Enhancing the Efficiency of Distraction Osteogenesis through Rate-Varying Distraction: A Computational Study
Abstract
:1. Introduction
2. Results
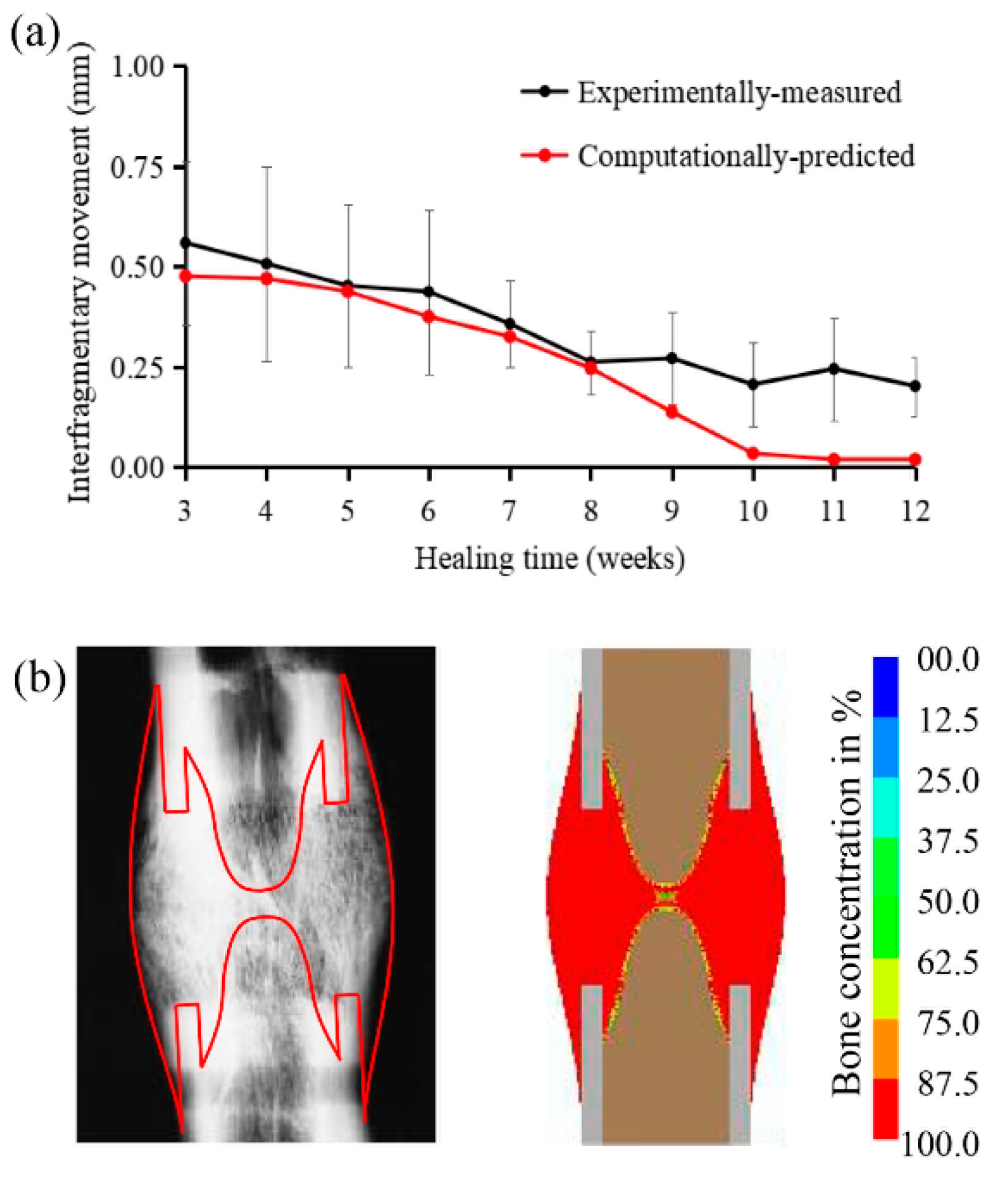
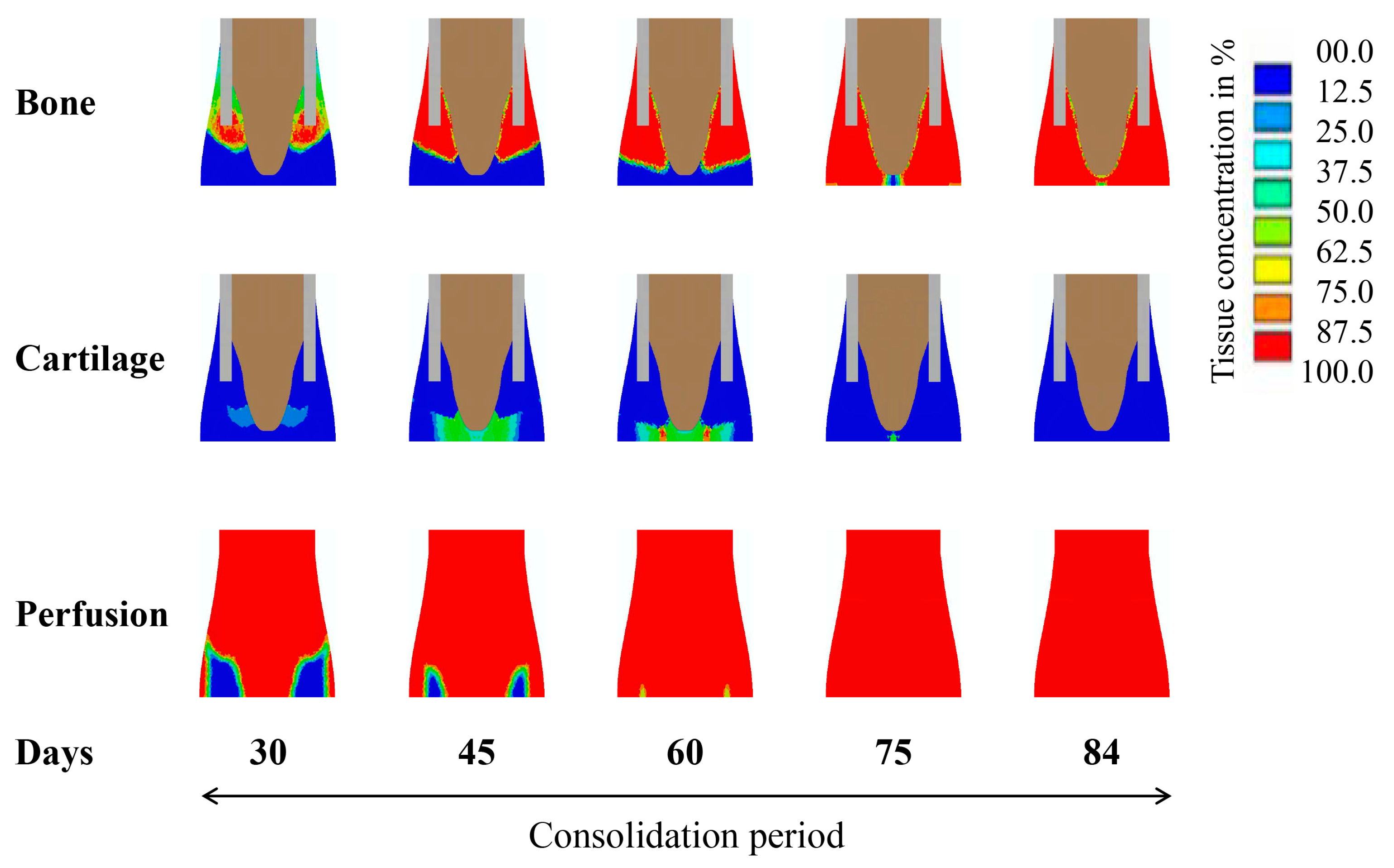
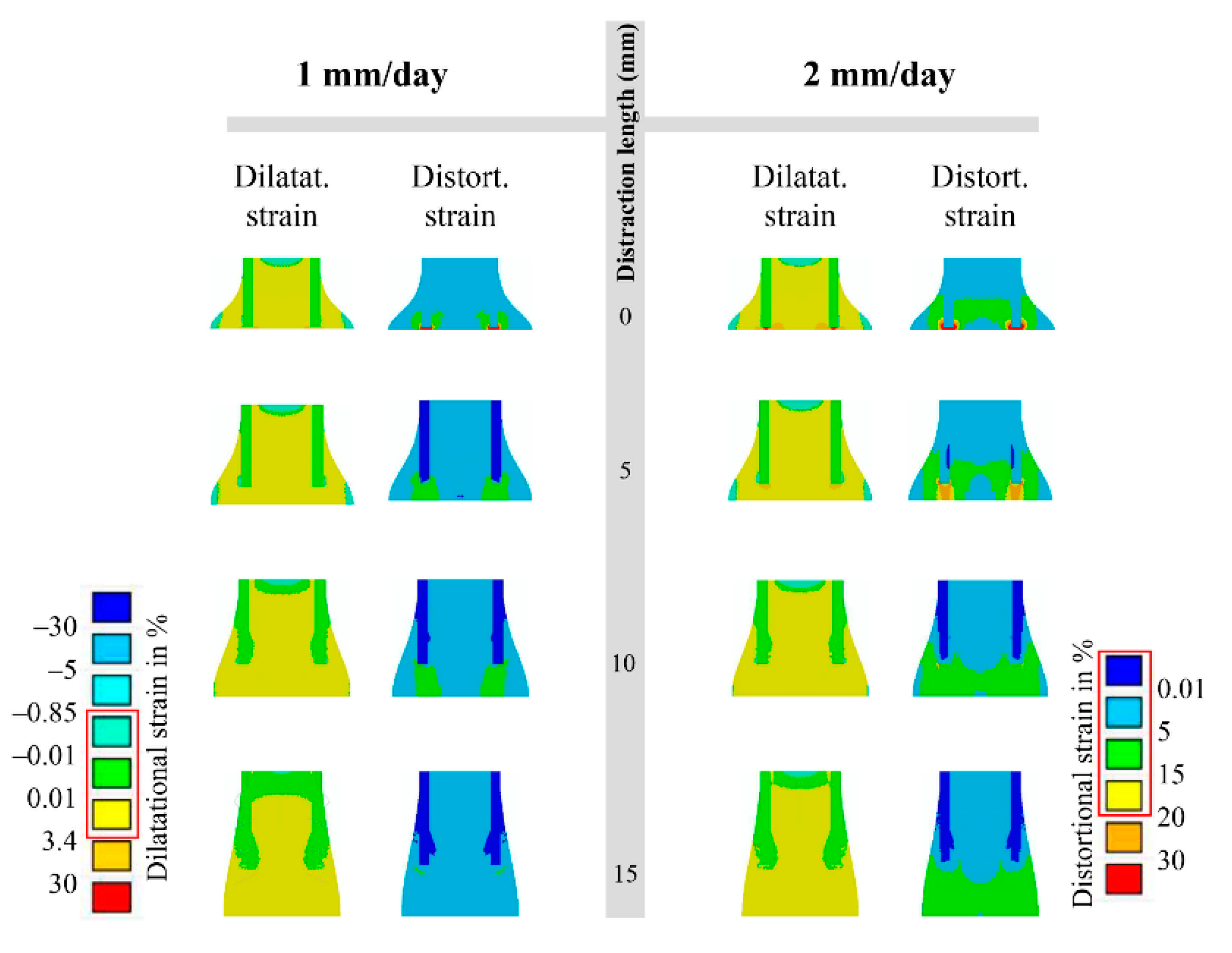
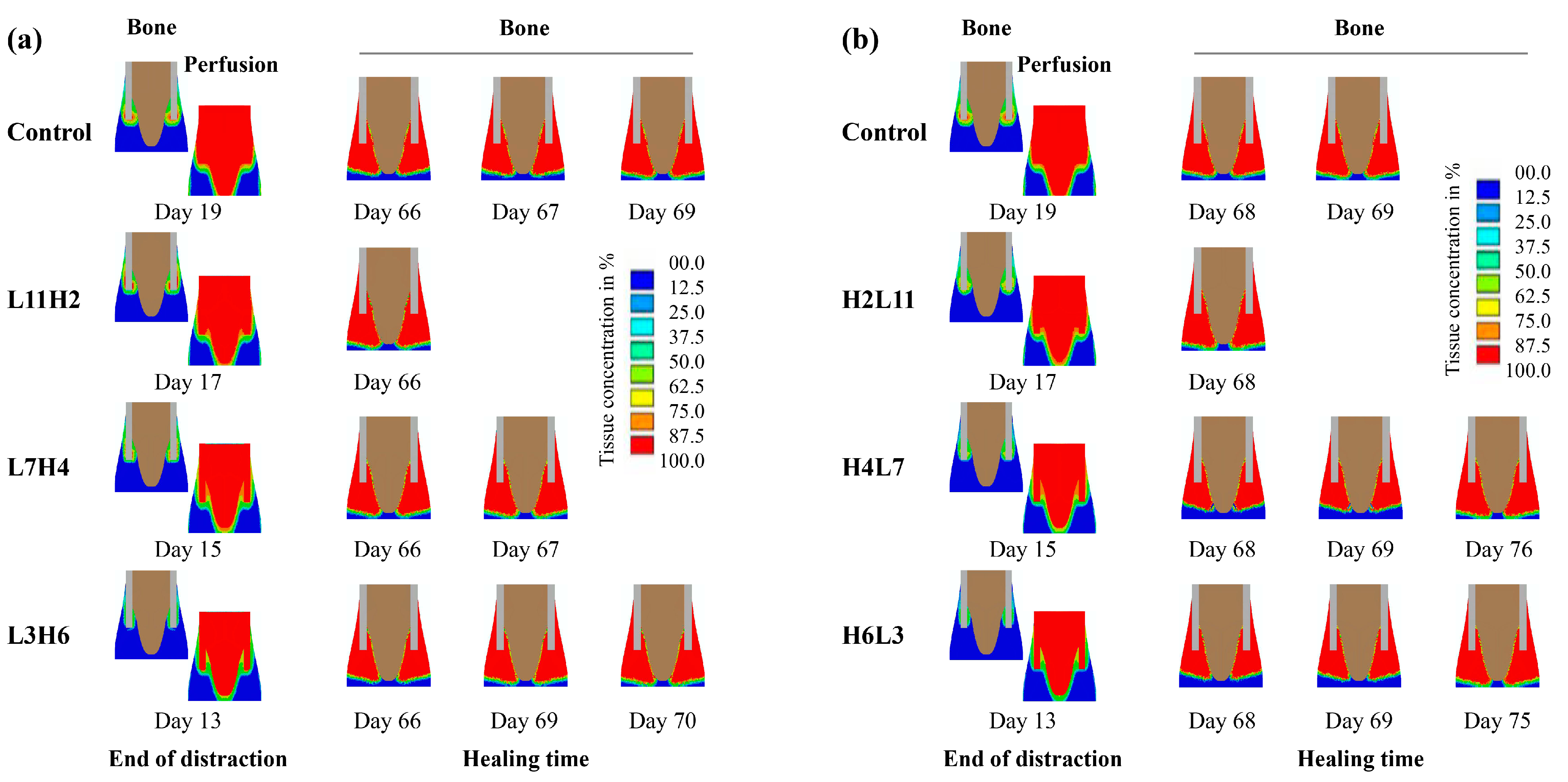
2.1. Computational Predictions and Experimental Validation of the Bone Regeneration Process under the Constant Low-Rate Distraction Protocol
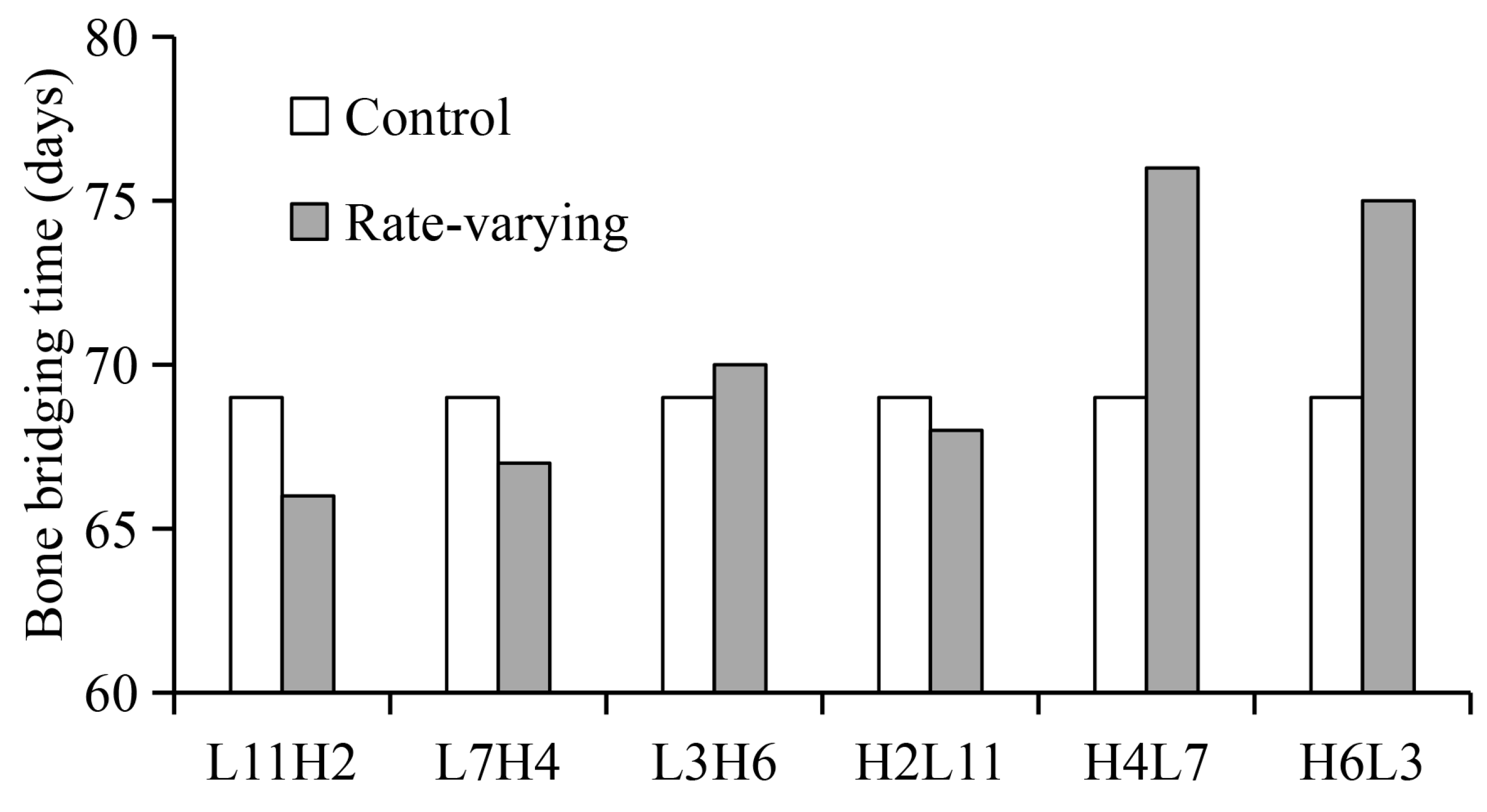
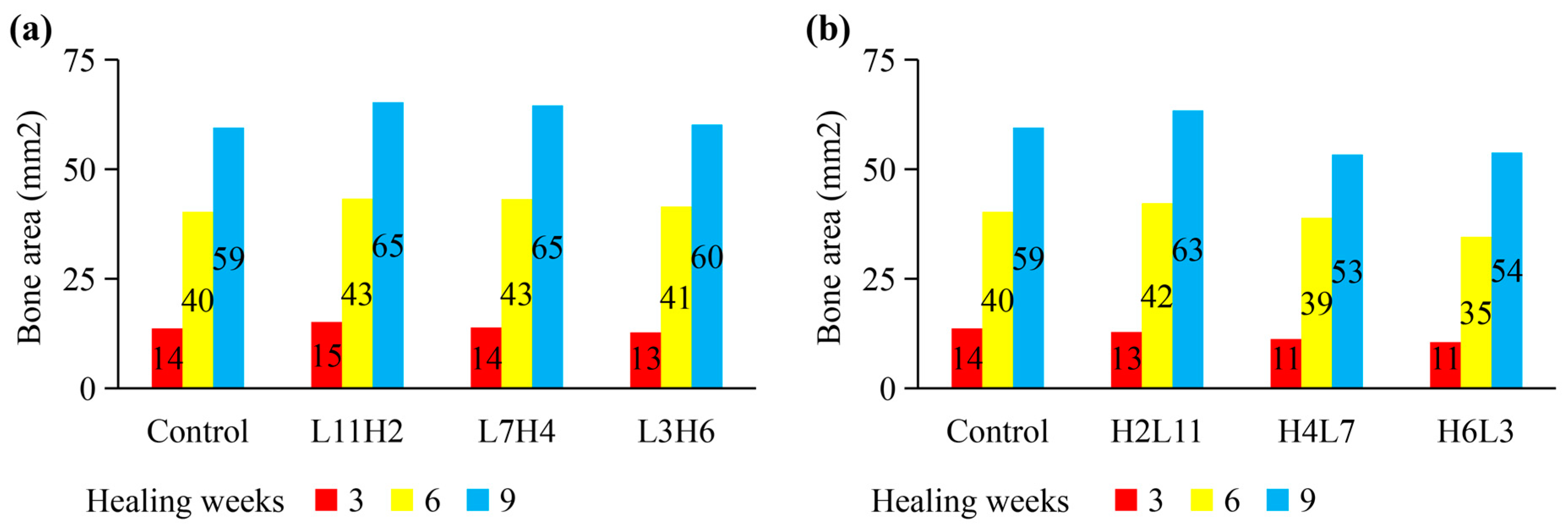
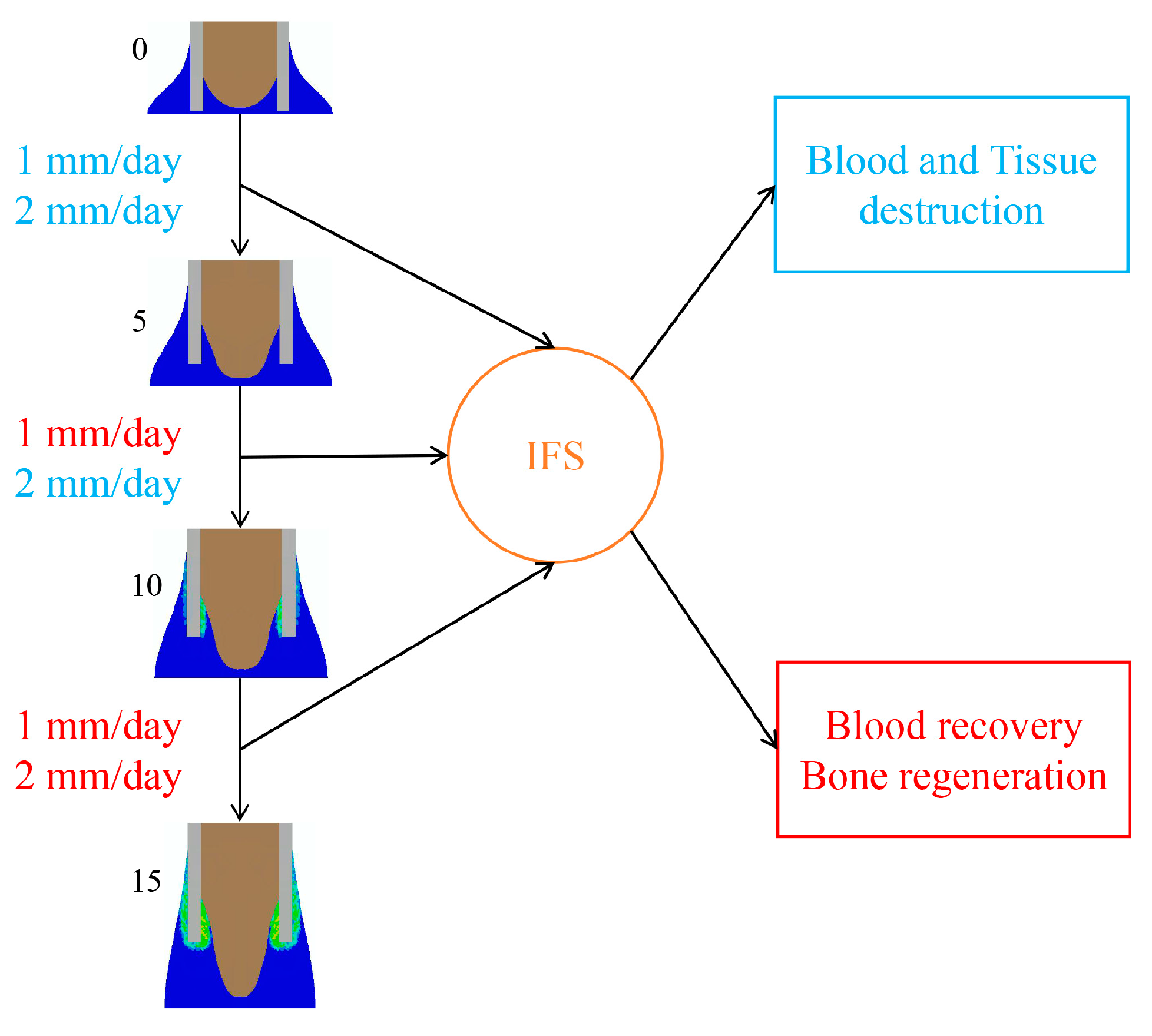
2.2. Influence of “Rate-Varying” Distractions on Bone Healing
3. Discussion
4. Materials and Methods
4.1. Finite Element Modeling of the Distraction Site
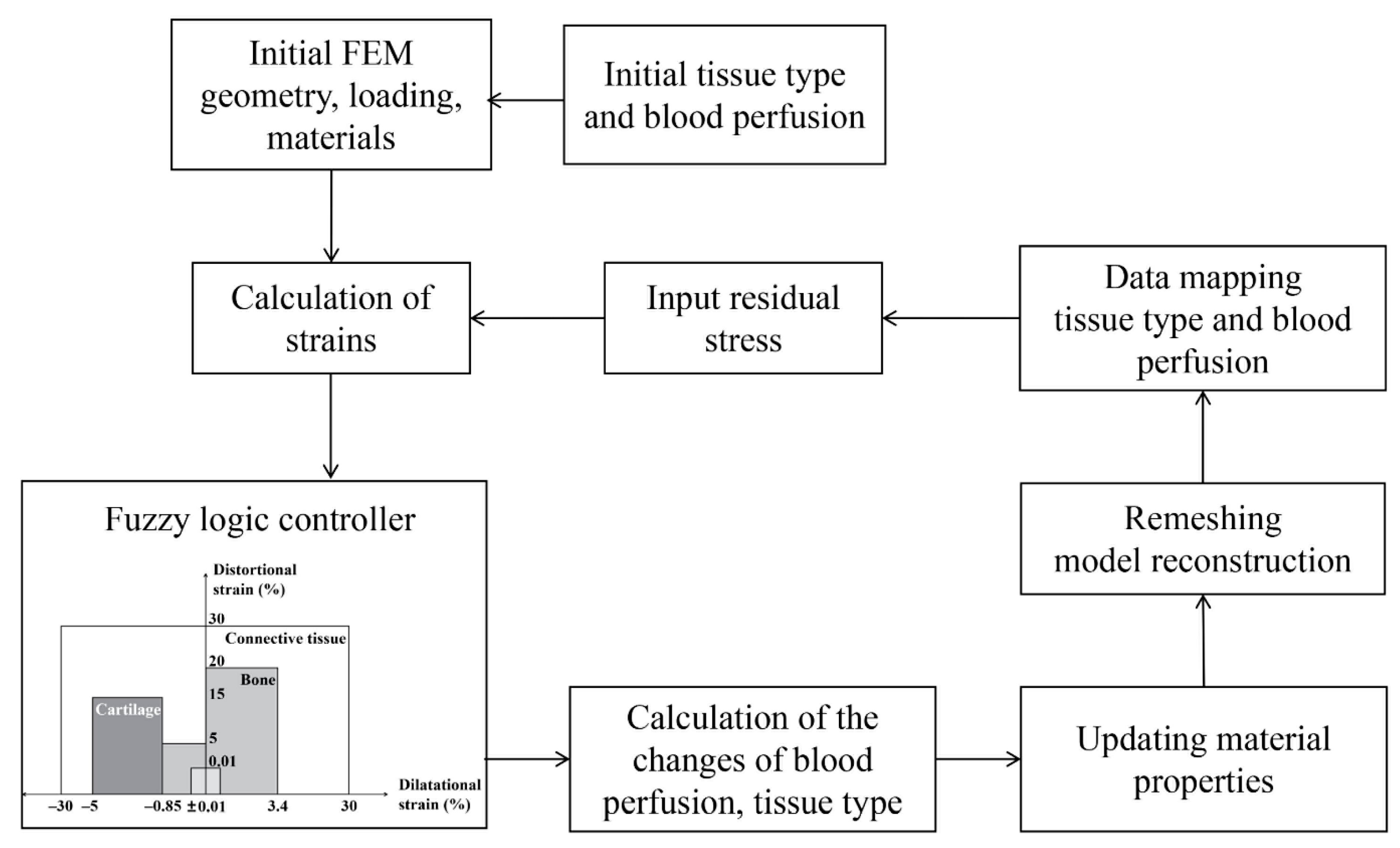
4.2. Mechanobiological Simulation of Distraction Osteogenesis
4.3. Model Implementation and Validation
4.4. Rate-Varying Distraction Protocols
