Chilling Requirement Validation and Physiological and Molecular Responses of the Bud Endodormancy Release in Paeonia lactiflora ‘Meiju’
Abstract
:1. Introduction
2. Results
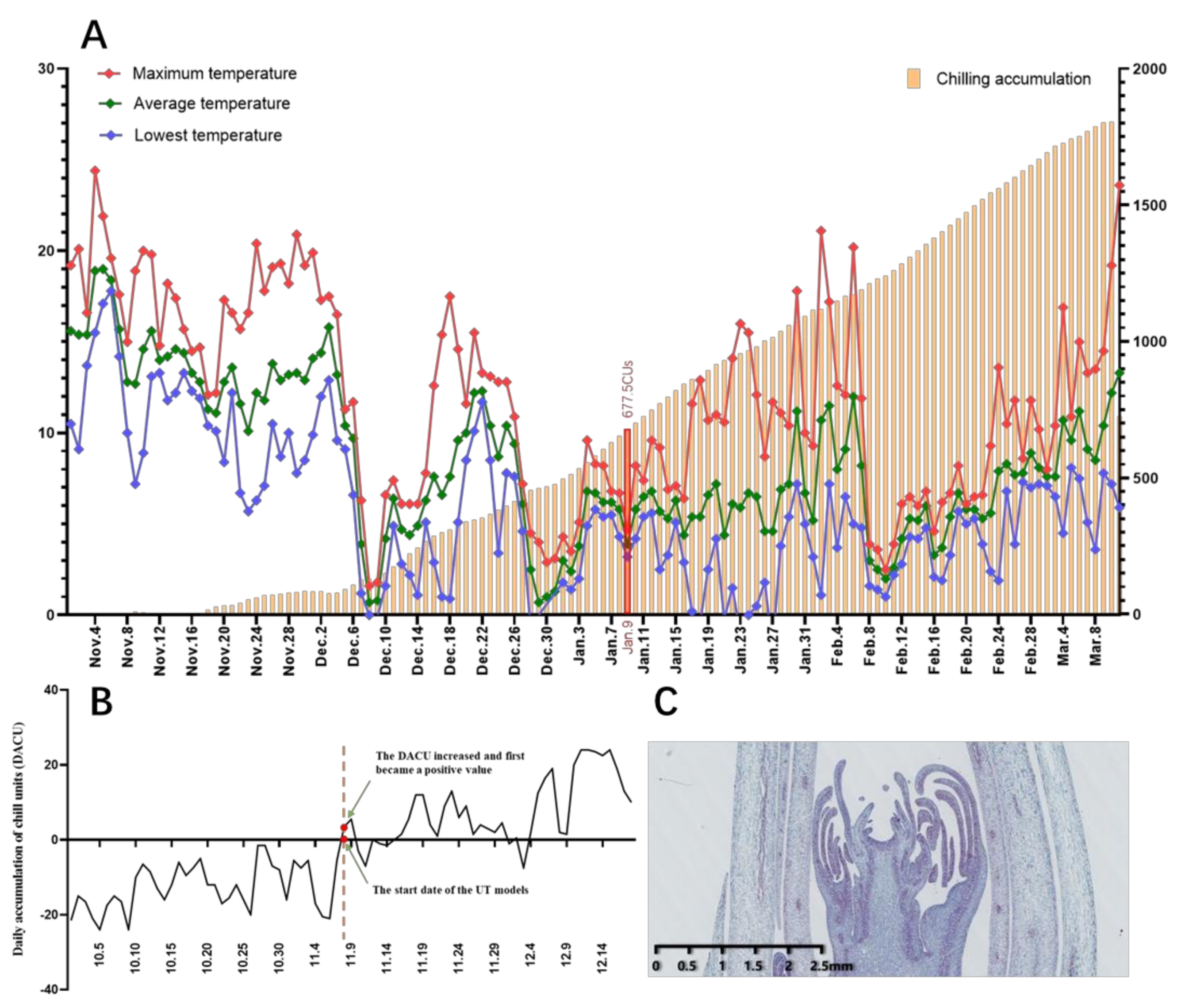
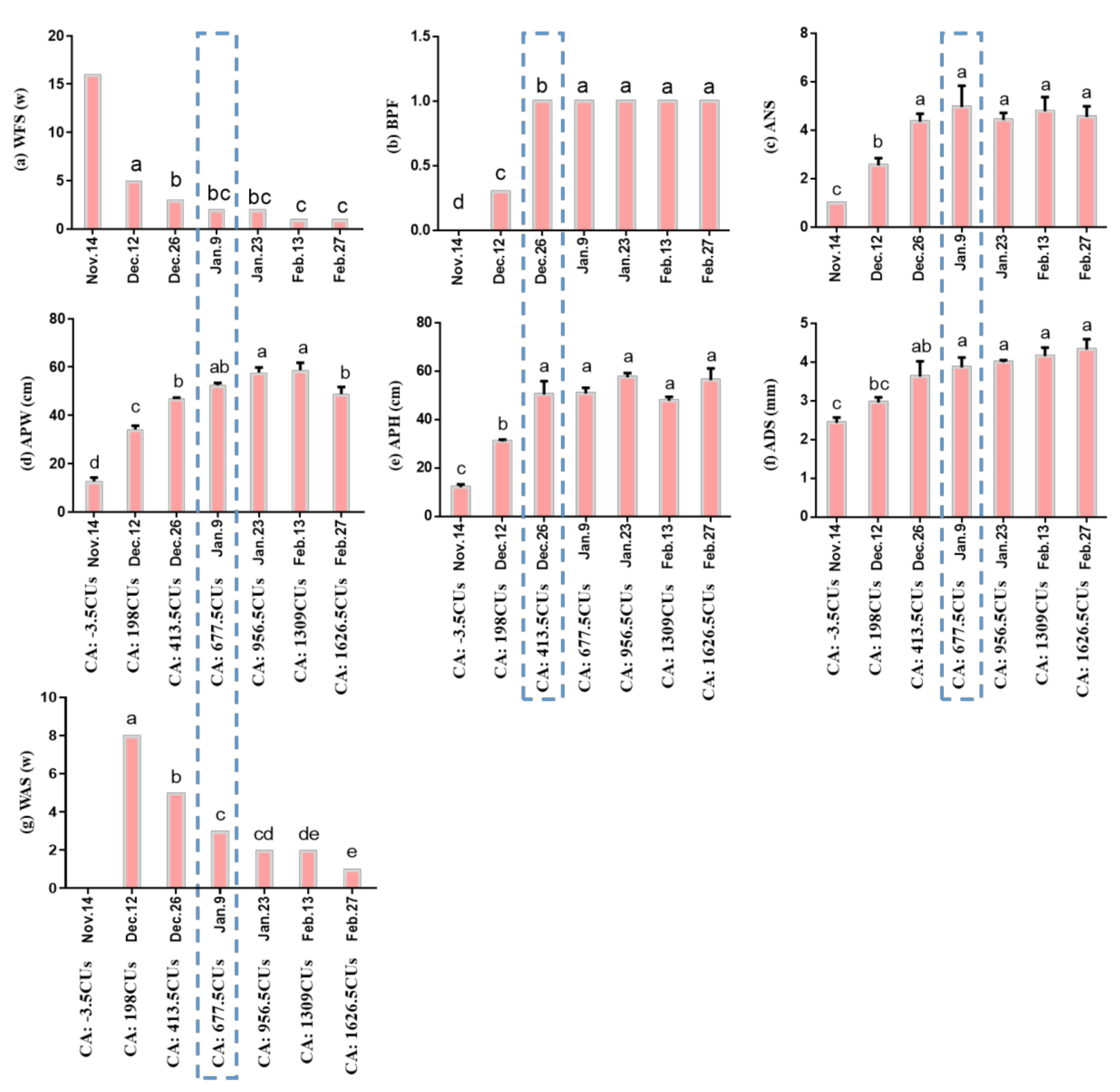
2.1. Morphology and CR Evaluation during Bud Dormancy and Sprouting
2.1.1. Morphological Observations of Bud Dormancy and Sprouting
2.1.2. Comprehensive Evaluation of the CR of ‘Meiju’ to Break Bud Endodormancy
2.2. Physiological Responses of the Underground Buds during the Whole Dormancy Process
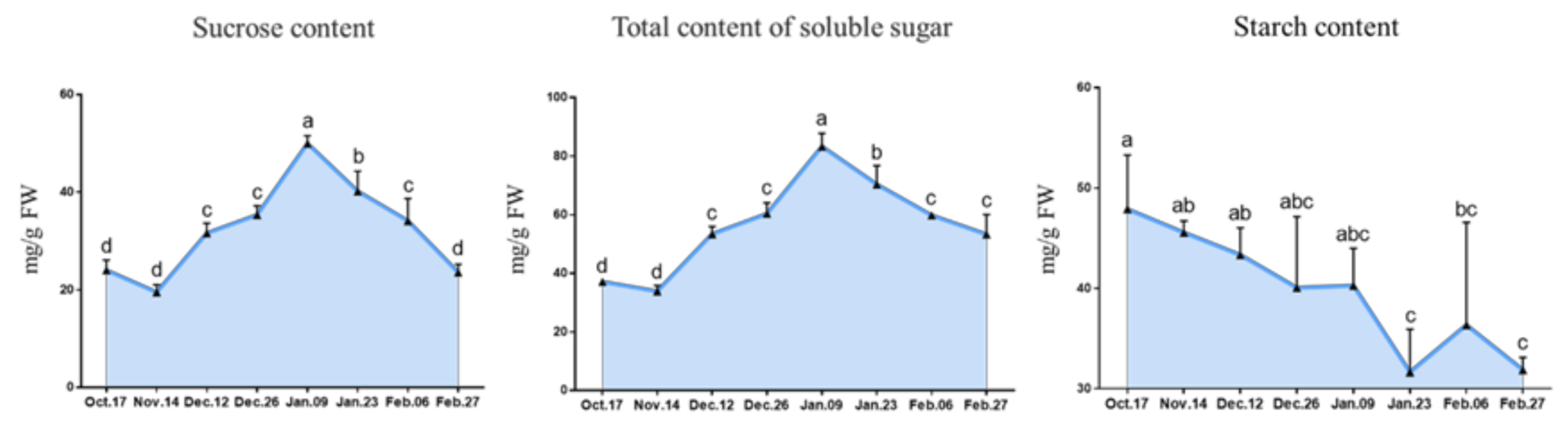
2.2.1. Change in the Carbohydrate Contents
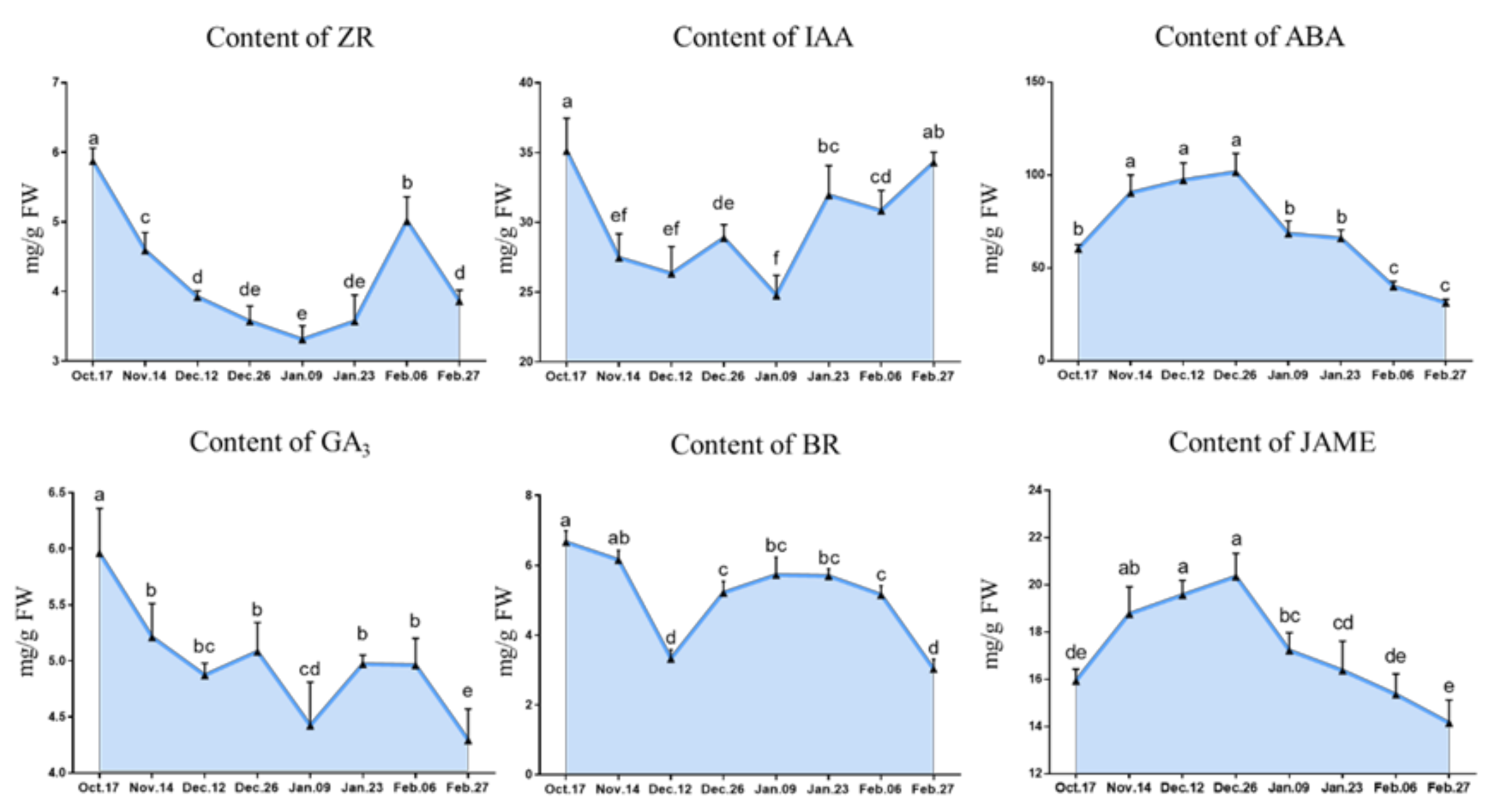
2.2.2. Change in the Phytohormone Contents
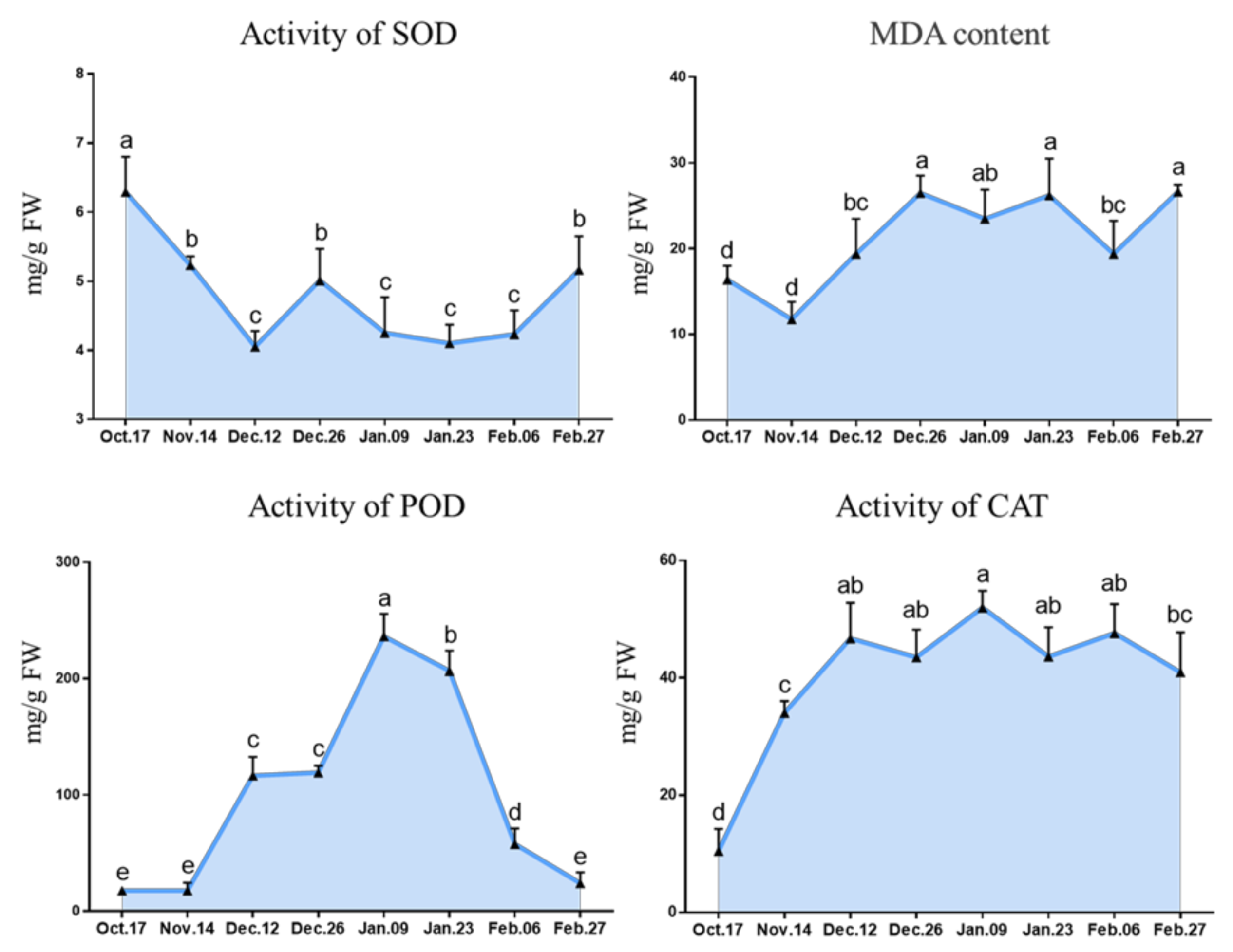
2.2.3. Change in the Enzyme Activity of Antioxidants
2.3. Correlation Analysis among All Morphological and Physiological Indices
2.4. Expression Analysis of Representative Dormancy-Related Genes in ‘Meiju’ Underground Buds
2.4.1. Selection and Annotation of the Representative Dormancy-Related Genes
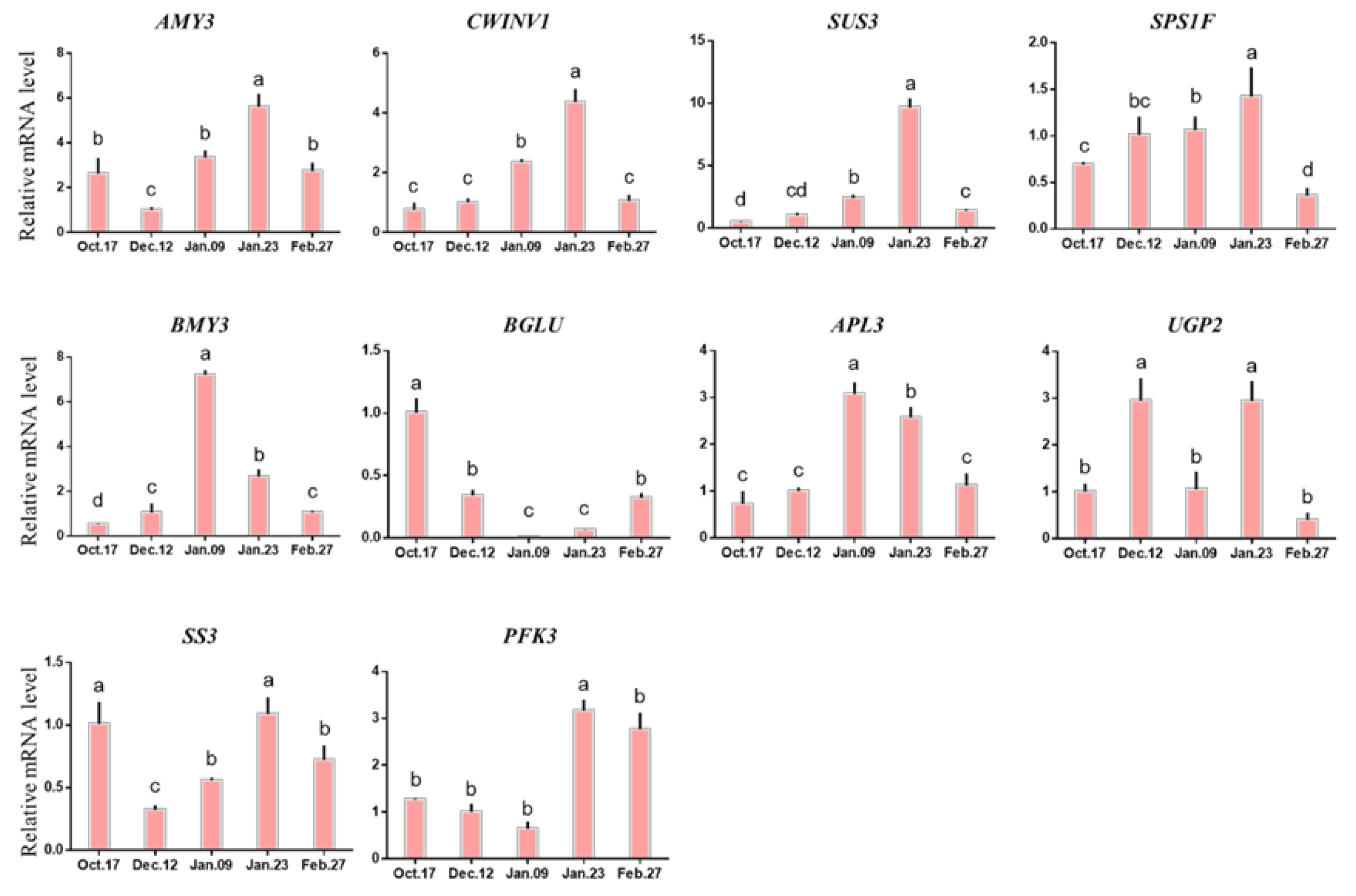
2.4.2. Expression of Genes Related to Carbohydrate Metabolism in Different Dormancy Stages
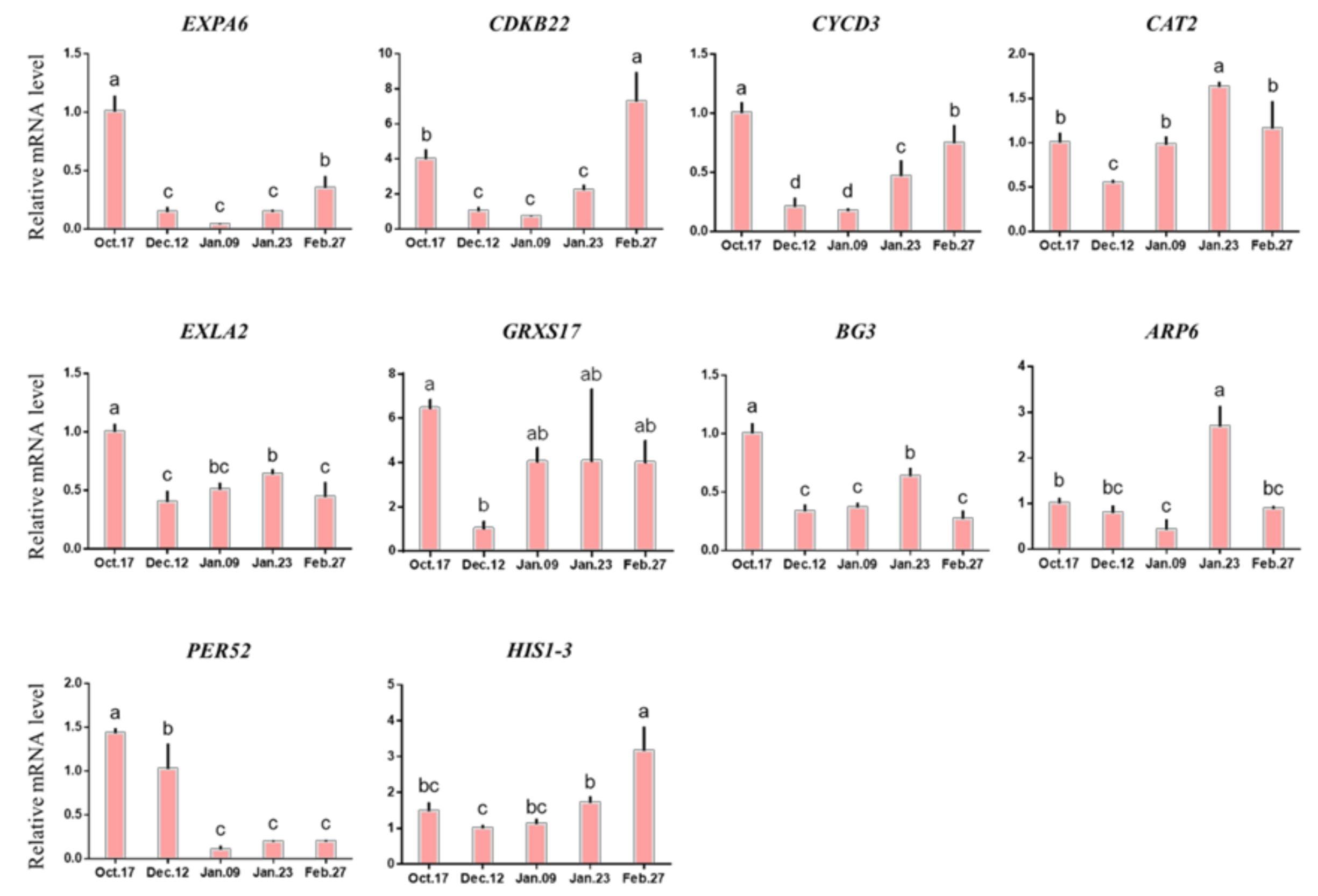
2.4.3. Expression of Genes Related to Antioxidant Metabolism in Different Dormancy Stages
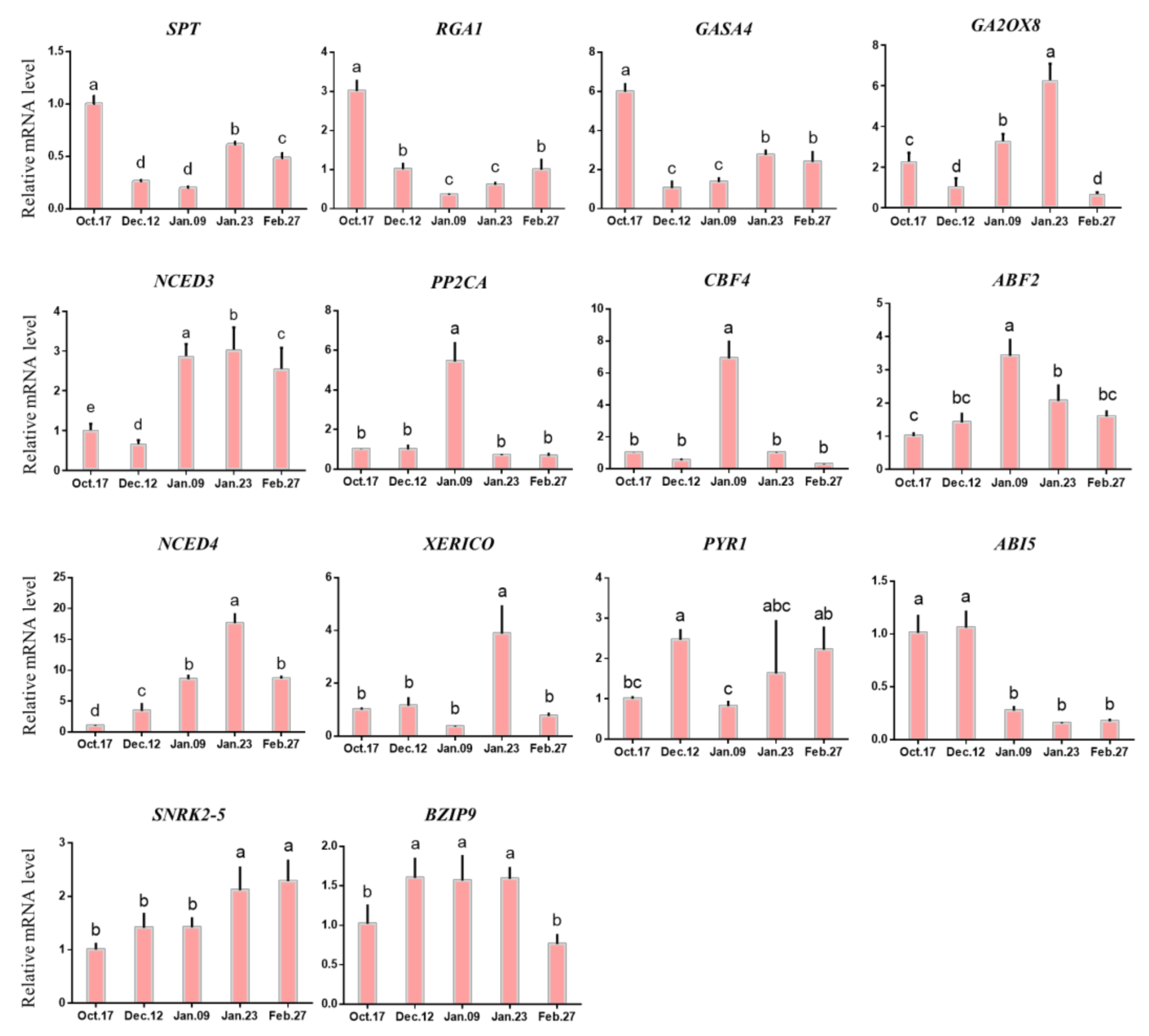
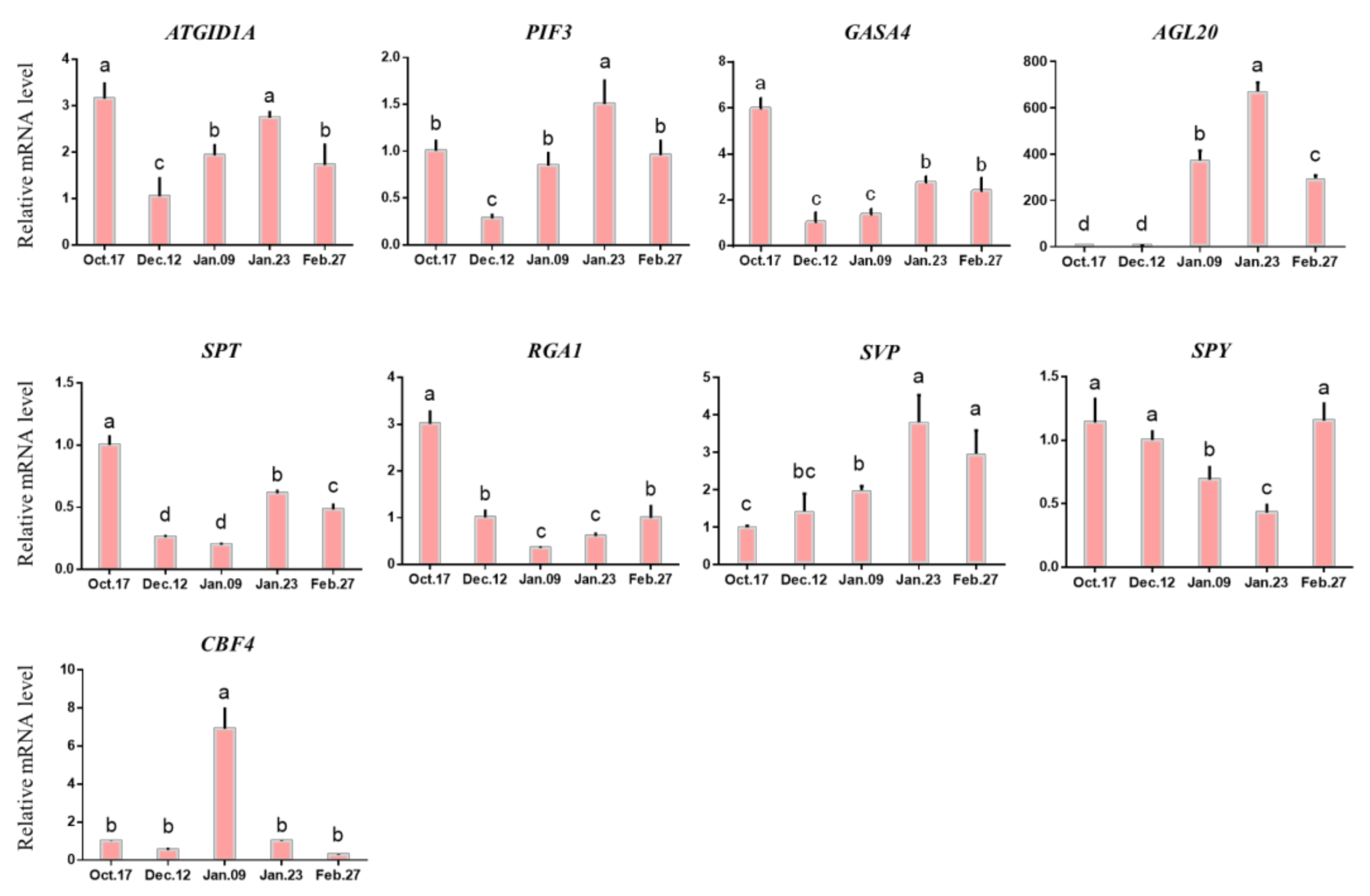
2.4.4. Expression of Genes Related to Hormone Metabolism in Different Dormancy Stages
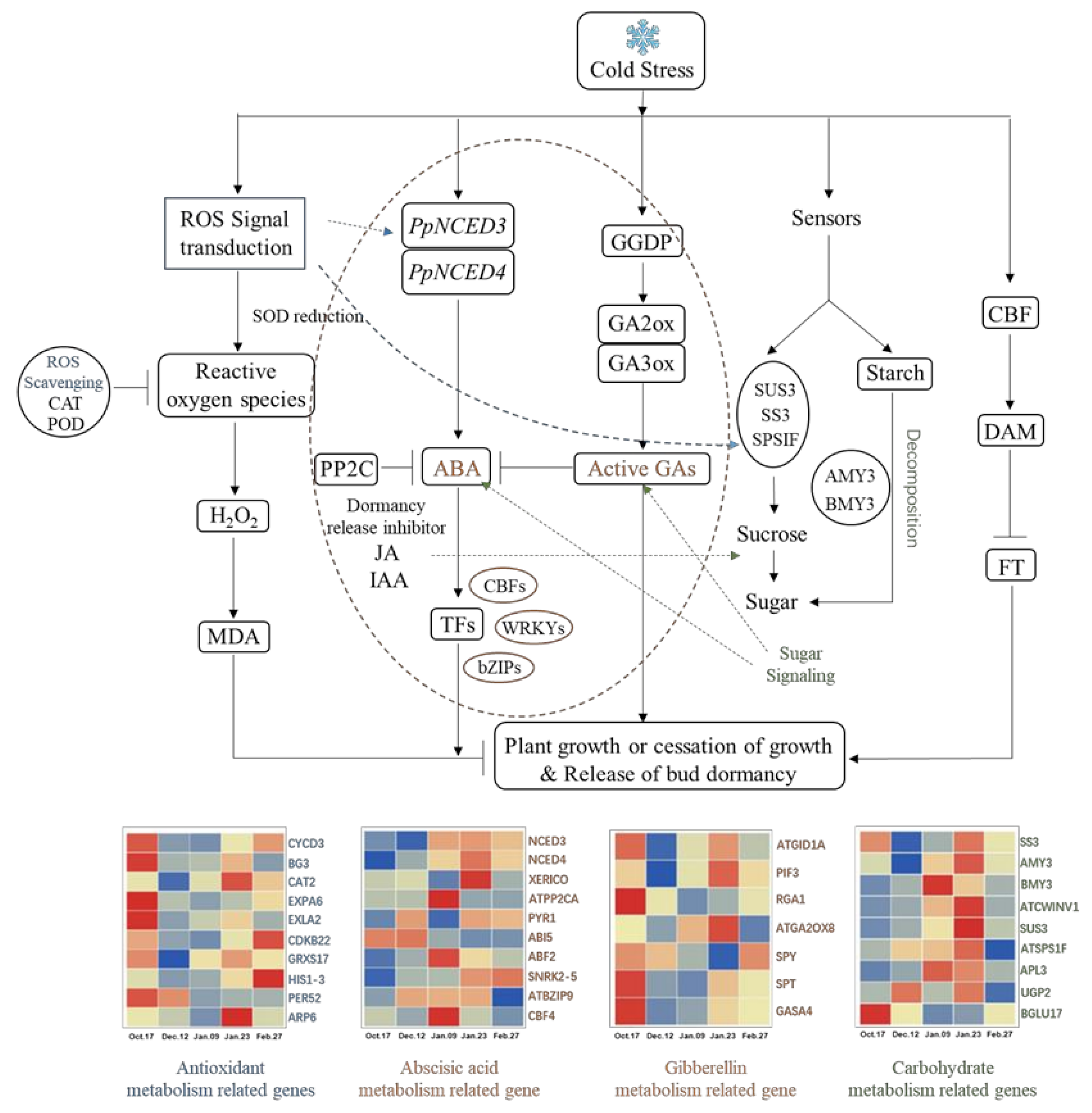
2.4.5. Changes in the DAM-SOC1-AP1 Pathway and Others in Different Dormancy Stages
3. Discussion
3.1. CR Evaluation Is an Important Method to Popularize Herbaceous Peony at Low Latitudes
3.2. Carbohydrate Metabolism Participates in the Regulation of Endodormancy Release
3.3. Relationship between Hormone Metabolism and Endodormancy Release
3.4. Antioxidant Metabolism Involved in Endodormancy Release Regulation
3.5. Judgment of Endodormancy Release Should Be Based on Comprehensive Research
3.6. Possible Regulatory Networks for Dormancy Release of Underground Buds in Herbaceous Peony
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of the Dormancy Release Date and Morphological Observations
4.3. Preparation of Paraffin Sections
4.4. Selection of an Evaluation Model
4.5. Determination of Related Physiological Indices and Gene Expression
4.5.1. Measurements of the Soluble Sugar Contents
4.5.2. Measurements of the Starch Contents
4.5.3. Measurement of Hormone Contents
4.5.4. Determination of ROS-Related Physiological Indices
4.5.5. Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faust, M.; Erez, A.; Rowland, L.; Wang, S.; Norman, H. Bud Dormancy in Perennial Fruit Trees: Physiological Basis for Dormancy Induction, Maintenance, and Release. HortScience 1997, 32, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Y.; Bräutigam, K.; Hüner, N.P.A.; Ensminger, I. Champions of winter survival: Cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 2021, 229, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endodormancy, paradormancy, and ecodormancy-physiological terminology and classification for dormancy research. Hortscience 1987, 22, 371–377. [Google Scholar]
