Significance of Circulating Cell-Free DNA Species in Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Results
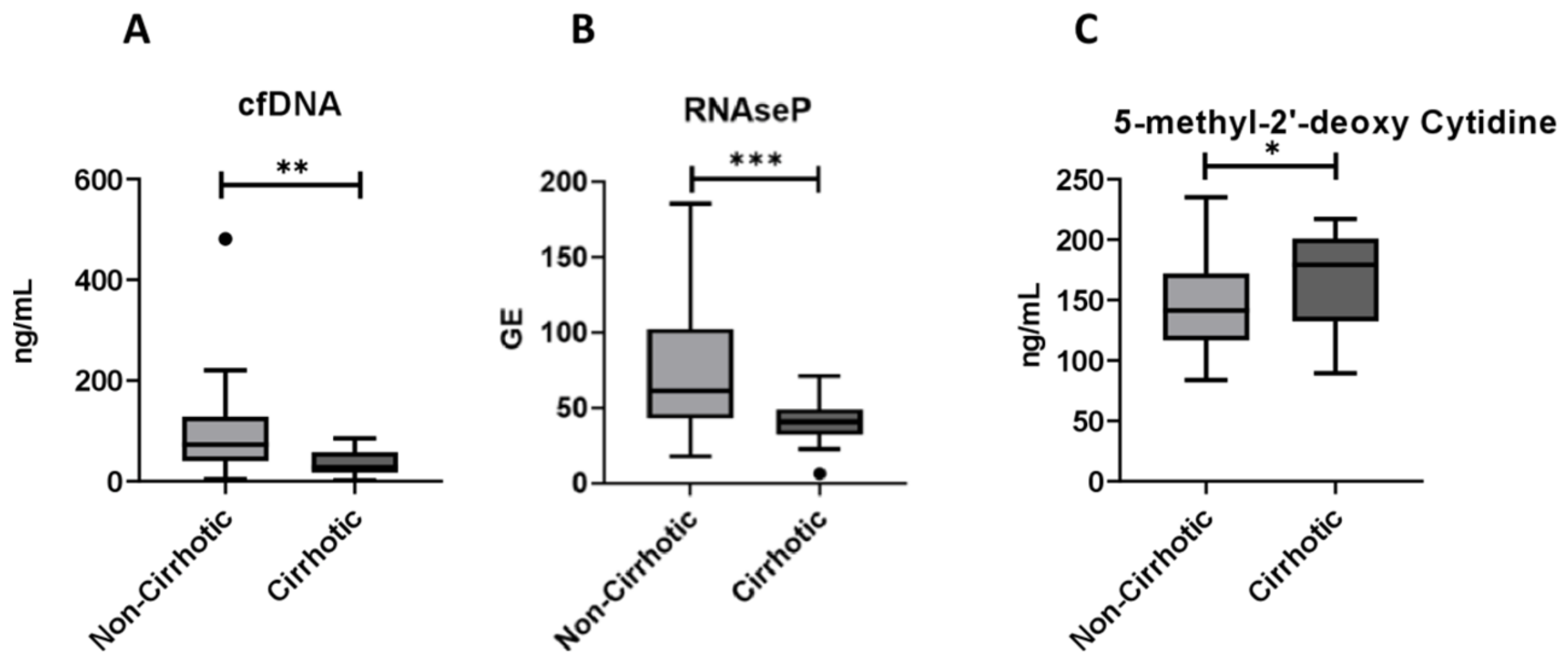
2.1. Circulating cf-DNA and DNA Methylation Markers in Relation to NAFLD Severity
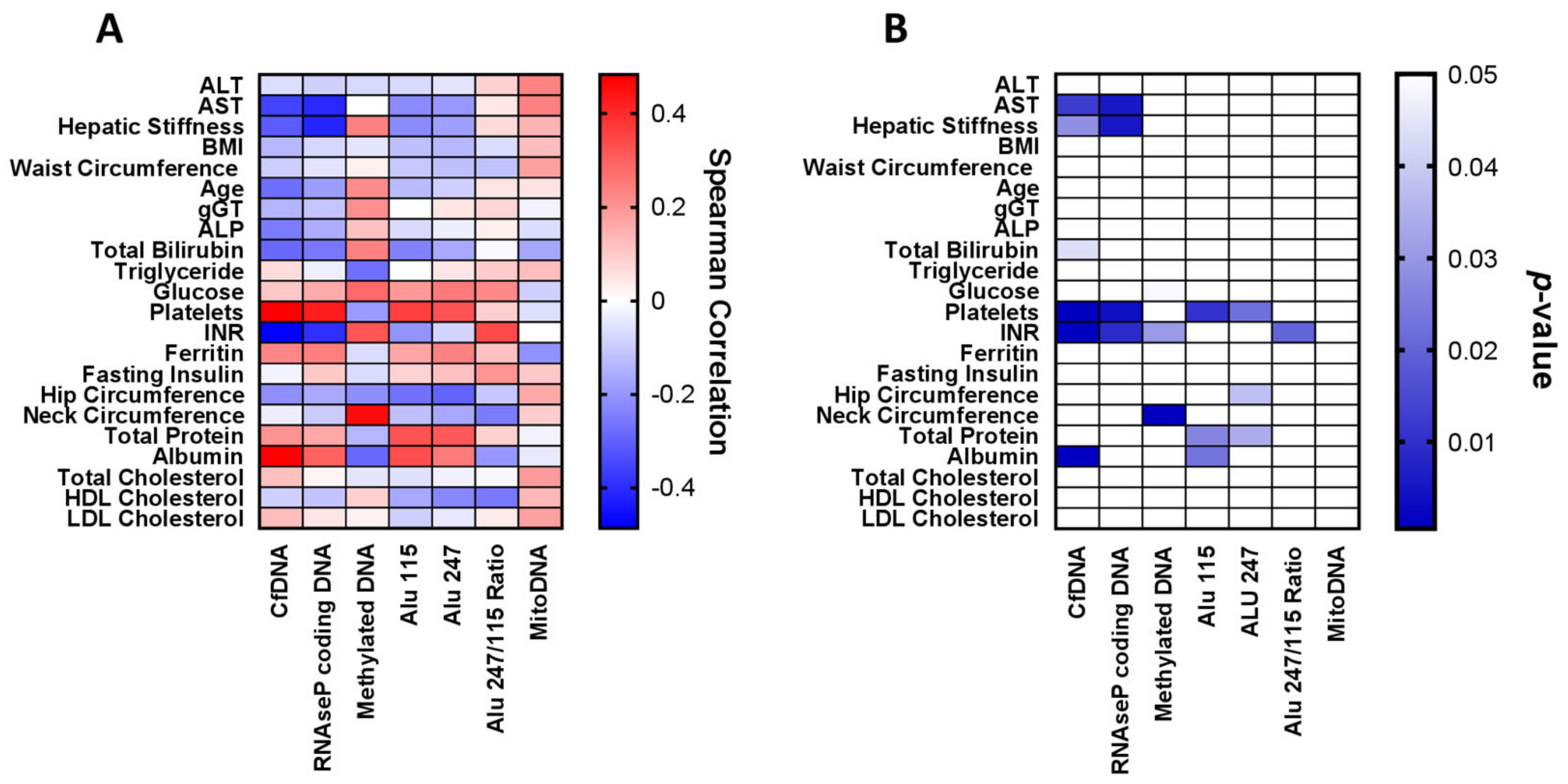
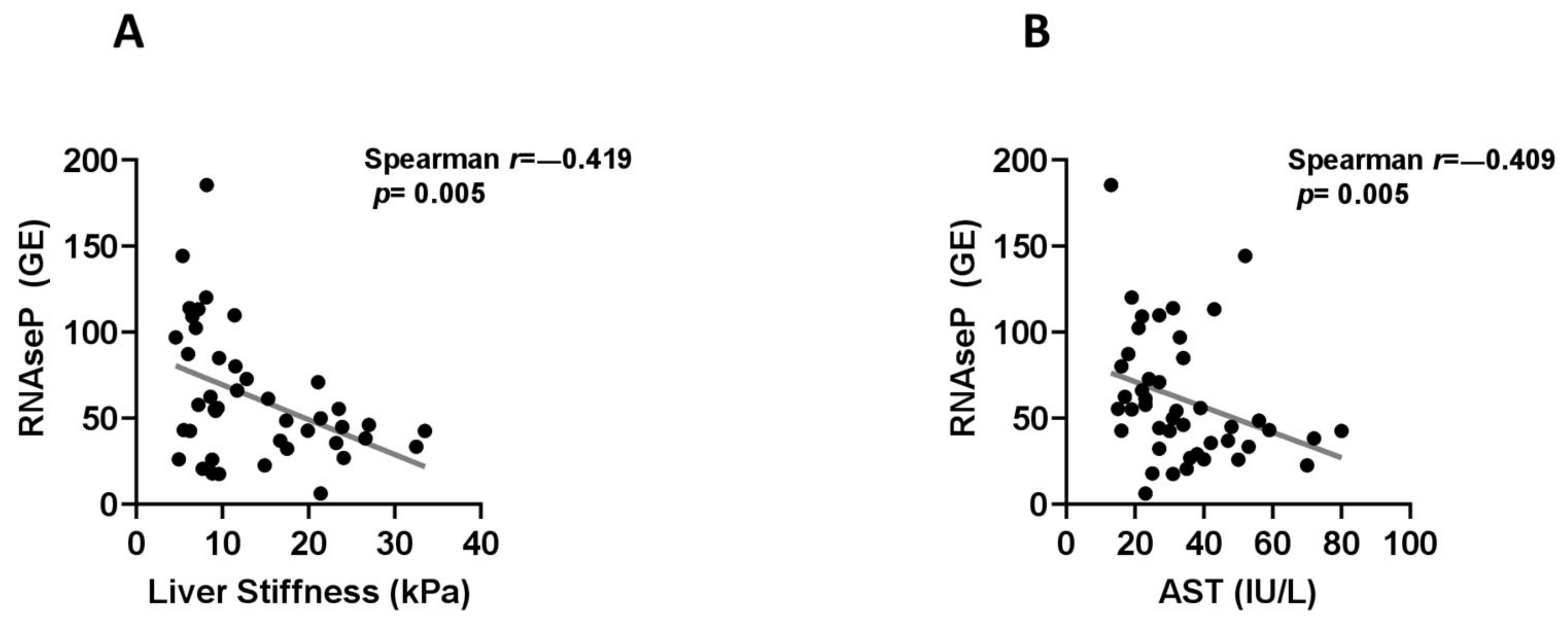
2.2. Associations between Circulating cf-DNA and DNA Methylation Markers with Other Patient Characteristics, Laboratory Parameters and Histological Findings
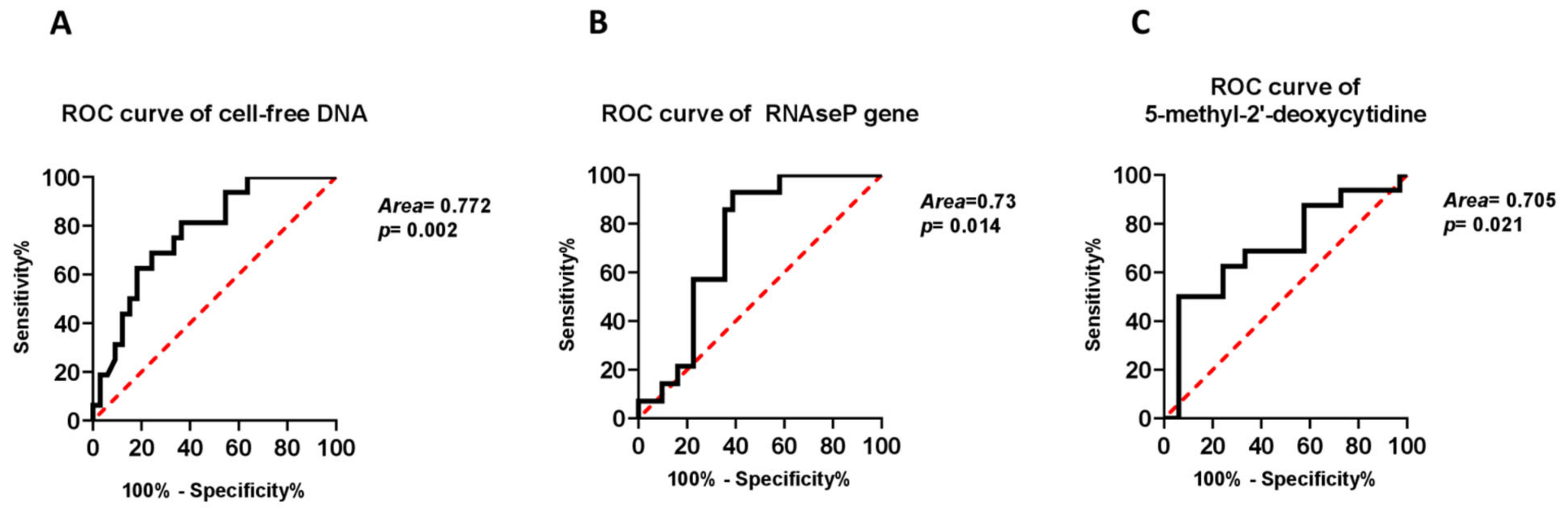
2.3. Independent Associations of cf-DNA Parameters with Presence of Cirrhosis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. NAFLD Diagnosis and Staging
4.3. Demographic and Laboratory Data
4.4. Circulating cf-DNA Species and DNA Methylation Markers
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef]
- Chatzigeorgiou, A.; Chavakis, T. Immune Cells and Metabolism. Handb. Exp. Pharmacol. 2016, 233, 221–249. [Google Scholar] [PubMed]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef]
- Beck, J.; Urnovitz, H.B.; Riggert, J.; Clerici, M.; Schutz, E. Profile of the circulating DNA in apparently healthy individuals. Clin. Chem. 2009, 55, 730–738. [Google Scholar] [CrossRef]
- Patel, K.M.; Tsui, D.W. The translational potential of circulating tumour DNA in oncology. Clin. Biochem. 2015, 48, 957–961. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Ungerer, V.; Holdenrieder, S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol. Detect. Quantif. 2019, 17, 100087. [Google Scholar] [CrossRef]
