The Combination of Cigarette Smoking and Alcohol Consumption Synergistically Increases Reactive Carbonyl Species in Human Male Plasma
Abstract
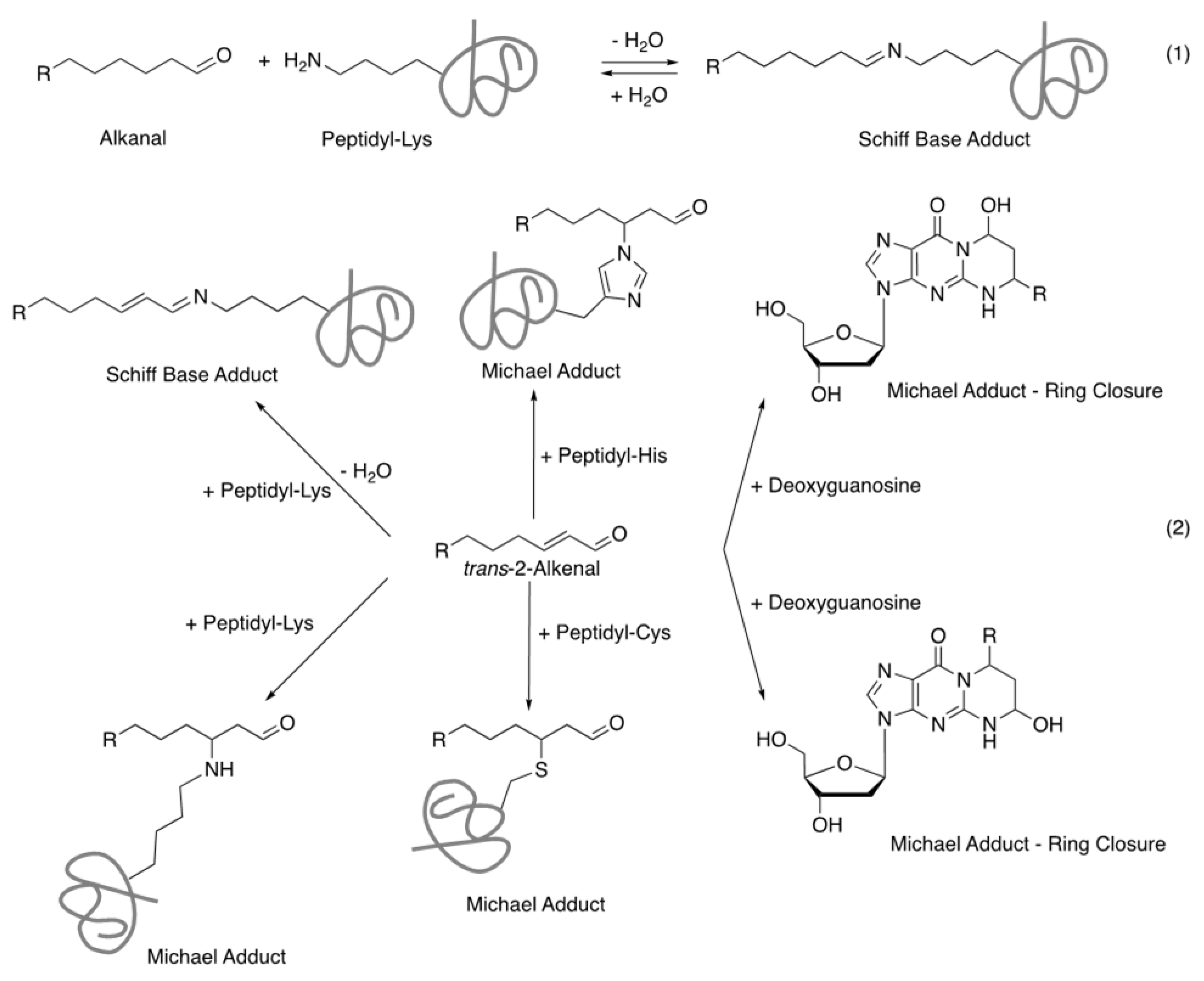
:1. Introduction
2. Results
2.1. Characteristics of Subjects and Bubble Charts of RCS Detected in Human Plasma Samples
2.2. Heatmap of the Levels of RCS Detected in the Plasma Samples
2.3. The Relative Levels of RCS Detected in the Plasma Samples of Each Group Compared to Those of the No-Smoking/No-Drinking Group
2.4. Body Mass Index, Alcohol Drinking, and Cigarette Smoking Are Associated with RCS Levels
2.5. Smoking and Drinking Synergetically Produce RCS
3. Discussion
4. Materials and Methods
4.1. The Study Subjects and Sample Collection
4.2. Chemicals
4.3. Extraction of RCS from Plasma and LC/ESI-MS/MS Analysis
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Tobacco. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 4 December 2020).