- Li, J.; Xu, Y.; Niu, Q.; He, L.; Teng, Y.; Bai, S. Abscisic Acid (ABA ) Promotes the Induction and Maintenance of Pear (Pyrus pyrifolia White Pear Group) Flower Bud Endodormancy. Int. J. Mol. Sci. 2018, 19, 310. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Gao, Y.; Wu, X.; Moriguchi, T.; Bai, S.; Teng, Y. Bud endodormancy in deciduous fruit trees: Advances and prospects. Hortic. Res. 2021, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, B.; Li, J.; Wang, Y.; Tao, R.; Yang, F.; Wu, X.; Yan, X.; Ahmad, M.; Shen, J.; et al. ABA-responsive ABRE-BINDING FACTOR3 activates DAM3 expression to promote bud dormancy in Asian pear. Plant, Cell Environ. 2020, 43, 1360–1375. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.-C.; Li, P.H.; Sun, L.-H.; Chen, T.H. Alterations in ultrastructure and subcellular localization of Ca2+in poplar apical bud cells during the induction of dormancy. J. Exp. Bot. 1997, 48, 1195–1207. [Google Scholar] [CrossRef] [Green Version]
- Heide, O.M.; Prestrud, A.K. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol. 2005, 25, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghelardini, L.; Santini, A.; Black-Samuelsson, S.; Myking, T.; Falusi, M. Bud dormancy release in elm (Ulmus spp.) clones—A case study of photoperiod and temperature responses. Tree Physiol. 2010, 30, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Campoy, J.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Perez, F.J.; Kuhn, N.; Vergara, R. Expression analysis of phytochromes A, B and floral integrator genes during the entry and exit of grapevine-buds from endodormancy. J. Plant Physiol. 2011, 168, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.P.; Anderson, J.; Chao, W.S.; Foley, M.E. Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci. 2003, 8, 534–540. [Google Scholar] [CrossRef] [PubMed]
- E Hedley, P.; Russell, J.R.; Jorgensen, L.; Gordon, S.; A Morris, J.; A Hackett, C.; Cardle, L.; Brennan, R. Candidate genes associated with bud dormancy release in blackcurrant (Ribes nigrum L.). BMC Plant Biol. 2010, 10, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, R.; Yamane, H.; Ooka, T.; Jotatsu, H.; Kitamura, Y.; Akagi, T.; Tao, R. Functional and Expressional Analyses of PmDAM Genes Associated with Endodormancy in Japanese Apricot. Plant Physiol. 2011, 157, 485–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, R.; Rowland, L.J.; Tanino, K. Induction and Release of Bud Dormancy in Woody Perennials: A Science Comes of Age. HortScience 2003, 38, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Horvath, D.P. Role of mature leaves in inhibition of root bud growth inEuphorbia esulaL. Weed Sci. 1999, 47, 544–550. [Google Scholar] [CrossRef]
