Altered Secretome of Diabetic Monocytes Could Negatively Influence Fracture Healing—An In Vitro Study
Abstract
:1. Introduction
2. Results
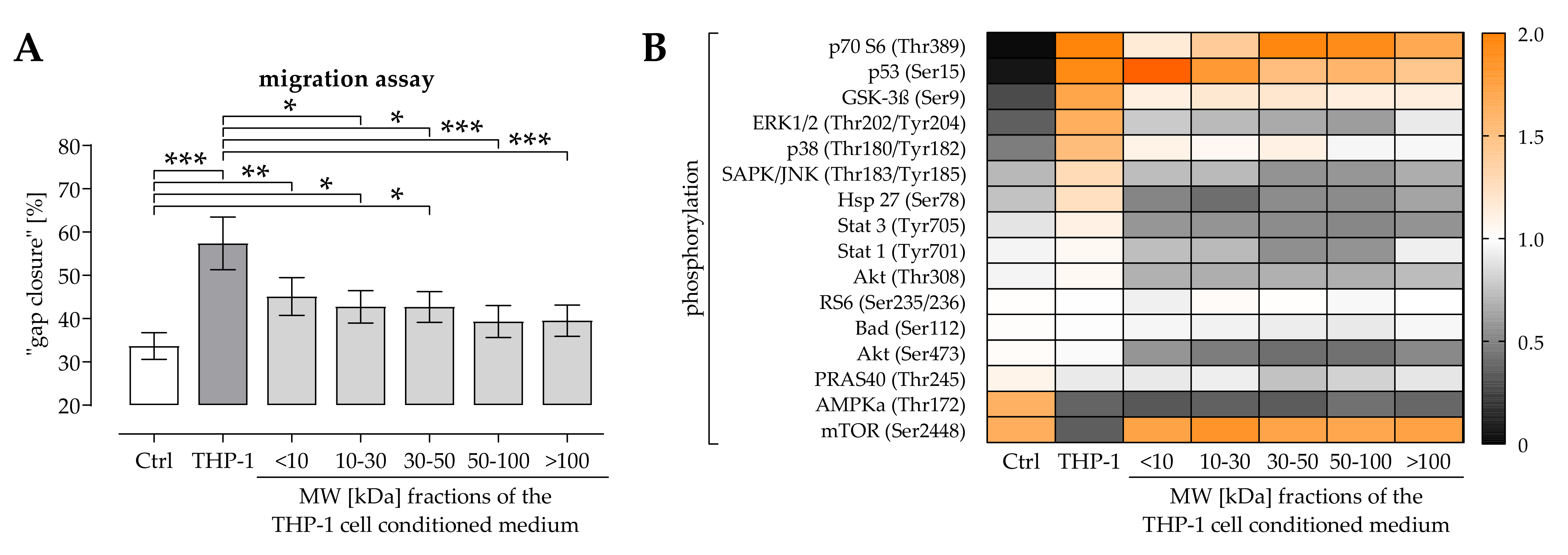
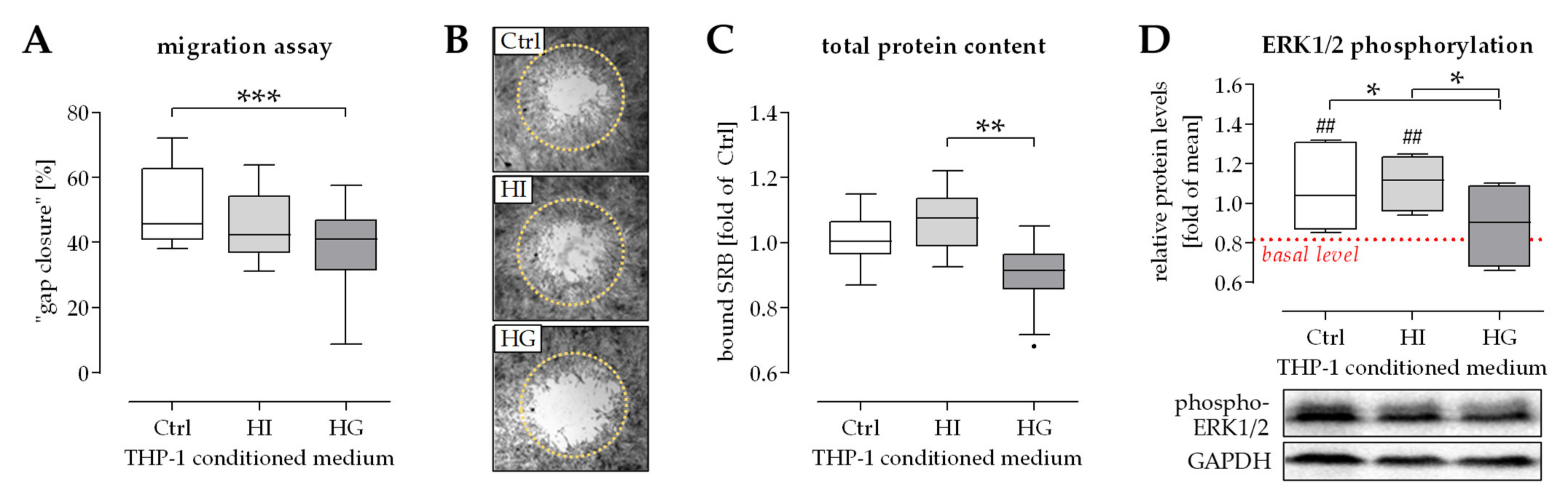
2.1. THP-1 Cells Secreted Factors Induced Migration of Osteoprogenitor Cells
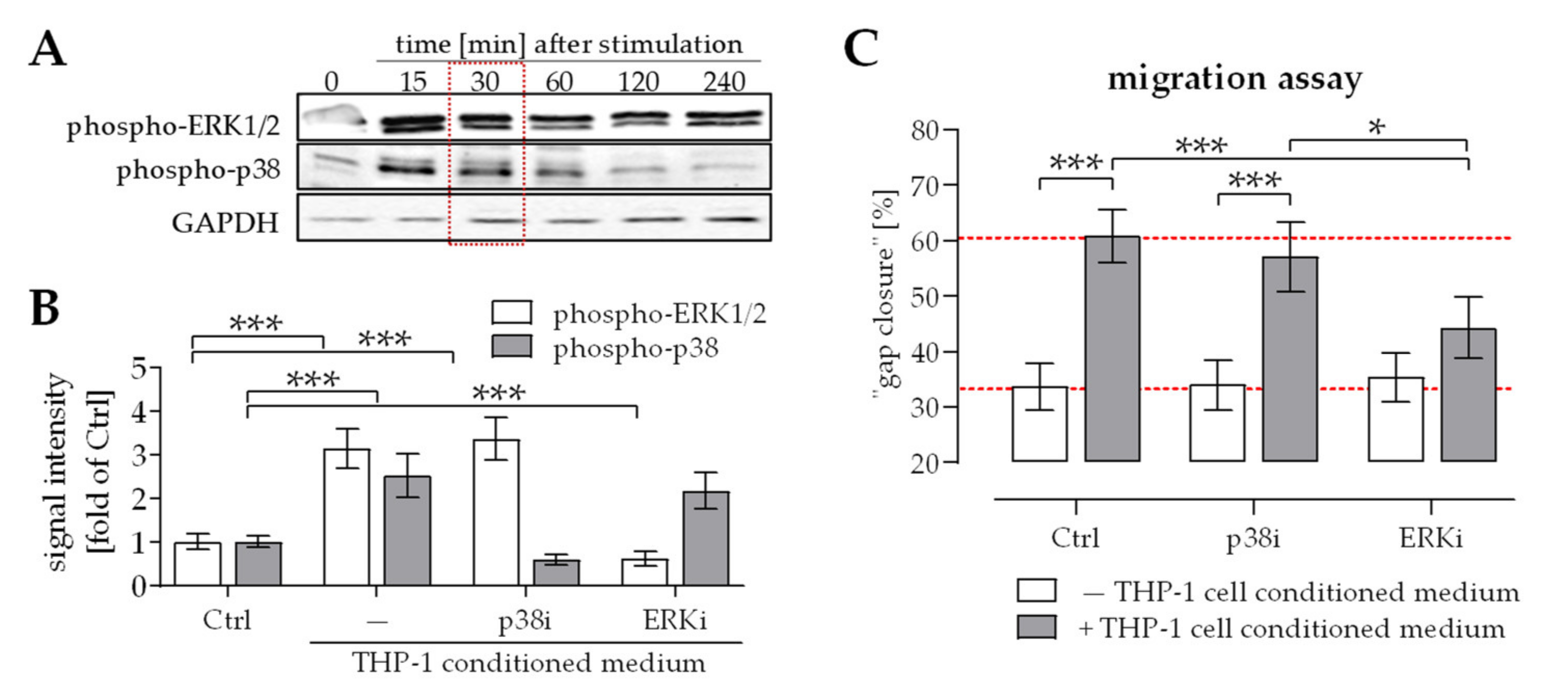
2.2. Activation of ERK1/2 Signaling Was Required for the Positive