- Shapiro, B.; Chakrabarty, M.; Cohn, E.M.; Leon, S.A. Determination of circulating DNA levels in patients with benign or malignant gastrointestinal disease. Cancer 1983, 51, 2116–2120. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Cell-free DNA methylation as liquid biopsy for the assessment of fibrosis in patients with nonalcoholic steatohepatitis: A gap between innovation and implementation. Hepatobiliary Surg. Nutr. 2017, 6, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera-Saldana, H.A.; Fernandez-Garza, L.E.; Barrera-Barrera, S.A. Liquid biopsy in chronic liver disease. Ann. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridi, A.M.; Chrysavgis, L.; Koutsilieris, M.; Chatzigeorgiou, A. The Role of Senescence in the Development of Nonalcoholic Fatty Liver Disease and Progression to Nonalcoholic Steatohepatitis. Hepatology 2020, 71, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Katsarou, A.; Moustakas, I.I.; Pyrina, I.; Lembessis, P.; Koutsilieris, M.; Chatzigeorgiou, A. Metabolic inflammation as an instigator of fibrosis during non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 1993–2011. [Google Scholar] [CrossRef] [PubMed]
- Buzova, D.; Maugeri, A.; Liguori, A.; Napodano, C.; Lo Re, O.; Oben, J.; Alisi, A.; Gasbarrini, A.; Grieco, A.; Cerveny, J.; et al. Circulating histone signature of human lean metabolic-associated fatty liver disease (MAFLD). Clin. Epigenetics 2020, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Meroni, M.; Longo, M.; Fargion, S.; Fracanzani, A.L. miRNA Signature in NAFLD: A Turning Point for a Non-Invasive Diagnosis. Int. J. Mol. Sci. 2018, 19, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Jiang, P.; Chan, K.C.; Wong, J.; Cheng, Y.K.; Liang, R.H.; Chan, W.K.; Ma, E.S.; Chan, S.L.; Cheng, S.H.; et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc. Natl. Acad. Sci. USA 2015, 112, E5503–E5512. [Google Scholar] [CrossRef] [Green Version]