- Horvath, D.; Chao, W.S.; Anderson, J. Molecular Analysis of Signals Controlling Dormancy and Growth in Underground Adventitious Buds of Leafy Spurge. Plant Physiol. 2002, 128, 1439–1446. [Google Scholar] [CrossRef] [Green Version]
- Destefano-Beltrán, L.; Knauber, D.; Huckle, L.; Suttle, J.C. Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol. Biol. 2006, 61, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Halaly, T.; Acheampong, A.K.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Or, E. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 2015, 66, 1527–1542. [Google Scholar] [CrossRef]
- Tuan, P.A.; Bai, S.; Saito, T.; Ito, A.; Moriguchi, T. Dormancy-Associated MADS-Box (DAM) and the Abscisic Acid Pathway Regulate Pear Endodormancy Through a Feedback Mechanism. Plant Cell Physiol. 2017, 58, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.L.; Welling, A.; Vahala, J.; Ripel, L.; Ruonala, R.; Kangasjärvi, J.; van der Schoot, C. Chilling of Dormant Buds Hyperinduces FLOWERING LOCUS T and Recruits GA-Inducible 1,3-β-Glucanases to Reopen Signal Conduits and Release Dormancy in Populus. Plant Cell 2011, 23, 130–146. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Liu, G.; Liu, D.; Ahmed, M.; Hussain, N.; Teng, Y. Study on the expression of dehydrin genes and activities of antioxidativeenzymes in floral buds of two sand pear (Pyrus pyrifolia Nakai) cultivarsrequiring different chilling hours for bud break. Turk. J. Agric. For. 2015, 39, 930–939. [Google Scholar] [CrossRef]
- Niu, Q.; Li, J.; Cai, D.; Qian, M.; Jia, H.; Bai, S.; Hussain, S.; Liu, G.; Teng, Y.; Zheng, X. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifoliawhite pear group) flower bud. J. Exp. Bot. 2016, 67, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielenberg, D.G.; Wang, Y.; Li, Z.; Zhebentyayeva, T.; Fan, S.; Reighard, G.L.; Scorza, R.; Abbott, A.G. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet. Genomes 2008, 4, 495–507. [Google Scholar] [CrossRef]
- Ubi, B.; Sakamoto, D.; Ban, Y.; Shimada, T.; Ito, A.; Nakajima, I.; Takemura, Y.; Tamura, F.; Saito, T.; Moriguchi, T. Molecular Cloning of Dormancy-associated MADS-box Gene Homologs and Their Characterization during Seasonal Endodormancy Transitional Phases of Japanese Pear. J. Am. Soc. Hortic. Sci. 2010, 135, 174–182. [Google Scholar] [CrossRef]
- Yamane, H.; Kashiwa, Y.; Ooka, T.; Tao, R.; Yonemori, K. Suppression Subtractive Hybridization and Differential Screening Reveals Endodormancy-associated Expression of an SVP/AGL24-type MADS-box Gene in Lateral Vegetative Buds of Japanese Apricot. J. Am. Soc. Hortic. Sci. 2008, 133, 708–716. [Google Scholar] [CrossRef]