Effect THP-1 Cell Conditioned Medium Has on Migration of Osteoprogenitor Cells
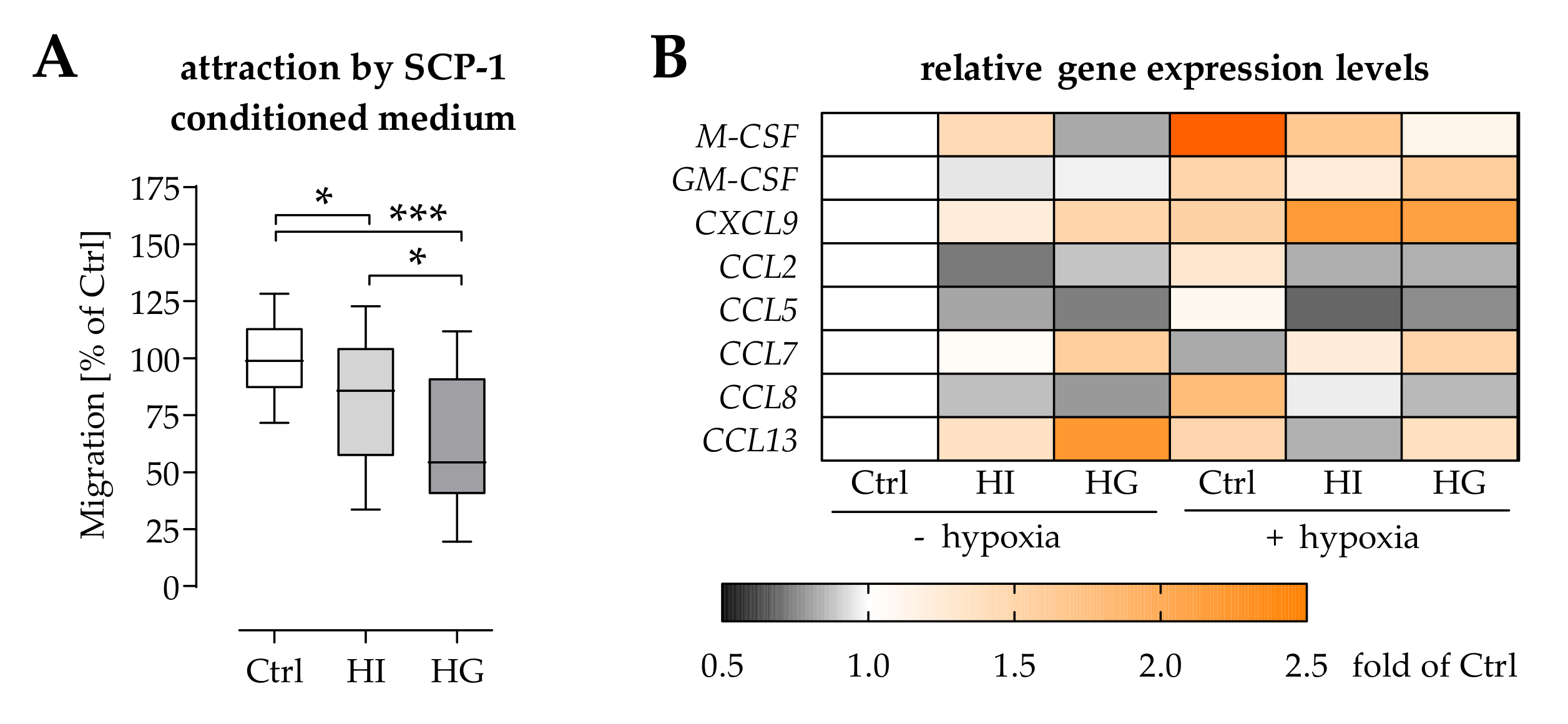
2.3. Pre-/Diabetic Conditions Affected Recruitment of Mononuclear Cells by Osteoprogenitor Cells
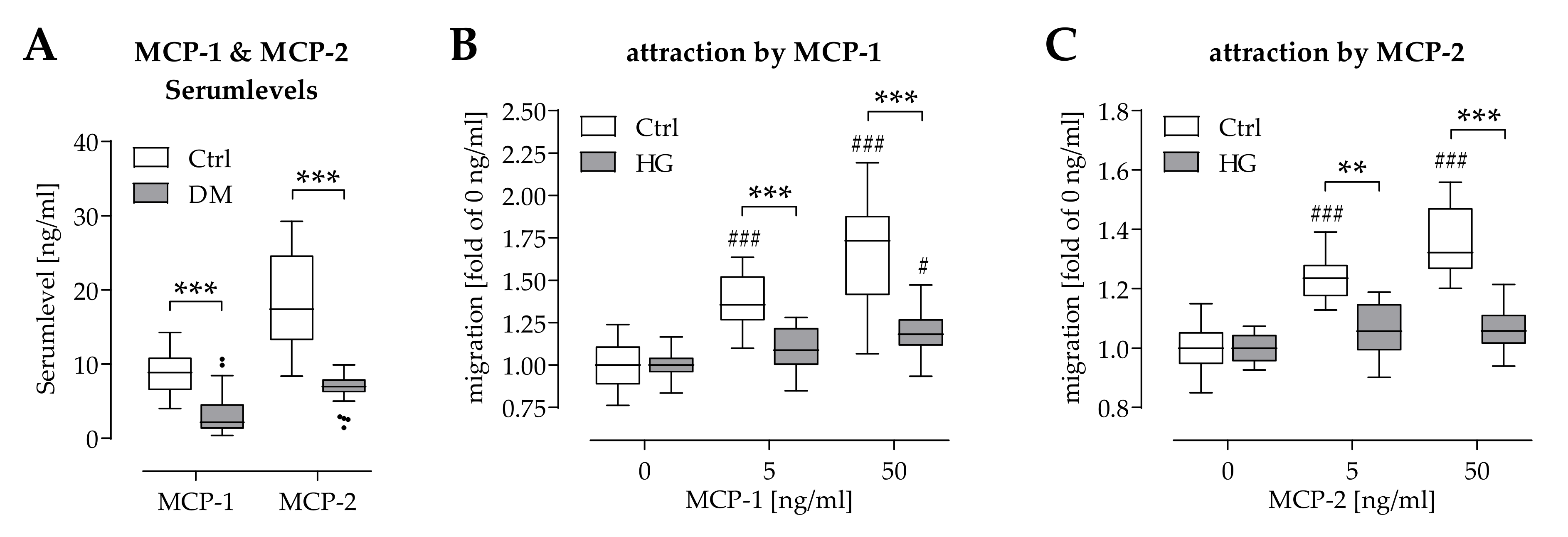
2.4. Hyperglycemia Affected Chemoattraction of Mononuclear Cells by MCP-1 and MCP-2
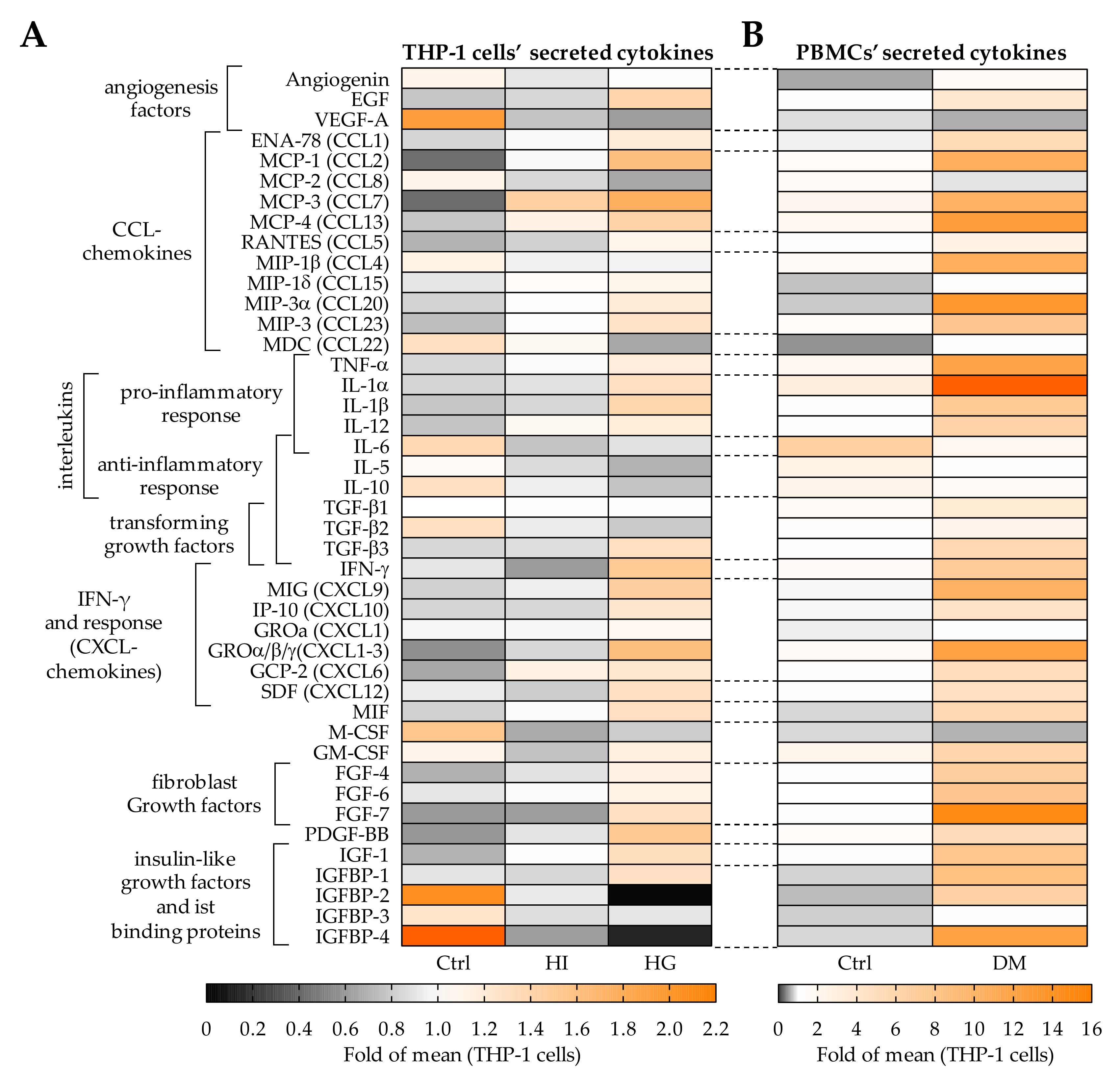
2.5. Pre-/Diabetic Conditions Altered the Secretome of THP-1 Cells
2.6. THP-1 Cells Failed to Stimulate SCP-1 Cells Migration under Pre-/Diabetic Conditions
3. Discussion
4. Materials and Methods
4.1. Patient Material
4.1.1. PBMCs
4.1.2. Osteoprogenitor Cells
4.2. Cell Lines
4.2.1. THP-1 Cells
4.2.2. SCP-1 Cells
4.3. Cell Culture Conditions
4.3.1. Simulation of Pre-/Diabetic Conditions
- Normoglycemic control conditions (Ctrl): To equalize osmolarity compared to hyperglycemic conditions (HG) mannitol was added to the medium to reach a concentration of 25 mM glucose + mannitol.
- Hyperinsulinemic “prediabetic” conditions (HI): Ctrl medium was supplemented with 160 I.U./L insulin (Actrapid, NovoNordisk, Bagsværd, Denmark).
- Hyperglycemic “diabetic” conditions (HG): glucose concentration of the basal medium was increased to 25 mM.