- Green, M.R.; Sambrook, J. Isolation and Quantification of DNA. Cold Spring Harb. Protoc. 2018, 2018, 6. [Google Scholar] [CrossRef]
- Karlas, T.; Weise, L.; Kuhn, S.; Krenzien, F.; Mehdorn, M.; Petroff, D.; Linder, N.; Schaudinn, A.; Busse, H.; Keim, V.; et al. Correlation of cell-free DNA plasma concentration with severity of non-alcoholic fatty liver disease. J. Transl. Med. 2017, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Dezso, K.; Rokusz, A.; Bugyik, E.; Szucs, A.; Szuak, A.; Dorogi, B.; Kiss, M.; Nemeskeri, A.; Nagy, P.; Paku, S. Human liver regeneration in advanced cirrhosis is organized by the portal tree. J. Hepatol. 2017, 66, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Huang, H.Y.; Chen, Y.C.; Bristow, R.E.; Kassauei, K.; Cheng, C.C.; Roden, R.; Sokoll, L.J.; Chan, D.W.; Shih Ie, M. Increased plasma DNA integrity in cancer patients. Cancer Res. 2003, 63, 3966–3968. [Google Scholar]
- Li, L.; Hann, H.W.; Wan, S.; Hann, R.S.; Wang, C.; Lai, Y.; Ye, X.; Evans, A.; Myers, R.E.; Ye, Z.; et al. Cell-free circulating mitochondrial DNA content and risk of hepatocellular carcinoma in patients with chronic HBV infection. Sci. Rep. 2016, 6, 23992. [Google Scholar] [CrossRef]
- Hardy, T.; Zeybel, M.; Day, C.P.; Dipper, C.; Masson, S.; McPherson, S.; Henderson, E.; Tiniakos, D.; White, S.; French, J.; et al. Plasma DNA methylation: A potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut 2017, 66, 1321–1328. [Google Scholar] [CrossRef]