- World Health Organization. Alcohol. 2020. Available online: https://www.who.int/health-topics/alcohol#tab=tab_1 (accessed on 4 December 2020).
- Knight, J.A.; Fan, J.; Malone, K.E.; John, E.M.; Lynch, C.F.; Langballe, R.; Bernstein, L.; Shore, R.E.; Brooks, J.D.; Reiner, A.S.; et al. Alcohol consumption and cigarette smoking in combination: A predictor of contralateral breast cancer risk in the WECARE study. Int. J. Cancer 2017, 141, 916–924. [Google Scholar] [CrossRef]
- Nechuta, S.J.; Shu, X.O.; Li, H.L.; Yang, G.; Xiang, Y.B.; Cai, H.; Chow, W.H.; Ji, B.; Zhang, X.; Wen, W.; et al. Combined impact of lifestyle-related factors on total and cause-specific mortality among Chinese women: Prospective cohort study. PLoS Med. 2010, 7, e1000339. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Pai, L.; Sun, P.K.; Hsu, L.L.; Sun, C.A. Joint effects of alcohol consumption and cigarette smoking on atherogenic lipid and lipoprotein profiles: Results from a study of Chinese male population in Taiwan. Eur. J. Epidemiol. 2001, 17, 629–635. [Google Scholar] [CrossRef]
- Baker, R.R.; Massey, E.D.; Smith, G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem. Toxicol. 2004, 42, S53–S83. [Google Scholar] [CrossRef] [PubMed]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Podlutsky, A.; Wolin, M.S.; Losonczy, G.; Pacher, P.; Ungvari, Z. Oxidative stress and accelerated vascular aging: Implications for cigarette smoking. Front. Biosci. 2009, 14, 3128–3144. [Google Scholar] [CrossRef] [Green Version]
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive Carbonyl Species: A Missing Link in ROS Signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.; Lewis, A.C. Carbonyl compounds in gas and particle phases of mainstream cigarette smoke. Sci. Total Environ. 2011, 409, 5000–5009. [Google Scholar] [CrossRef]
- Carmines, E.L. Evaluation of the potential effects of ingredients added to cigarettes. Part 1: Cigarette design, testing approach, and review of results. Food Chem. Toxicol. 2002, 40, 77–91. [Google Scholar] [CrossRef]