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wee, C.; Ruter, D.; Schulz, S.; Sisk, G.; West, J.; Tintle, S.; Valerio, I. Reconstruction of extremity long bone defects with vascularized fibula bone grafts. Plast. Aesthetic Res. 2019, 6, 12. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, L.; Laule, J.; Wenger, K.; Suger, G.; Liener, U.; Kinzl, L. The influence of stiffness of the fixator on maturation of callus after segmental transport. J. Bone Jt. Surg.Ser. B 2000, 82, 142–148. [Google Scholar] [CrossRef]
- Stoneburner, J.; Azadgoli, B.; Howell, A.C.; Tucker, D.; Marecek, G.; Carey, J. Review of soft tissue coverage options in distraction osteogenesis of the extremity. Plast. Aesthetic Res. 2020, 7, 13. [Google Scholar] [CrossRef]
- Fu, R.; Feng, Y.; Liu, Y.; Yang, H. Mechanical regulation of bone regeneration during distraction osteogenesis. Med. Nov. Technol. Devices 2021, 11, 100077. [Google Scholar] [CrossRef]
- Singh, M.; Vashistha, A.; Chaudhary, M.; Kaur, G. Biological basis of distraction osteogenesis—A review. J. Oral Maxillofac. Surg. Med. Pathol. 2016, 28, 1–7. [Google Scholar] [CrossRef]