- Murphy, S.K.; Yang, H.; Moylan, C.A.; Pang, H.; Dellinger, A.; Abdelmalek, M.F.; Garrett, M.E.; Ashley-Koch, A.; Suzuki, A.; Tillmann, H.L.; et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 1076–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.; Khin, S.; Kopp, W.C. Characterization of effect of repeated freeze and thaw cycles on stability of genomic DNA using pulsed field gel electrophoresis. Biopreserv. Biobank 2012, 10, 4–11. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]

| NAFL (n = 10) | NASH (n = 23) | No Cirrhosis 1 (NAFL+NASH) | Cirrhosis 2 (n = 16) | p-Value (1 vs. 2) | |
|---|---|---|---|---|---|
| Age, years | 52.9 (8.6) | 55.6 (11.1) | 54.7 (10.4) | 68.1 (8.6) | <0.001 |
| Sex, males (%) | 7 (70) | 10 (43.5) | 17 (51.5) | 9 (56.3) | 0.755 |
| BMI, kg/m2 | 28.3 (3.1) | 32.6 (5.4) | 31.3 (5.2) | 32.0 (5.2) | 0.649 |
| WC, cm | 103 (5) | 111 (11) | 108 (10) | 113 (13) | 0.176 |
| Alcohol, n (%) No use Mild use | 8 (80) 2 (20) | 20 (90.9) 2 (9.1) | 28 (87.5) 4 (12.5) | 14 (93.3) 1 (6.7) | 0.545 |
| Smoking, n (%) Never Former Current | 5 (50) 2 (20) 3 (30) | 7 (30.4) 8 (34.8) 8 (34.8) | 12 (36.4) 10 (30.3) 11 (33.3) | 7 (43.8) 6 (37.5) 3 (18.8) | 0.570 |
| T2DM, n (%) | 3 (30) | 13 (59.1) | 16 (50) | 13 (81.3) | 0.037 |
| LSM, kPa | 6.7 1.6} | 9.6 {4.7} | 8.8 {4.9} | 22.3 {10} | <0.001 |
| ALT, IU/L | 34 {30} | 35 {34} | 35 {37} | 32 {28} | 0.394 |
| AST, IU/L | 22.5 {13} | 31 {15} | 27 {15} | 47 {39} | 0.002 |
| ALP, IU/L | 86 {64} | 72 {38} | 73 {43} | 123 {53} | <0.001 |