- Ding, Y.S.; Yan, X.; Wong, J.; Chan, M.; Watson, C.H. In Situ Derivatization and Quantification of Seven Carbonyls in Cigarette Mainstream Smoke. Chem. Res. Toxicol. 2016, 29, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Hatai, M.; Horiyama, S.; Yoshikawa, N.; Kinoshita, E.; Kagota, S.; Shinozuka, K.; Nakamura, K. trans-2-Pentenal, an Active Compound in Cigarette Smoke, Identified via Its Ability to Form Adducts with Glutathione. Chem. Pharm. Bull. 2019, 67, 1000–1005. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.K.; Hile, G.A.; Capella, K.M.; Espenship, M.F.; Smith, M.M.; De Jesus, V.R.; Blount, B.C. Quantification of 19 Aldehydes in Human Serum by Headspace SPME/GC/High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2018, 52, 10571–10579. [Google Scholar] [CrossRef]
- Calejo, I.; Moreira, N.; Araujo, A.M.; Carvalho, M.; Bastos Mde, L.; de Pinho, P.G. Optimisation and validation of a HS-SPME-GC-IT/MS method for analysis of carbonyl volatile compounds as biomarkers in human urine: Application in a pilot study to discriminate individuals with smoking habits. Talanta 2016, 148, 486–493. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, K.C.; Souza-Silva, E.A.; Assumpcao, C.F.; Zini, C.A.; Welke, J.E. Carbonyl compounds and furan derivatives with toxic potential evaluated in the brewing stages of craft beer. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2020, 37, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.J.; Ochoa, A.; Kerth, C.R.; Miller, R.K.; Murray, S.C. Assessing the impact of corn variety and Texas terroir on flavor and alcohol yield in new-make bourbon whiskey. PLoS ONE 2019, 14, e0220787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani Gorji, S.; Calingacion, M.; Smyth, H.E.; Fitzgerald, M. Comprehensive profiling of lipid oxidation volatile compounds during storage of mayonnaise. J. Food Sci. Technol. 2019, 56, 4076–4090. [Google Scholar] [CrossRef]