- Wu, R.-M.; Walton, E.F.; Richardson, A.; Wood, M.; Hellens, R.; Varkonyi-Gasic, E. Conservation and divergence of four kiwifruit SVP-like MADS-box genes suggest distinct roles in kiwifruit bud dormancy and flowering. J. Exp. Bot. 2011, 63, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zhao, S.; Tan, F.; Zhao, H.; Wang, D.; Si, H.; Chen, Q. Changes in ROS production and antioxidant capacity during tuber sprouting in potato. Food Chem. 2017, 237, 205–213. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wang, C.; Gai, S.; Mu, P. Correlation Analysis Between C/N and the Amount of Chilling Treatment During the Release of Dormant Floral Buds in Paeonia suffruticosa. Chin. Agric. Sci. Bull. 2012, 28, 231–235. [Google Scholar]
- Liu, B.; Zheng, G.-S.; Yan, Z.-P.; Wang, Z.-Z. Effect of different chilling treatments on forcing culture of tree peony in winter and changes of nutrient substance. Xibei Zhiwu Xuebao 2004, 24, 1635–1639. [Google Scholar]
- Destefano-Beltrán, L.; Knauber, D.; Huckle, L.; Suttle, J. Chemically forced dormancy termination mimics natural dormancy progression in potato tuber meristems by reducing ABA content and modifying expression of genes involved in regulating ABA synthesis and metabolism. J. Exp. Bot. 2006, 57, 2879–2886. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Si, F.; Zhang, Y.; Gai, S. Effect of Exogenous Gibberellin on DNA Methylation Level and Expression of Related Enzyme Genes in Tree Peony Floral Buds. Sci. Agric. Sin. 2018, 51, 3561–3569. [Google Scholar]
- Xin, H.; Zhang, Y.; Wang, X.; Liu, C.; Feng, W.; Gai, S. Morphological, anatomical and DNA methylation changes of tree peony buds during chilling induced dormancy release. Plant Physiol. Biochem. 2019, 144, 64–72. [Google Scholar] [CrossRef]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chu, C. Brassinosteroid Regulates Cell Elongation by Modulating Gibberellin Metabolism in Rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Tang, Y.; Xia, X.; Sun, J.; Meng, J.; Shang, J.; Tao, J. Integration of Transcriptome, Proteome, and Metabolome Provides Insights into How Calcium Enhances the Mechanical Strength of Herbaceous Peony Inflorescence Stems. Cells 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.; Catley, J.; Walton, E. The effect of forcing temperature on peony shoot and flower development. Sci. Hortic. 2007, 113, 188–195. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Zhang, D.; Shi, X.; Wu, Y.; Qi, Z.; Ding, H.; Zhu, K.; Xia, Y.; Zhang, J. Improving crucial details and selecting the optimal model for evaluating the chilling requirement of Paeonia lactiflora Pall. at low latitudes during four winters. Sci. Hortic. 2020, 265, 109175. [Google Scholar] [CrossRef]
- Yuxi, Z.; Dan, Y.; Chunying, L.; Shupeng, G. Dynamic of carbohydrate metabolism and the related genes highlights PPP pathway activation during chilling induced bud dormancy release in tree peony (Paeonia suffruticosa). Sci. Hortic. 2018, 242, 36–43. [Google Scholar] [CrossRef]