| GGT, IU/L | 132 {136} | 30 {43} | 51 {150} | 100 {214} | 0.018 |
| Albumin, g/L | 46 {5} | 47 {4} | 46 {4} | 36 {9} | <0.001 |
| Bilirubin, mg/dl | 1.0 {1.0} | 0.5 {0.5} | 0.7 {0.7} | 1.0 {0.8} | 0.013 |
| Ferritin, mg/dl | 230 {238} | 105 {198} | 130 {186} | 50 {99} | 0.030 |
| Platelets, ×109/L | 226 (58) | 254 (81) | 245 (75) | 134 (64) | <0.001 |
| INR | 0.98 (0.06) | 1.01 (0.08) | 1.00 (0.07) | 1.29 (0.45) | 0.030 |
| Glucose, mg/dl | 104 (20) | 107 (17) | 106 (18) | 141 (54) | 0.020 |
| Insulin, pmol/L | 17 {34} | 18 {9} | 18 {13} | 23 {16} | 0.272 |
| LDL, mg/dl | 95 (36) | 106 (42) | 103 (40) | 80 (21) | 0.055 |
| HDL, mg/dl | 54 (22) | 54 (14) | 54 (16) | 50 (16) | 0.447 |
| TG, mg/dl | 122 (47) | 119 (60) | 120 (56) | 120 (39) | 0.964 |
| NAFL (n = 10) | NASH (n = 23) | No Cirrhosis 1 (NAFL + NASH) | Cirrhosis 2 (n = 16) | p-Value (1 vs. 2) | |
|---|---|---|---|---|---|
| cfDNA, ng/mL | 72.9 {95.2} | 68.4 {86.9} | 72.6 {88.0} | 27.9 {40.7} | 0.002 |
| RNAse P, GE | 83.6 (46.9) | 63.8 (36.7) | 70.2 (40.6) | 39.9 (15.5) | <0.001 |
| Alu115, GE | 131.4 (81.8) | 104.8 (53.8) | 112.9 (63.5) | 89.7 (45.4) | 0.199 |
| Alu247, GE | 39.9 (27.7) | 31.1 (16.7) | 33.8 (20.6) | 29.9 (19.0) | 0.525 |
| Alu247/Alu115 ratio | 0.29 (0.05) | 0.30 (0.05) | 0.30 (0.05) | 0.31(0.06) | 0.297 |
| Mitochondrial DNA, copies | 19 {17} | 26 {40} | 25 {37} | 27 {27} | 0.725 |
| 5′methyl-2-deoxy-cytidine, ng/mL | 144.2 (39.9) | 145.6 (34.6) | 145.1 (35.7) | 170.3 (38.9) | 0.029 |
| Parameters Included in the Model | Adjusted Odds Ratio (95% CI) | p-Value |
|---|---|---|
| 1st model Age (per 1 year) T2DM (yes vs. no) Cell-free DNA (per 1 ng/mL) | 1.130 (1.024–1.247) 3.512 (0.588–20.965) 0.974 (0.951–0.997) | 0.015 0.168 0.026 |
