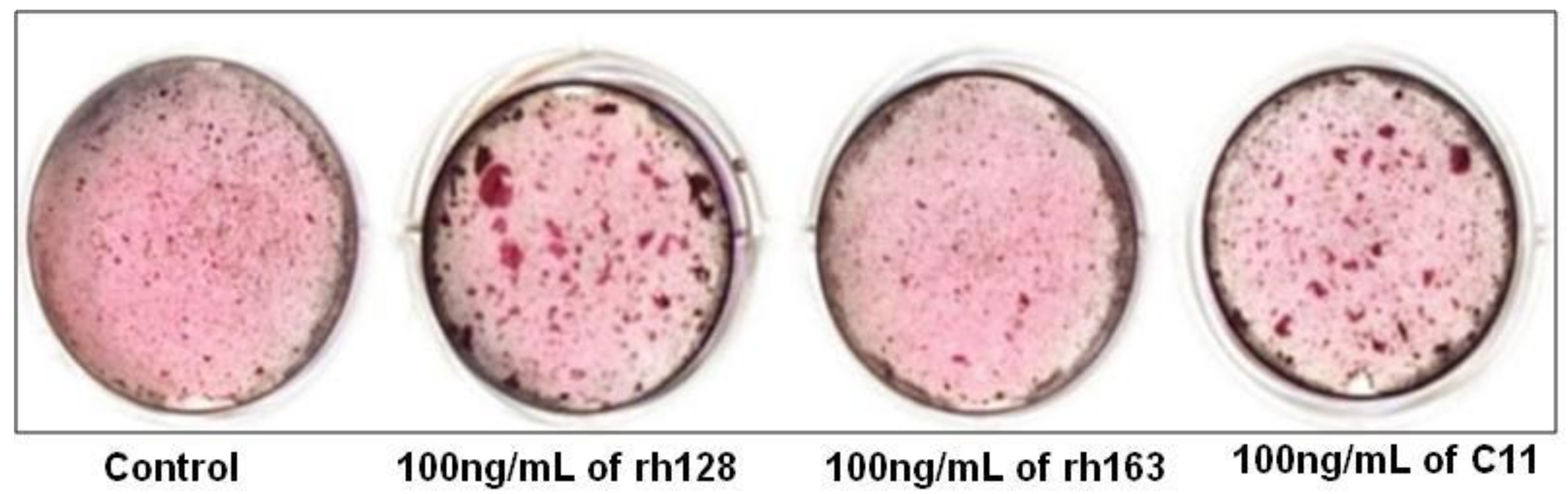
| Kuminatsu et al. (2017) | C11 | HCEM | 0, 10,100 or 1000 ng/mL | 1, 7, 14, 21 d | Osteogenic differentiation was significantly enhanced by treatment with rh128 and C11 peptide but not with rh163. | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorino, A.; Marturano, A.; Placella, G.; Staderini, E.; Domingo, L.I.; Cerulli, G.G.; Tiribuzi, R.; Blasi, P. Amelogenin-Derived Peptides in Bone Regeneration: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9224. https://doi.org/10.3390/ijms22179224
Fiorino A, Marturano A, Placella G, Staderini E, Domingo LI, Cerulli GG, Tiribuzi R, Blasi P. Amelogenin-Derived Peptides in Bone Regeneration: A Systematic Review. International Journal of Molecular Sciences. 2021; 22(17):9224. https://doi.org/10.3390/ijms22179224
Chicago/Turabian StyleFiorino, Antonino, Alessandro Marturano, Giacomo Placella, Edoardo Staderini, Lorena Igual Domingo, Giuliano G. Cerulli, Roberto Tiribuzi, and Paolo Blasi. 2021. "Amelogenin-Derived Peptides in Bone Regeneration: A Systematic Review" International Journal of Molecular Sciences 22, no. 17: 9224. https://doi.org/10.3390/ijms22179224
APA StyleFiorino, A., Marturano, A., Placella, G., Staderini, E., Domingo, L. I., Cerulli, G. G., Tiribuzi, R., & Blasi, P. (2021). Amelogenin-Derived Peptides in Bone Regeneration: A Systematic Review. International Journal of Molecular Sciences, 22(17), 9224. https://doi.org/10.3390/ijms22179224