4.3.2. Hypoxia Induction
4.4. Cell Migration Assays
4.4.1. Scratch Assay and ORIS™ Migration Assay for Osteoprogenitor Cells
4.4.2. Boyden Chamber Assay for Migration of Mononuclear Cells
4.5. Quantification of Protein Levels
4.5.1. PathScan® Intracellular Signaling Array with SCP-1 Cell Lysates
4.5.2. Fractionation of THP-1 Cell Conditioned Medium
4.5.3. Western Blot with SCP-1 Cell Lysates
4.5.4. Human Cytokine Array C5 and Human Growth Factor Array with Media Conditioned by Mononuclear Cells
4.5.5. Enzyme-Linked Immunosorbent Assay (ELISA) with Serum Samples
4.6. RNA Isolation and RT-PCR
4.7. Sulforhodamine B (SRB) Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation endproducts |
| BMD | Bone mineral density |
| CCL | C-C motif chemokine ligand |
| CXCL | C-X-C motif chemokine ligand |
| DEPC | Diethyl pyrocarbonate |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| EtOH | Ethanol |
| FCS | Fetal calf serum |
| FGF | Fibroblast growth factor |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GM-CSF | Granulocyte-macrophage colony stimulating factor |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HG | High glucose |
| HI | High insulin |
| IFN-γ | Interferon gamma |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| MAPkinase | Mitogen activated protein kinase |
| M-CSF | Macrophage colony stimulating factor |
| MCP | Monocyte chemoattractant protein |
| MEM | Minimum essential medium |
| MSCs | mesenchymal stem cells |
| PBMCs | peripheral blood mononuclear cells |
| PBS | Phosphate buffered saline |
| PDGF | Platelet-derived growth factor |
| RIPA | Radioimmunoprecipitation assay |
| SRB | Sulforhodamine B |
| T2DM | Diabetes Mellitus Type 2 |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| VEGF | Vascular endothelial growth factor |
References
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R. Diabetes and global ageing among 65-99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9 th edition. Diabetes Res. Clin. Pract. 2020, 162, 108078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, Q.; Nie, B.; Zhang, H.; Zhai, H.; Zhao, L.; Xia, P.; Lu, Y.; Wang, N. Trends in Bone Mineral Density, Osteoporosis, and Osteopenia Among U.S. Adults With Prediabetes, 2005–2014. Diabetes Care 2020, 43, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, S.; Kostev, K.; Kohlmann, T. Prevalence of diabetic foot syndrome and its risk factors in the UK. J. Wound Care 2010, 19, 333–337. [Google Scholar] [CrossRef]
- Pscherer, S.; Dippel, F.W.; Lauterbach, S.; Kostev, K. Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real-life conditions in Germany. Prim. Care Diabetes 2012, 6, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—A meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef]
- Picke, A.K.; Campbell, G.; Napoli, N.; Hofbauer, L.C.; Rauner, M. Update on the impact of type 2 diabetes mellitus on bone metabolism and material properties. Endocr. Connect. 2019, 8, R55–R70. [Google Scholar] [CrossRef]
- Marin, C.; Luyten, F.P.; Van der Schueren, B.; Kerckhofs, G.; Vandamme, K. The Impact of Type 2 Diabetes on Bone Fracture Healing. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 2005, 48, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. The effect of diabetes mellitus on osseous healing. Clin. Oral Implant. Res. 2010, 21, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.K.; Do, T.P.; Critchlow, C.W.; Dent, R.E.; Jick, S.S. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012, 83, 653–660. [Google Scholar] [CrossRef]
- Kahm, K.; Laxy, M.; Schneider, U.; Rogowski, W.H.; Lhachimi, S.K.; Holle, R. Health Care Costs Associated With Incident Complications in Patients With Type 2 Diabetes in Germany. Diabetes Care 2018, 41, 971–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mildner, M.; Hacker, S.; Haider, T.; Gschwandtner, M.; Werba, G.; Barresi, C.; Zimmermann, M.; Golabi, B.; Tschachler, E.; Ankersmit, H.J. Secretome of peripheral blood mononuclear cells enhances wound healing. PLoS ONE 2013, 8, e60103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care (New Rochelle) 2013, 2, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellowley, C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. BoneKEy Rep. 2013, 2, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacker, S.; Mittermayr, R.; Traxler, D.; Keibl, C.; Resch, A.; Salminger, S.; Leiss, H.; Hacker, P.; Gabriel, C.; Golabi, B.; et al. The secretome of stressed peripheral blood mononuclear cells increases tissue survival in a rodent epigastric flap model. Bioeng. Transl. Med. 2021, 6, e10186. [Google Scholar] [CrossRef]
- Wagner, T.; Traxler, D.; Simader, E.; Beer, L.; Narzt, M.S.; Gruber, F.; Madlener, S.; Laggner, M.; Erb, M.; Vorstandlechner, V.; et al. Different pro-angiogenic potential of gamma-irradiated PBMC-derived secretome and its subfractions. Sci. Rep. 2018, 8, 18016. [Google Scholar] [CrossRef] [Green Version]