- Hariri, F.; Chin, S.Y.; Rengarajoo, J.; Foo, Q.C.; Abidin, S.N.N.Z.; Badruddin, A.F.A. Distraction Osteogenesis in Oral and Craniomaxillofacial Reconstructive Surgery. In Osteogenesis and Bone Regeneration; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Domingos, G.; Armés, H.; Dias, I.; Viegas, C.; Requicha, J. Distraction Osteogenesis: Biological Principles and Its Application in Companion Animals. In Osteogenesis and Bone Regeneration; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ilizarov, G.A. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin. Orthop. Relat. Res. 1989, 249–281. [Google Scholar] [CrossRef]
- Ilizarov, G.A. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin. Orthop. Relat. Res. 1989, 263–285. [Google Scholar] [CrossRef]
- Li, G.; Simpson, A.H.R.W.; Kenwright, J.; Triffitt, J.T. Assessment of cell proliferation in regenerating bone during distraction osteogenesis at different distraction rates. J. Orthop. Res. 1997, 15, 765–772. [Google Scholar] [CrossRef]
- Li, G.; Simpson, A.H.R.W.; Kenwright, J.; Triffitt, J.T. Effect of lengthening rate on angiogenesis during distraction osteogenesis. J. Orthop. Res. 1999, 17, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kiss, S.; Pap, K.; Vízkelety, T.; Terebessy, T.; Balla, M.; Szőke, G. The humerus is the best place for bone lengthening. Int. Orthop. 2008, 32, 385–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, B.; Gosheger, G.; Wirth, T.; Horn, J.; Rödl, R. Leg Length Discrepancy—Treatment Indications and Strategies. Dtsch. Arztebl. Int. 2020, 117, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Li, P.; Du, H.M.; Liu, L.; Zheng, X.H.; Lin, Y.F.; Wang, H.; Jing, W.; Tang, W.; Chen, W.H.; et al. Effects of bone morphogenetic protein 2 gene therapy on new bone formation during mandibular distraction osteogenesis at rapid rate in rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.R.; Moore, D.C.; Ehrlich, M.G. Increased lengthening rate decreases expression of fibroblast growth factor 2, platelet-derived growth factor, vascular endothelial growth factor, and CD31 in a rat model of distraction osteogenesis. J. Pediatr. Orthop. 