- Moumtaz, S.; Percival, B.C.; Parmar, D.; Grootveld, K.L.; Jansson, P.; Grootveld, M. Toxic aldehyde generation in and food uptake from culinary oils during frying practices: Peroxidative resistance of a monounsaturate-rich algae oil. Sci. Rep. 2019, 9, 4125. [Google Scholar] [CrossRef] [Green Version]
- Hamberg, M.; Sanz, A.; Castresana, C. alpha-oxidation of fatty acids in higher plants: Identification of a pathogen-inducible oxygenase (piox) as an alpha-dioxygenase and biosynthesis of 2-hydroperoxylinolenic acid. J. Biol. Chem. 1999, 274, 24503–24513. [Google Scholar] [CrossRef] [Green Version]
- Onyango, A.N. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chem. Phys. Lipids 2012, 165, 777–786. [Google Scholar] [CrossRef]
- Catala, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Dattmore, D.A.; Wang, W.; Pohnert, G.; Wolfram, S.; Zhang, J.; Yang, R.; Decker, E.A.; Lee, K.S.S.; Zhang, G. trans, trans-2,4-Decadienal, a lipid peroxidation product, induces inflammatory responses via Hsp90- or 14-3-3zeta-dependent mechanisms. J. Nutr. Biochem. 2020, 76, 108286. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Reactive carbonyl species in vivo: Generation and dual biological effects. Sci. World J. 2014, 2014, 417842. [Google Scholar] [CrossRef] [Green Version]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Cecilia, O.M.; Jose Alberto, C.G.; Jose, N.P.; Ernesto German, C.M.; Ana Karen, L.C.; Luis Miguel, R.P.; Ricardo Raul, R.R.; Adolfo Daniel, R.C. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, C.J.; Zhen, Z. Reactive Carbonyl Species: Diabetic Complication in the Heart and Lungs. Trends Endocrinol. Metab. 2019, 30, 546–556. [Google Scholar] [CrossRef]
- Marginean, C.; Popescu, M.S.; Vladaia, M.; Tudorascu, D.; Pirvu, D.C.; Petrescu, F. Involvement of Oxidative Stress in COPD. Curr. Health Sci. J. 2018, 44, 48–55. [Google Scholar] [PubMed]