- Luedeling, E. Climate change impacts on winter chill for temperate fruit and nut production: A review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, D.; Shi, X.; Zhang, D.; Qiu, S.; Wei, J.; Zhang, J.; Zhou, J.; Zhu, K.; Xia, Y. Mining and expression analysis of candidate genes involved in regulating the chilling requirement fulfillment of Paeonia lactiflora ‘Hang Baishao’. BMC Plant Biol. 2017, 17, 262. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhang, N.; Qiu, S.; Wei, J.; Guo, J.; Li, D.; Xia, Y. Evaluating the Comprehensive Performance of Herbaceous Peonies at low latitudes by the Integration of Long-running Quantitative Observation and Multi-Criteria Decision Making Approach. Sci. Rep. 2019, 9, 15079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Sechet, J.; To, A.; North, H.M.; Marion-Poll, A. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012, 70, 501–512. [Google Scholar] [CrossRef]
- Mouhu, K.; Kurokura, T.; Koskela, E.; Albert, V.A.; Elomaa, P.; Hytönen, T. The Fragaria vesca Homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 Represses Flowering and Promotes Vegetative Growth. Plant Cell 2013, 25, 3296–3310. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Tang, H.; Wang, B.; Yue, C.; Wang, L.; Zeng, J.; Yang, Y.; Wang, X. Integrative transcriptional and metabolic analyses provide insights into cold spell response mechanisms in young shoots of the tea plant. Tree Physiol. 2018, 38, 1655–1671. [Google Scholar] [CrossRef]
- Anderson, J.V.; Horvath, D.P.; Chao, W.S.; Foley, M.E. Bud Dormancy in Perennial Plants: A Mechanism for Survival. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Topics in Current Genetics: Weed Biology Research Unit; USDA-Agricultural Research Service: Fargo, ND, USA, 2010; Volume 21, pp. 69–90. [Google Scholar]
- Philip, M.M.; FangYun, C.; HongYan, L. Chronological changes in plant hormone and sugar contents in cv. Ao-Shuang autumn flowering tree peony. Hortic. Sci. 2011, 38, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Guy, C.L.; Huber, J.L.A.; Huber, S.C. Sucrose Phosphate Synthase and Sucrose Accumulation at Low Temperature. Plant Physiol. 1992, 100, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Ichimura, K.; Oda, M. Changes in Sugar Content during Cold Acclimation and Deacclimation of Cabbage Seedlings. Ann. Bot. 1996, 78, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant, Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2013, 239, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Cheng, H.; King, K.E.; Wang, W.; He, Y.; Hussain, A.; Lo, J.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002, 16, 646–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouquin, T.; Meier, C.; Foster, R.; Nielsen, M.E.; Mundy, J. Control of Specific Gene Expression by Gibberellin and Brassinosteroid. Plant Physiol. 2001, 127, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Andujar, C.; Ordiz, M.I.; Huang, Z.; Nonogaki, M.; Beachy, R.N. Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc. Natl. Acad. Sci. USA 2011, 108, 17225–17229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Gao, J.; Zhu, X.; Song, Y.; Li, Z.; Ren, G.; Zhou, X.; Kuai, B. ABF2, ABF3, and ABF4 Promote ABA-Mediated Chlorophyll Degradation and Leaf Senescence by Transcriptional Activation of Chlorophyll Catabolic Genes and Senescence-Associated Genes in Arabidopsis. Mol. Plant 2016, 9, 1272–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-J.; Kang, J.-Y.; Park, H.-J.; Kim, M.D.; Bae, M.S.; Choi, H.-I.; Kim, S.Y. DREB2C Interacts with ABF2, a bZIP Protein Regulating Abscisic Acid-Responsive Gene Expression, and Its Overexpression Affects Abscisic Acid Sensitivity. Plant Physiol. 2010, 153, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Jin, Y.; Liu, C.; Vonapartis, E.; Liang, J.; Wu, W.; Gazzarrini, S.; He, J.; Yi, M. GhNAC83 inhibits corm dormancy release by regulating ABA signaling and cytokinin biosynthesis in Gladiolus hybridus. J. Exp. Bot. 2019, 70, 1221–1237. [Google Scholar] [CrossRef] [Green Version]
- Cutler, A.J.; E Krochko, J. Formation and breakdown of ABA. Trends Plant Sci. 1999, 4, 472–478. [Google Scholar] [CrossRef]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional analysis of ArabidopsisNCED6andNCED9genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, W.; Yang, W. APETALA 2-domain-containing transcription factors: Focusing on abscisic acid and gibberellins antagonism. New Phytol. 2018, 217, 977–983. [Google Scholar] [CrossRef]
- An, F.; Zhang, X.; Zhu, Z.; Ji, Y.; He, W.; Jiang, Z.; Li, M.; Guo, H. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 2012, 22, 915–927. [Google Scholar] [CrossRef] [Green Version]
- Zawaski, C.; Kadmiel, M.; Pickens, J.; Ma, C.; Strauss, S.; Busov, V. Repression of gibberellin biosynthesis or signaling produces striking alterations in poplar growth, morphology, and flowering. Planta 2011, 234, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.V.; Mendiondo, G.M.; Maskin, L.; Gudesblat, G.E.; Iusem, N.D.; Benech-Arnold, R.L. Expression of ABA signalling genes and ABI5 protein levels in imbibed Sorghum bicolor caryopses with contrasting dormancy and at different developmental stages. Ann. Bot. 2009, 104, 975–985. [Google Scholar] [CrossRef]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J.J. DELAY OF GERMINATION1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar] [CrossRef]
- Steber, C.M.; McCourt, P. A Role for Brassinosteroids in Germination in Arabidopsis. Plant Physiol. 2001, 125, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.-Q.; Luan, S.; Li, J.; He, Z.-H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Truong, T.T.; Barrero, J.M.; Jacobsen, J.V.; Hocart, C.H.; Gubler, F. A role for jasmonates in the release of dormancy by cold stratification in wheat. J. Exp. Bot. 2016, 67, 3497–3508. [Google Scholar] [CrossRef] [Green Version]
- Suttle, J.C.; Banowetz, G.M. Changes in cis -zeatin and cis -zeatin riboside levels and biological activity during potato tuber dormancy. Physiol. Plant. 2000, 109, 68–74. [Google Scholar] [CrossRef]