2007, 27, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Bleek, K.; Willie, B.M.; Schwabe, P.; Seemann, P.; Duda, G.N. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 2016, 27, 141–148. [Google Scholar] [CrossRef]
- Hatefi, S.; Hatefi, K.; Le Roux, F.; Alizargar, J.; Behdadipour, Z.; Yihun, Y.; Abou-El-Hossein, K. Review of automatic continuous distraction osteogenesis devices for mandibular reconstruction applications. Biomed. Eng. Online 2020, 19, 17. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, F. Simulation of Fracture Healing Applied to Distraction Osteogenesis. Ph.D. Thesis, Universität Ulm. Medizinische Fakultät, Ulm, Germany, 2013. [Google Scholar]
- Epari, D.R.; Taylor, W.R.; Heller, M.O.; Duda, G.N. Mechanical conditions in the initial phase of bone healing. Clin. Biomech. 2006, 21, 646–655. [Google Scholar] [CrossRef]
- Claes, L.; Blakytny, R.; Göckelmann, M.; Schoen, M.; Ignatius, A.; Willie, B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J. Orthop. Res. 2009, 27, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, H.; Comas, O.; van Donkelaar, C.C.; Mediavilla, J.; Wilson, W.; Huiskes, R.; Ito, K. Bone regeneration during distraction osteogenesis: Mechano-regulation by shear strain and fluid velocity. J. Biomech. 2007, 40, 2002–2011. [Google Scholar] [CrossRef]
- Reina-Romo, E.; Gómez-Benito, M.J.; Domínguez, J.; Niemeyer, F.; Wehner, T.; Simon, U.; Claes, L.E. Effect of the fixator stiffness on the young regenerate bone after bone transport: Computational approach. J. Biomech. 2011, 44, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Reina-Romo, E.; Gómez-Benito, M.J.; García-Aznar, J.M.; Domínguez, J.; Doblaré, M. Modeling distraction osteogenesis: Analysis of the distraction rate. Biomech. Model. Mechanobiol. 2009, 8, 323–335. [Google Scholar] [CrossRef]
- Mora-Macías, J.; Reina-Romo, E.; Domínguez, J. Model of the distraction callus tissue behavior during bone transport based in experiments in vivo. J. Mech. Behav. Biomed. Mater. 2016, 61, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.E.; Heigele, C.A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 1999, 32, 255–266. [Google Scholar] [CrossRef]