- Menini, S.; Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G. Diabetes and Pancreatic Cancer—A Dangerous Liaison Relying on Carbonyl Stress. Cancers 2021, 13, 313. [Google Scholar] [CrossRef]
- Tomono, S.; Miyoshi, N.; Ohshima, H. Comprehensive analysis of the lipophilic reactive carbonyls present in biological specimens by LC/ESI-MS/MS. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 988, 149–156. [Google Scholar] [CrossRef]
- Onuma, W.; Tomono, S.; Miyamoto, S.; Fujii, G.; Hamoya, T.; Fujimoto, K.; Miyoshi, N.; Fukai, F.; Wakabayashi, K.; Mutoh, M. Irsogladine maleate, a gastric mucosal protectant, suppresses intestinal polyp development in Apc-mutant mice. Oncotarget 2016, 7, 8640–8652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoya, T.; Miyamoto, S.; Tomono, S.; Fujii, G.; Nakanishi, R.; Komiya, M.; Tamura, S.; Fujimoto, K.; Toshima, J.; Wakaayashi, K.; et al. Chemopreventive effects of a low-side-effect antibiotic drug, erythromycin, on mouse intestinal tumors. J. Clin. Biochem. Nutr. 2017, 60, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame Lignans Suppress Age-Related Cognitive Decline in Senescence-Accelerated Mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Jurgens, G.; Quehenberger, O.; Koller, E. Autoxidation of human low density lipoprotein: Loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J. Lipid Res. 1987, 28, 495–509. [Google Scholar] [CrossRef]
- Girona, J.; La Ville, A.E.; Heras, M.; Olive, S.; Masana, L. Oxidized lipoproteins including HDL and their lipid peroxidation products inhibit TNF-alpha secretion by THP-1 human macrophages. Free Radic. Biol. Med. 1997, 23, 658–667. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef]
- Orso, E.; Schmitz, G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin. Res. Cardiol. Suppl. 2017, 12 (Suppl. 1), 31–37. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.M.; Domingues, P.; Melo, T.; Perez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J. Proteom. 2013, 92, 110–131. [Google Scholar] [CrossRef]