- Hacker, S.; Mittermayr, R.; Nickl, S.; Haider, T.; Lebherz-Eichinger, D.; Beer, L.; Mitterbauer, A.; Leiss, H.; Zimmermann, M.; Schweiger, T.; et al. Paracrine Factors from Irradiated Peripheral Blood Mononuclear Cells Improve Skin Regeneration and Angiogenesis in a Porcine Burn Model. Sci. Rep. 2016, 6, 25168. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Uludağ, H.; Wang, Z.; Rezansoff, A.; Jiang, H. Macrophages Inhibit Migration, Metabolic Activity and Osteogenic Differentiation of Human Mesenchymal Stem Cells in vitro. Cells Tissues Organs 2012, 195, 473–483. [Google Scholar] [CrossRef]
- Olekson, M.P.; Faulknor, R.A.; Hsia, H.C.; Schmidt, A.M.; Berthiaume, F. Soluble Receptor for Advanced Glycation End Products Improves Stromal Cell-Derived Factor-1 Activity in Model Diabetic Environments. Adv. Wound Care (New Rochelle) 2016, 5, 527–538. [Google Scholar] [CrossRef]
- Ehnert, S.; Freude, T.; Ihle, C.; Mayer, L.; Braun, B.; Graeser, J.; Flesch, I.; Stockle, U.; Nussler, A.K.; Pscherer, S. Factors circulating in the blood of type 2 diabetes mellitus patients affect osteoblast maturation - description of a novel in vitro model. Exp. Cell Res. 2015, 332, 247–258. [Google Scholar] [CrossRef]
- Ehnert, S.; Linnemann, C.; Aspera-Werz, R.H.; Bykova, D.; Biermann, S.; Fecht, L.; De Zwart, P.M.; Nussler, A.K.; Stuby, F. Immune Cell Induced Migration of Osteoprogenitor Cells Is Mediated by TGF-beta Dependent Upregulation of NOX4 and Activation of Focal Adhesion Kinase. Int. J. Mol. Sci. 2018, 19, 2239. [Google Scholar] [CrossRef] [Green Version]
- Panahipour, L.; Kargarpour, Z.; Laggner, M.; Mildner, M.; Ankersmit, H.J.; Gruber, R. TGF-beta in the Secretome of Irradiated Peripheral Blood Mononuclear Cells Supports In Vitro Osteoclastogenesis. Int. J. Mol. Sci. 2020, 21, 8569. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.; Gaber, T.; Strehl, C.; Jakstadt, M.; Hoff, H.; Schmidt-Bleek, K.; Lang, A.; Rohner, E.; Huscher, D.; Matziolis, G.; et al. A Pronounced Inflammatory Activity Characterizes the Early Fracture Healing Phase in Immunologically Restricted Patients. Int. J. Mol. Sci. 2017, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.; Gaber, T.; Strehl, C.; Schmidt-Bleek, K.; Lang, A.; Huscher, D.; Burmester, G.R.; Schmidmaier, G.; Perka, C.; Duda, G.N.; et al. Immunological characterization of the early human fracture hematoma. Immunol. Res. 2016, 64, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, P.; Li, J.; Li, Y.; Wang, S.; Wu, X.; Sun, S.; Cen, S.; Su, H.; Deng, W.; et al. MCP1 triggers monocyte dysfunctions during abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. J. Mol. Med. (Berl.) 2017, 95, 143–154. [Google Scholar] [CrossRef]
- Häussling, V.; Aspera-Werz, R.H.; Rinderknecht, H.; Springer, F.; Arnscheidt, C.; Menger, M.M.; Histing, T.; Nussler, A.K.; Ehnert, S. 3D Environment Is Required In Vitro to Demonstrate Altered Bone Metabolism Characteristic for Type 2 Diabetics. Int. J. Mol. Sci. 2021, 22, 2925. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Haider, T.; Höftberger, R.; Rüger, B.; Mildner, M.; Blumer, R.; Mitterbauer, A.; Buchacher, T.; Sherif, C.; Altmann, P.; Redl, H.; et al. The secretome of apoptotic human peripheral blood mononuclear cells attenuates secondary damage following spinal cord injury in rats. Exp. Neurol. 2015, 267, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Lichtenauer, M.; Mildner, M.; Hoetzenecker, K.; Zimmermann, M.; Podesser, B.K.; Sipos, W.; Berényi, E.; Dworschak, M.; Tschachler, E.; Gyöngyösi, M.; et al. Secretome of apoptotic peripheral blood cells (APOSEC) confers cytoprotection to cardiomyocytes and inhibits tissue remodelling after acute myocardial infarction: A preclinical study. Basic Res. Cardiol. 2011, 106, 1283–1297. [Google Scholar] [CrossRef] [Green Version]
- Lind, M.; Trindade, M.C.; Nakashima, Y.; Schurman, D.J.; Goodman, S.B.; Smith, L. Chemotaxis and activation of particle-challenged human monocytes in response to monocyte migration inhibitory factor and C-C chemokines. J. Biomed. Mater. Res. 1999, 48, 246–250. [Google Scholar] [CrossRef]
- Toso, C.; Emamaullee, J.A.; Merani, S.; Shapiro, A.M. The role of macrophage migration inhibitory factor on glucose metabolism and diabetes. Diabetologia 2008, 51, 1937–1946. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, A.W., Jr. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar] [CrossRef]
- Freude, T.; Braun, K.F.; Haug, A.; Pscherer, S.; Stöckle, U.; Nussler, A.K.; Ehnert, S. Hyperinsulinemia reduces osteoblast activity in vitro via upregulation of TGF-β. J. Mol. Med. (Berl.) 2012, 90, 1257–1266. [Google Scholar] [CrossRef]
- Lerman, O.Z.; Galiano, R.D.; Armour, M.; Levine, J.P.; Gurtner, G.C. Cellular dysfunction in the diabetic fibroblast: Impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am. J. Pathol. 2003, 162, 303–312. [Google Scholar] [CrossRef]