- Noriega, X.; Burgos, B.; Pérez, F.J. Short day-photoperiod triggers and low temperatures increase expression of peroxidase RNA transcripts and basic peroxidase isoenzyme activity in grapevine buds. Phytochemistry 2007, 68, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, L.; Hancock, R.D.; Haupt, S.; Walker, P.G.; Pont, S.D.A.; McNicol, J.; Cardle, L.; Morris, J.; Viola, R.; Brennan, R.; et al. Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J. Exp. Bot. 2007, 58, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Xu, C.; Wang, Z.; Zhang, C.; Lin, M. Physiological changes of three jujube cultivars with different cooling requirements during dormancy. J. Arid. Land Resour. Environ. 2020, 34, 187–193. [Google Scholar]
- Atkinson, C.; Brennan, R.; Jones, H. Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 2013, 91, 48–62. [Google Scholar] [CrossRef]
- Welling, A.; Palva, E.T. Molecular control of cold acclimation in trees. Physiol. Plant. 2006, 127, 167–181. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Yi, G.-X.; Wang, B.-M.; Deng, A.-X.; Nan, T.-G.; Li, Z.-H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef] [PubMed]



| ZR | IAA | ABA | GA3 | BR | JAME | SUC | TSS | STA | POD | SOD | CAT | MDA | BFP | WFS | ANS | APW | APH | ADS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZR | 1.00 | ||||||||||||||||||
| IAA | 0.52 | 1.00 | |||||||||||||||||
| ABA | −0.27 | −0.64 | 1.00 | ||||||||||||||||
| GA3 | 0.77 * | 0.35 | 0.26 | 1.00 | |||||||||||||||
| BR | 0.41 | 0.02 | 0.16 | 0.66 | 1.00 | ||||||||||||||
| JAME | −0.31 | −0.69 | 0.98 ** | 0.17 | 0.08 | 1.00 | |||||||||||||
| SUC | −0.6 | −0.48 | 0.07 | −0.41 | 0.1 | 0.1 | 1.00 | ||||||||||||
| TSS | −0.72 ** | −0.38 | −0.11 | −0.63 | −0.11 | −0.06 | 0.95 ** | 1.00 | |||||||||||
| STA | 0.52 | −0.27 | 0.54 | 0.66 | 0.45 | 0.50 | −0.32 | −0.56 | 1.00 | ||||||||||
| POD | −0.73 ** | −0.52 | 0.24 | −0.4 | 0.08 | 0.23 | 0.94 * | 0.89 ** | −0.31 | 1.00 | |||||||||
| SOD | 0.64 | 0.57 | −0.1 | 0.62 | 0.37 | −0.15 | −0.66 | −0.71 * | 0.5 | −0.68 | 1.00 | ||||||||
| CAT | −0.79 ** | −0.62 | 0.07 | −0.83 * | −0.48 | 0.18 | 0.63 | 0.74 * | −0.56 | 0.61 | −0.90 ** | 1.00 | |||||||
| MDA | −0.70 | 0.14 | −0.21 | −0.58 | −0.42 | −0.17 | 0.55 | 0.71 * | −0.77 * | 0.54 | −0.36 | 0.49 | 1.00 | ||||||
| BPF | −0.77 * | −0.49 | 0.07 | −0.84 * | −0.65 | 0.19 | 0.76 * | 0.81 * | −0.75 | 0.71 | −0.90 ** | −0.93 ** | 0.74 | 1.00 | |||||
| WFS | 0.77 * | 0.6 | −0.13 | 0.87 * | 0.52 | −0.25 | −0.57 | −0.65 | 0.74 | −0.58 | 0.89 ** | −0.96 ** | −0.51 | −0.90 ** | 1.00 | ||||
| ANS | −0.80 * | −0.49 | 0.14 | −0.82 * | −0.6 | 0.26 | 0.7 | 0.76 * | −0.78 * | 0.68 | −0.87 * | 0.93 ** | 0.75 | 0.98 ** | −0.94 ** | 1.00 | |||
| APW | −0.88 ** | −0.56 | 0.10 | −0.90 ** | −0.46 | 0.19 | 0.79 * | 0.84 * | −0.80 * | 0.80 * | −0.89 ** | 0.94 ** | 0.73 | 0.95 ** | −0.95 ** | 0.96 ** | 1.00 | ||
| APH | −0.75 | −0.61 | 0.02 | −0.92 ** | −0.6 | 0.17 | 0.75 | 0.80 * | −0.69 | 0.66 | −0.90 ** | 0.98 ** | 0.62 | 0.97 ** | −0.93 ** | 0.95 ** | 0.94 ** | 1.00 | |