- Kawai, Y.; Nuka, E. Abundance of DNA adducts of 4-oxo-2-alkenals, lipid peroxidation-derived highly reactive genotoxins. J. Clin. Biochem. Nutr. 2018, 62, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.S.; Yen, J.H.; Kou, M.C.; Wu, M.J. Luteolin and Apigenin Attenuate 4-Hydroxy-2-Nonenal-Mediated Cell Death through Modulation of UPR, Nrf2-ARE and MAPK Pathways in PC12 Cells. PLoS ONE 2015, 10, e0130599. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Soulage, C.O.; Pelletier, C.C.; Florens, N.; Lemoine, S.; Dubourg, L.; Juillard, L.; Guebre-Egziabher, F. Two Toxic Lipid Aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), Accumulate in Patients with Chronic Kidney Disease. Toxins 2020, 12, 567. [Google Scholar] [CrossRef]
- Thomas, C.E.; Jackson, R.L.; Ohlweiler, D.F.; Ku, G. Multiple lipid oxidation products in low density lipoproteins induce interleukin-1 beta release from human blood mononuclear cells. J. Lipid Res. 1994, 35, 417–427. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Lai, Y.; Lei, F.; Liu, S.; Liu, R.; Wang, T. Exploring the association between interleukin-1beta and its interacting proteins in Alzheimer’s disease. Mol. Med. Rep. 2015, 11, 3219–3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, P.C.; Mahler, J.; Peddada, S.; Lomnitski, L.; Nyska, A. Forestomach tumor induction by 2,4-hexadienal in F344N rats and B6C3F1 mice. Arch. Toxicol. 2003, 77, 511–520. [Google Scholar] [CrossRef]
- Young, S.C.; Chang, L.W.; Lee, H.L.; Tsai, L.H.; Liu, Y.C.; Lin, P. DNA damages induced by trans, trans-2,4-decadienal (tt-DDE), a component of cooking oil fume, in human bronchial epithelial cells. Environ. Mol. Mutagen. 2010, 51, 315–321. [Google Scholar] [CrossRef]