| 2nd model Age (per 1 year) T2DM (yes vs. no) RNAse P coding DNA (per 1 GE) | 1.140 (1.025–1.269) 5.724 (0.626–52.339) 0.945 (0.896–0.996) | 0.0160.1220.035 |
| 3rd model Age (per 1 year) T2DM (yes vs. no) 5-methyl-2′-deoxycytidine (per 1 ng/mL) | 1.142 (1.039–1.256) 2.727 (0.498–14.916) 1.014 (0.994–1.035) | 0.0060.2470.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysavgis, L.; Papatheodoridi, A.; Cholongitas, E.; Koutsilieris, M.; Papatheodoridis, G.; Chatzigeorgiou, A. Significance of Circulating Cell-Free DNA Species in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 8849. https://doi.org/10.3390/ijms22168849
Chrysavgis L, Papatheodoridi A, Cholongitas E, Koutsilieris M, Papatheodoridis G, Chatzigeorgiou A. Significance of Circulating Cell-Free DNA Species in Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2021; 22(16):8849. https://doi.org/10.3390/ijms22168849
Chicago/Turabian StyleChrysavgis, Lampros, Alkistis Papatheodoridi, Evangelos Cholongitas, Michael Koutsilieris, George Papatheodoridis, and Antonios Chatzigeorgiou. 2021. "Significance of Circulating Cell-Free DNA Species in Non-Alcoholic Fatty Liver Disease" International Journal of Molecular Sciences 22, no. 16: 8849. https://doi.org/10.3390/ijms22168849
APA StyleChrysavgis, L., Papatheodoridi, A., Cholongitas, E., Koutsilieris, M., Papatheodoridis, G., & Chatzigeorgiou, A. (2021). Significance of Circulating Cell-Free DNA Species in Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 22(16), 8849. https://doi.org/10.3390/ijms22168849