- Sarahrudi, K.; Mousavi, M.; Grossschmidt, K.; Sela, N.; König, F.; Vécsei, V.; Aharinejad, S. The impact of colony-stimulating factor-1 on fracture healing: An experimental study. J. Orthop. Res. 2009, 27, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Wu, C.S.; Chiu, M.H.; Wu, C.H.; Chang, Y.T.; Chen, G.S.; Lan, C.E. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: An important mechanism to delay the diabetic wound healing. J. Dermatol. Sci. 2019, 96, 159–167. [Google Scholar] [CrossRef]
- Ackerman, J.E.; Geary, M.B.; Orner, C.A.; Bawany, F.; Loiselle, A.E. Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PLoS ONE 2017, 12, e0181127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhao, Y.; Hao, H.; Dai, H.; Han, Q.; Tong, C.; Liu, J.; Han, W.; Fu, X. Mesenchymal stem cell-conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int. J. Low. Extremity Wounds 2015, 14, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, H.; Khan, M.T.; Sharan, K. Hyperglycemia impairs osteoblast cell migration and chemotaxis due to a decrease in mitochondrial biogenesis. Mol. Cell. Biochem. 2020, 469, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, V.; Wong, M.M.; Redpath, A.N.; Ersek, A.; Baban, D.F.; Williams, L.M.; Cope, A.P.; Horwood, N.J. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS ONE 2012, 7, e39871. [Google Scholar] [CrossRef] [Green Version]
- Laggner, M.; Copic, D.; Nemec, L.; Vorstandlechner, V.; Gugerell, A.; Gruber, F.; Peterbauer, A.; Ankersmit, H.J.; Mildner, M. Therapeutic potential of lipids obtained from γ-irradiated PBMCs in dendritic cell-mediated skin inflammation. EBioMedicine 2020, 55, 102774. [Google Scholar] [CrossRef]
- Simader, E.; Beer, L.; Laggner, M.; Vorstandlechner, V.; Gugerell, A.; Erb, M.; Kalinina, P.; Copic, D.; Moser, D.; Spittler, A.; et al. Tissue-regenerative potential of the secretome of γ-irradiated peripheral blood mononuclear cells is mediated via TNFRSF1B-induced necroptosis. Cell Death Dis. 2019, 10, 729. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Kaili, D.; Freeman, J.; Lei, C.Y.; Geng, B.C.; Tan, T.; He, J.F.; Shi, Z.; Ma, J.J.; Luo, Y.H.; et al. Diabetes inhibits corneal epithelial cell migration and tight junction formation in mice and human via increasing ROS and impairing Akt signaling. Acta Pharmacol. Sin. 2019, 40, 1205–1211. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ito, H.; Kitaori, T.; Murata, K.; Shibuya, H.; Furu, M.; Yoshitomi, H.; Fujii, T.; Yamamoto, K.; Matsuda, S. MCP/CCR2 signaling is essential for recruitment of mesenchymal progenitor cells during the early phase of fracture healing. PLoS ONE 2014, 9, e104954. [Google Scholar] [CrossRef] [Green Version]
- Khan, U.A.; Hashimi, S.M.; Bakr, M.M.; Forwood, M.R.; Morrison, N.A. CCL2 and CCR2 are Essential for the Formation of Osteoclasts and Foreign Body Giant Cells. J. Cell. Biochem. 2016, 117, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Kao, Y.T.; Huang, W.K.; Lin, K.Y.; Wu, S.C.; Hsu, S.C.; Schuyler, S.C.; Li, L.Y.; Lu, F.L.; Lu, J. CCL5/RANTES is important for inducing osteogenesis of human mesenchymal stem cells and is regulated by dexamethasone. Biosci. Trends 2014, 8, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintges, K.; Beil, F.T.; Albers, J.; Jeschke, A.; Schweizer, M.; Claass, B.; Tiegs, G.; Amling, M.; Schinke, T. Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J. Bone Miner. Res. 2013, 28, 2070–2080. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Christoffersson, G.; Massena, S.; Ahl, D.; Jansson, L.; Henriksnas, J.; Phillipson, M. Increased recruitment but impaired function of leukocytes during inflammation in mouse models of type 1 and type 2 diabetes. PLoS ONE 2011, 6, e22480. [Google Scholar] [CrossRef] [PubMed]
- Kostidou, E.; Koliakos, G.; Paletas, K.; Kaloyianni, M. Monocyte attachment and migration through collagen IV in diabetes mellitus. Mol. Cells 2008, 25, 452–456. [Google Scholar] [PubMed]
- Bouma, G.; Coppens, J.M.C.; Lam-Tse, W.-K.; Luini, W.; Sintnicolaas, K.; Levering, W.H.; Sozzani, S.; Drexhage, H.A.; Versnel, M.A. An increased MRP8/14 expression and adhesion, but a decreased migration towards proinflammatory chemokines of type 1 diabetes monocytes. Clin. Exp. Immunol. 2005, 141, 509–517. [Google Scholar] [CrossRef]
- Nandy, D.; Janardhanan, R.; Mukhopadhyay, D.; Basu, A. Effect of hyperglycemia on human monocyte activation. J. Investig. Med. 2011, 59, 661–667. [Google Scholar] [CrossRef]