- Stout, M.D.; Bodes, E.; Schoonhoven, R.; Upton, P.B.; Travlos, G.S.; Swenberg, J.A. Toxicity, DNA binding, and cell proliferation in male F344 rats following short-term gavage exposures to trans-2-hexenal. Toxicol. Pathol. 2008, 36, 232–246. [Google Scholar] [CrossRef] [Green Version]
- Nadasi, E.; Varjas, T.; Pajor, L.; Ember, I. Carcinogenic potential of trans-2-hexenal is based on epigenetic effect. In Vivo 2005, 19, 559–562. [Google Scholar]
- Ulker, Z.; Alpsoy, L.; Mihmanli, A. Assessment of cytotoxic and apoptotic effects of benzaldehyde using different assays. Hum. Exp. Toxicol. 2013, 32, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Bouza, M.; Gonzalez-Soto, J.; Pereiro, R.; de Vicente, J.C.; Sanz-Medel, A. Exhaled breath and oral cavity VOCs as potential biomarkers in oral cancer patients. J. Breath Res. 2017, 11, 016015. [Google Scholar] [CrossRef]
- Langenau, J.; Boeing, H.; Bergmann, M.M.; Nothlings, U.; Oluwagbemigun, K. The Association between Alcohol Consumption and Serum Metabolites and the Modifying Effect of Smoking. Nutrients 2019, 11, 2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stemmer, U.; Dunai, Z.A.; Koller, D.; Purstinger, G.; Zenzmaier, E.; Deigner, H.P.; Aflaki, E.; Kratky, D.; Hermetter, A. Toxicity of oxidized phospholipids in cultured macrophages. Lipids Health Dis. 2012, 11, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
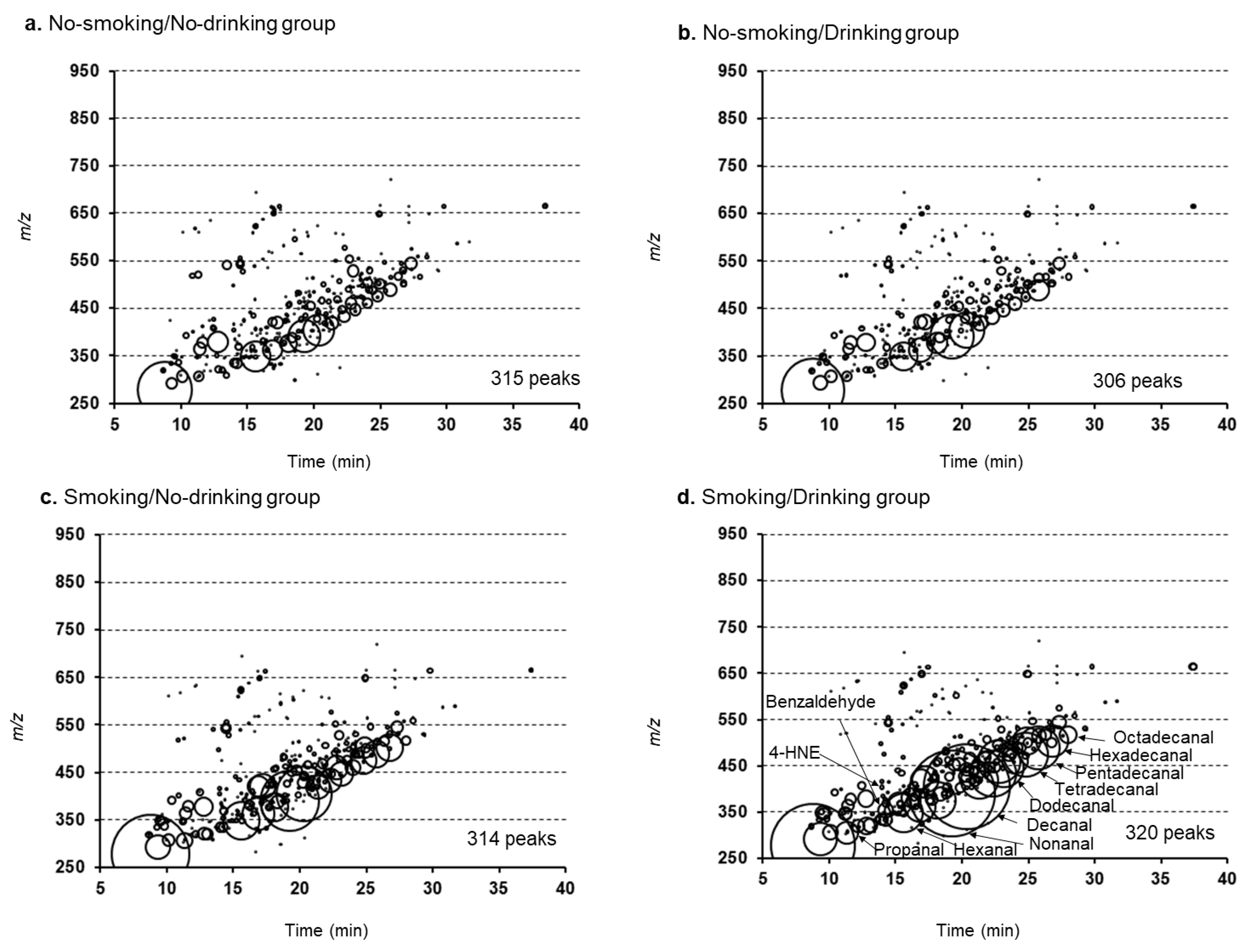

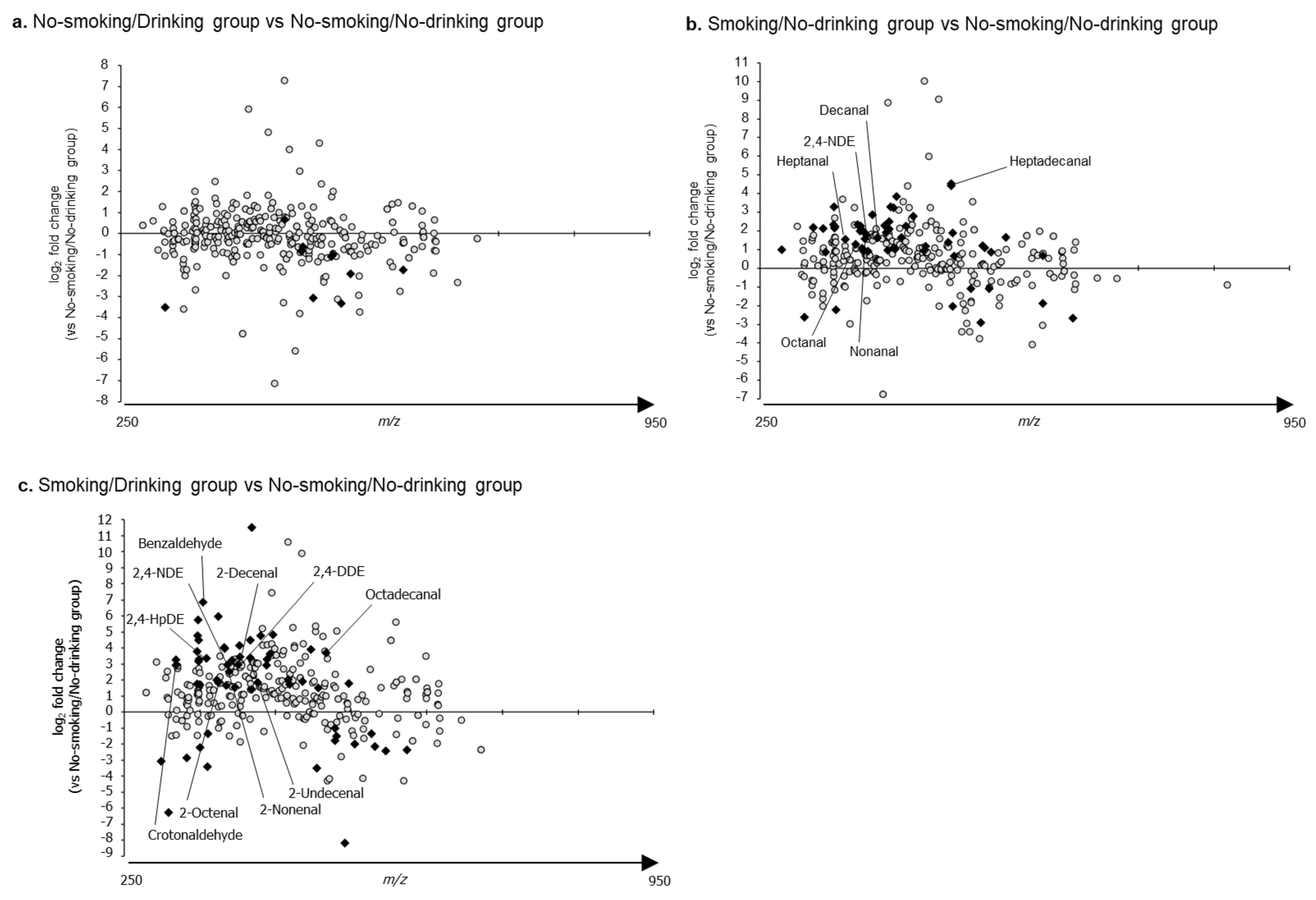
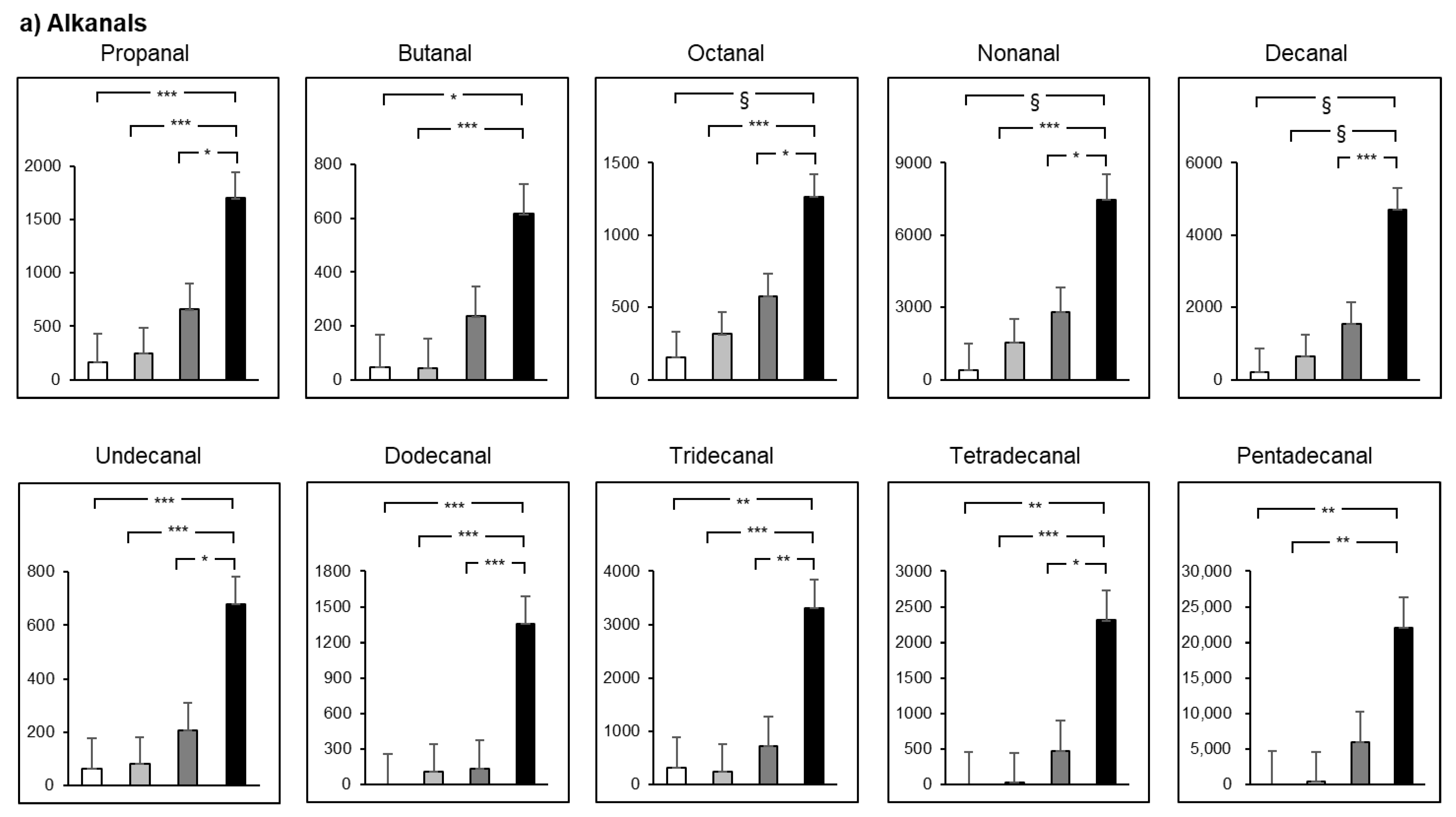
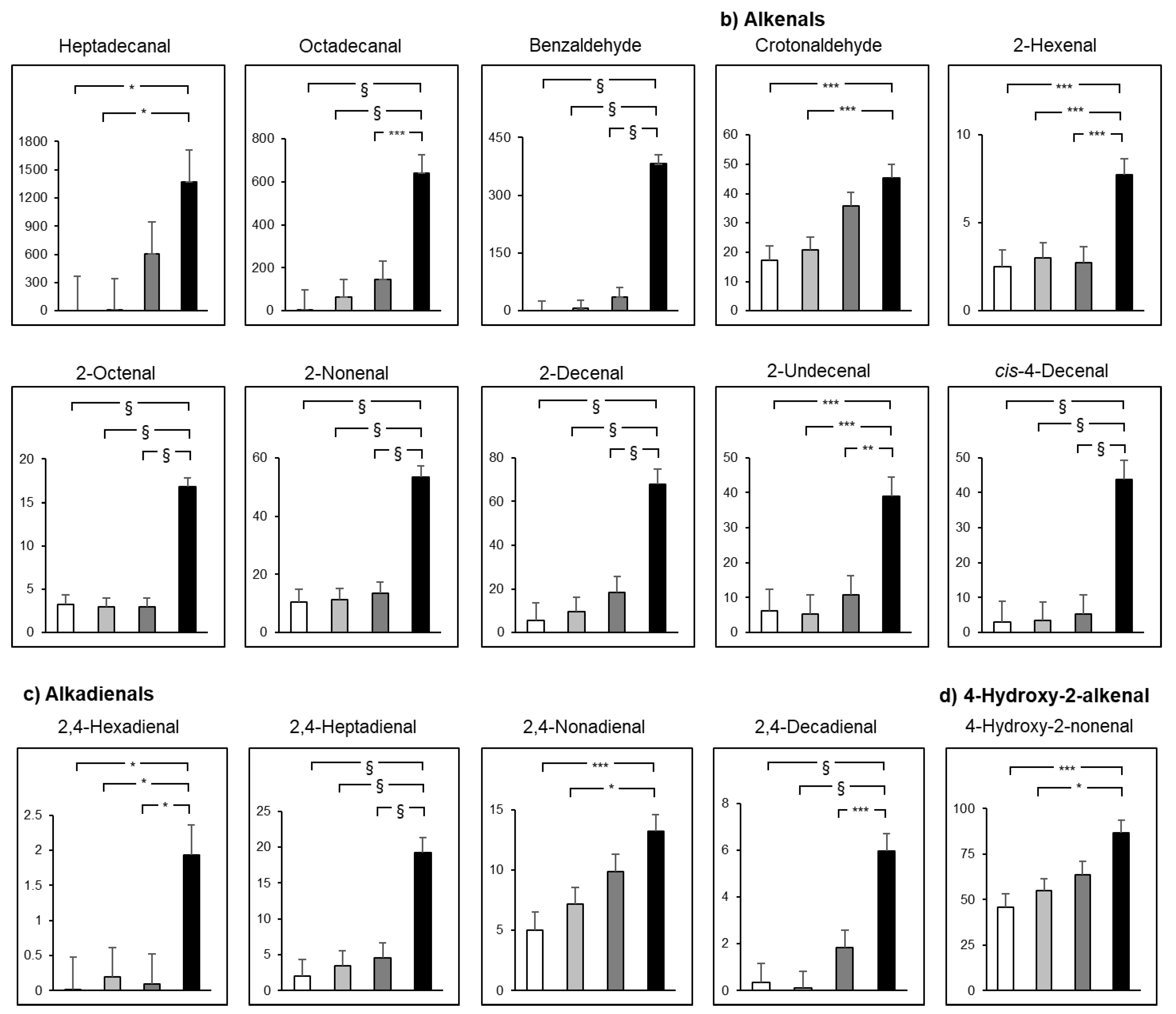
| No-Smoking/No-Drinking Group | No-Smoking/Drinking Group | Smoking/No-Drinking Group | Smoking/Drinking Group | P a | P b | P c | P d | P e | P f | |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 | ||||||
| Age (years) | 73.2 ± 7.6 | 63.2 ± 8.4 | 59.5 ± 10.8 | 59.6 ± 10.2 | 0.132 | 0.014 | 0.015 | 1.000 | 1.000 | 1.000 |
| BMI (kg/m2) 1 | 22.9 ± 3.0 | 23.5 ± 3.0 | 24.4 ± 2.3 | 23.2 ± 2.7 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Triglyceride (mg/dL) | 89.2 ± 38.0 | 107.4 ± 55.0 | 126.6 ± 60.2 | 237.3 ± 215.4 | 1.000 | 1.000 | 0.045 | 1.000 | 0.106 | 0.246 |