- Meyers, N.; Schülke, J.; Ignatius, A.; Claes, L. Evolution of callus tissue behavior during stable distraction osteogenesis. J. Mech. Behav. Biomed. Mater. 2018, 85, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Carmona, P.; Mora-Macías, J.; Sanz-Herrera, J.A.; Morgaz, J.; Navarrete-Calvo, R.; Domínguez, J.; Reina-Romo, E. Mechanical Influence of Surrounding Soft Tissue on Bone Regeneration Processes: A Bone Lengthening Study. Ann. Biomed. Eng. 2020, 49, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, F.; Claes, L.; Ignatius, A.; Meyers, N.; Simon, U. Simulating lateral distraction osteogenesis. PLoS ONE 2018, 13, e0194500. [Google Scholar] [CrossRef]
- Fu, R.; Feng, Y.; Liu, Y.; Willie, B.; Yang, H. The Interactive Effects of Dynamization Time and Degree on Bone Healing. J. Orthop. Res. 2021. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, R.; Feng, Y.; Bertrand, D.; Du, T.; Liu, Y.; Willie, B.M.; Yang, H. Enhancing the Efficiency of Distraction Osteogenesis through Rate-Varying Distraction: A Computational Study. Int. J. Mol. Sci. 2021, 22, 11734. https://doi.org/10.3390/ijms222111734
Fu R, Feng Y, Bertrand D, Du T, Liu Y, Willie BM, Yang H. Enhancing the Efficiency of Distraction Osteogenesis through Rate-Varying Distraction: A Computational Study. International Journal of Molecular Sciences. 2021; 22(21):11734. https://doi.org/10.3390/ijms222111734
Chicago/Turabian StyleFu, Ruisen, Yili Feng, David Bertrand, Tianming Du, Youjun Liu, Bettina M. Willie, and Haisheng Yang. 2021. "Enhancing the Efficiency of Distraction Osteogenesis through Rate-Varying Distraction: A Computational Study" International Journal of Molecular Sciences 22, no. 21: 11734. https://doi.org/10.3390/ijms222111734
APA StyleFu, R., Feng, Y., Bertrand, D., Du, T., Liu, Y., Willie, B. M., & Yang, H. (2021). Enhancing the Efficiency of Distraction Osteogenesis through Rate-Varying Distraction: A Computational Study. International Journal of Molecular Sciences, 22(21), 11734. https://doi.org/10.3390/ijms222111734