- Xiang, G.; Huang, X.; Wang, T.; Wang, J.; Zhao, G.; Wang, H.; Feng, Y.; Lei, W.; Hu, X. The impact of sitagliptin on macrophage polarity and angiogenesis in the osteointegration of titanium implants in type 2 diabetes. Biomed. Pharmacother. 2020, 126, 110078. [Google Scholar] [CrossRef]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Moser, L.B.; Neubauer, M.; Lacza, Z.; Nehrer, S.; De Luna, A. Effects of Extracellular Vesicles from Blood-Derived Products on Osteoarthritic Chondrocytes within an Inflammation Model. Int. J. Mol. Sci. 2021, 22, 7224. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ye, Y.P.; Dong, Y.H.; Guo, F.J.; Chen, A.M.; Huang, S.L. Inhibitory effects of high glucose/insulin environment on osteoclast formation and resorption in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, K.H.; Park, C.H.; Shin, S.Y.; Rhyu, I.C.; Lee, Y.M.; Seol, Y.J. Enhanced Bone Regeneration by Diabetic Cell-Based Adenoviral BMP-2 Gene Therapy in Diabetic Animals. Tissue Eng. Part A 2018, 24, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Pscherer, S.; Freude, T.; Forst, T.; Nussler, A.K.; Braun, K.F.; Ehnert, S. Anti-diabetic treatment regulates pro-fibrotic TGF-β serum levels in type 2 diabetics. Diabetol. Metab. Syndr. 2013, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Wei, J.; Hettinghouse, A.; Li, G.; Li, X.; Einhorn, T.A.; Liu, C.-j. Progranulin promotes bone fracture healing via TNFR pathways in mice with type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. USA 2021, 1490, 77–89. [Google Scholar] [CrossRef]
- Tevlin, R.; Seo, E.Y.; Marecic, O.; McArdle, A.; Tong, X.; Zimdahl, B.; Malkovskiy, A.; Sinha, R.; Gulati, G.; Li, X.; et al. Pharmacological rescue of diabetic skeletal stem cell niches. Sci. Transl. Med. 2017, 9, eaag2809. [Google Scholar] [CrossRef] [Green Version]
- Rahbar Layegh, E.; Fadaei Fathabadi, F.; Lotfinia, M.; Zare, F.; Mohammadi Tofigh, A.; Abrishami, S.; Piryaei, A. Photobiomodulation therapy improves the growth factor and cytokine secretory profile in human type 2 diabetic fibroblasts. J. Photochem. Photobiol. B 2020, 210, 111962. [Google Scholar] [CrossRef]
- De Mayo, T.; Conget, P.; Becerra-Bayona, S.; Sossa, C.L.; Galvis, V.; Arango-Rodríguez, M.L. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS ONE 2017, 12, e0177533. [Google Scholar] [CrossRef] [Green Version]
- Saheli, M.; Bayat, M.; Ganji, R.; Hendudari, F.; Kheirjou, R.; Pakzad, M.; Najar, B.; Piryaei, A. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch. Dermatol. Res. 2020, 312, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Zimmermann, M.; Mitterbauer, A.; Ellinger, A.; Gruber, F.; Narzt, M.S.; Zellner, M.; Gyöngyösi, M.; Madlener, S.; Simader, E.; et al. Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration. Sci. Rep. 2015, 5, 16662. [Google Scholar] [CrossRef] [PubMed]
- Bocker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Camp, J.P.; Capitano, A.T. Induction of zone-like liver function gradients in HepG2 cells by varying culture medium height. Biotechnol. Prog. 2007, 23, 1485–1491. [Google Scholar] [CrossRef]
| Target | Gene Bank Accession Number | Sequence Forward Primer | Sequence Reverse Primer | Ta [°C] | No. of Cycles | Amplicon Size [bp] |
|---|---|---|---|---|---|---|
| GAPDH | NM_002046.7 | GTCAGTGGTG GACCTGACCT | AGGGGTCTAC ATGGCAACTG | 56 | 25 | 420 |
| CCL2/MCP1 | NM_002982.4 | CCTTCATTCC CCAAGGGCTC | GGTTTGCTTG TCCAGGTGGT | 60 | 35 | 236 |
| CCL5 | NM_002985.3 | ATCCTCATTG CTACTGCCCTC | GCCACTGGTTA GAAATACTCC | 60 | 40 | 135 |
| CCL7/MCP3 | NM_006273.4 | CTTGCTCAGC CAGTTGGGATT | CCACTTCGTG TGGGGTCAG | 60 | 35 | 183 |
| CCL8/MCP2 | NM_005623.3 | ACTTGCTCAGC CAGATTCAGTT | CCCATCTCTC CTTGGGGTCA | 60 | 35 | 185 |
| CCL13/MCP4 | NM_005408.3 | CAGCCAGATG CACTCAACGTC | CTCCTTTGGG TCAGCACAGA | 60 | 35 | 168 |
| CXCL9 | NM_002416.3 | CAGGCTCAAAAT CCAATACAGGAG | TACTGGGGTT CCTTGCACTC | 58 | 40 | 124 |
| CSF1/M-CSF | NM_000757.6 | AAGTTTGCCT GGGTCCTCTC | CCACTCCAT CATGTGGCT | 60 | 40 | 289 |
| CSF2/GM-CSF | NM_000758.4 | GAGACACTGC TGCTGAGATGA | GAGGGCGTG CTGCTTGTA | 64 | 40 | 180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linnemann, C.; Savini, L.; Rollmann, M.F.; Histing, T.; Nussler, A.K.; Ehnert, S. Altered Secretome of Diabetic Monocytes Could Negatively Influence Fracture Healing—An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 9212. https://doi.org/10.3390/ijms22179212
Linnemann C, Savini L, Rollmann MF, Histing T, Nussler AK, Ehnert S. Altered Secretome of Diabetic Monocytes Could Negatively Influence Fracture Healing—An In Vitro Study. International Journal of Molecular Sciences. 2021; 22(17):9212. https://doi.org/10.3390/ijms22179212
Chicago/Turabian StyleLinnemann, Caren, Lorena Savini, Mika F. Rollmann, Tina Histing, Andreas K. Nussler, and Sabrina Ehnert. 2021. "Altered Secretome of Diabetic Monocytes Could Negatively Influence Fracture Healing—An In Vitro Study" International Journal of Molecular Sciences 22, no. 17: 9212. https://doi.org/10.3390/ijms22179212
APA StyleLinnemann, C., Savini, L., Rollmann, M. F., Histing, T., Nussler, A. K., & Ehnert, S. (2021). Altered Secretome of Diabetic Monocytes Could Negatively Influence Fracture Healing—An In Vitro Study. International Journal of Molecular Sciences, 22(17), 9212. https://doi.org/10.3390/ijms22179212