| HDL-C (mg/dL) 2 | 57.5 ± 16.0 | 59.8 ± 16.6 | 48.3 ± 9.6 | 55.4 ± 9.6 | 1.000 | 0.799 | 1.000 | 0.376 | 1.000 | 1.000 |
| LDL-C (mg/dL) 3 | 105.2 ± 28.2 | 104.5 ± 31.5 | 137.8 ± 37.7 | 101.0 ± 25.9 | 1.000 | 0.150 | 1.000 | 0.133 | 1.000 | 0.073 |
| Drinking habits 4 | no | yes | no | yes | ||||||
| Number of cigarettes/day | 0 | 0 | 26 ± 7.0 | 24 ± 5.7 | 0.492 | |||||
| Brinkman index | 0 | 0 | 1008 ± 355.7 | 882 ± 335.0 | 0.425 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mure, K.; Tomono, S.; Mure, M.; Horinaka, M.; Mutoh, M.; Sakai, T.; Ishikawa, H.; Wakabayashi, K. The Combination of Cigarette Smoking and Alcohol Consumption Synergistically Increases Reactive Carbonyl Species in Human Male Plasma. Int. J. Mol. Sci. 2021, 22, 9043. https://doi.org/10.3390/ijms22169043
Mure K, Tomono S, Mure M, Horinaka M, Mutoh M, Sakai T, Ishikawa H, Wakabayashi K. The Combination of Cigarette Smoking and Alcohol Consumption Synergistically Increases Reactive Carbonyl Species in Human Male Plasma. International Journal of Molecular Sciences. 2021; 22(16):9043. https://doi.org/10.3390/ijms22169043
Chicago/Turabian StyleMure, Kanae, Susumu Tomono, Minae Mure, Mano Horinaka, Michihiro Mutoh, Toshiyuki Sakai, Hideki Ishikawa, and Keiji Wakabayashi. 2021. "The Combination of Cigarette Smoking and Alcohol Consumption Synergistically Increases Reactive Carbonyl Species in Human Male Plasma" International Journal of Molecular Sciences 22, no. 16: 9043. https://doi.org/10.3390/ijms22169043
APA StyleMure, K., Tomono, S., Mure, M., Horinaka, M., Mutoh, M., Sakai, T., Ishikawa, H., & Wakabayashi, K. (2021). The Combination of Cigarette Smoking and Alcohol Consumption Synergistically Increases Reactive Carbonyl Species in Human Male Plasma. International Journal of Molecular Sciences, 22(16), 9043. https://doi.org/10.3390/ijms22169043