| ADS | −0.65 | −0.34 | −0.20 | −0.80 * | −0.45 | −0.06 | 0.75 | 0.81 * | −0.88 ** | 0.658 | −0.88 ** | 0.90 ** | 0.7 | 0.95 ** | −0.90 ** | 0.95 ** | 0.93 ** | 0.94 ** | 1.00 |
| Abbreviations | Full Names | Gene | Physiological Metabolic Pathways |
|---|---|---|---|
| SS3 | starch synthase 3 | AT1G11720 | Carbohydrate transport and metabolism |
| AMY3 | α-amylase-like 3 | AT1G69830 | Carbohydrate transport and metabolism |
| BMY3 | β-amylase 3 | AT5G18670 | putative beta-amylase |
| ATCWINV1 | Arabidopsis thalianacell wall invertase 1 | AT3G13790 | Carbohydrate transport and metabolism |
| SUS3 | sucrose synthase 3 | AT4G02280 | Cell all/membrane/envelope biogenesis |
| ATSPS1F | sucrose phosphate synthase 1f | AT5G20280 | Cell all/membrane/envelope biogenesis |
| PFK3 | phosphofructokinase 3 | AT4G26270 | Carbohydrate transport and metabolism |
| APL3 | / | AT4G39210 | Carbohydrate transport and metabolism |
| UGP2 | UDP-glucose pyrophosphorylase 2 | AT5G17310 | Carbohydrate transport and metabolism |
| BGLU17 | β-glucosidase 17 | AT2G44480 | Carbohydrate transport and metabolism |
| NCED3 | 9-cis-epoxycarotenoid dioxygenase 3 | AT3G14440 | Secondary metabolites biosynthesis, transport and catabolism |
| NCED4 | 9-cis-epoxycarotenoid dioxygenase 4 | AT4G19170 | Secondary metabolites biosynthesis, transport and catabolism |
| XERICO | AT2G04240 | Involved in ABA metabolism | |
| ATPP2CA | protein phosphatase 2CA | AT3G11410 | Signal transduction mechanisms |
| PYR1 | pyrabactin resistance 1 | AT4G17870 | PYR/PYL/RCAR family proteins function as abscisic acid sensors |
| ABI5 | ABA insensitive 5 | AT2G36270 | involved in ABA signalling during seed maturation and germination |
| ABF2 | abscisic acid responsive elements-binding factor 2 | AT1G45249 | Leucine zipper transcription factor that binds to the abscisic acid (ABA)–responsive element (ABRE) motif in the promoter region of ABA-inducible genes. |
| SNRK2-5 | SNF1-related protein kinase 2.5 | AT5G63650 | encodes a member of SNF1-related protein kinases (SnRK2) whose activity is activated by ionic (salt) and non-ionic (mannitol) osmotic stress. |
| ATBZIP9 | Arabidopsis thalianabasic leucine zipper 9 | AT5G24800 | |
| CYCD3 | cyclin d3 | AT4G34160 | Cell cycle control, cell division, chromosome partitioning |
| BG3 | β-1,3-glucanase 3 | AT3G57240 | encodes a member of glycosyl hydrolase family 17 |
| CAT2 | catalase 2 | AT4G35090 | Catalase domain-containing protein/Catalase-rel domain-containing protein [Cephalotus follicularis] |
| EXPA6 | Arabidopsis thalianatexpansin 6 | AT2G28950 | Encodes an expansin. Involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. |
| EXLA2 | expansin-like a2 | AT4G38400 | member of EXPANSIN-LIKE. |
| CDKB22 | cyclin-dependent kinase b2;2 | AT1G20930 | Expressed in the shoot apical meristem. Involved in regulation of the G2/M transition of the mitotic cell cycle. |
| GRXS17 | Arabidopsis thalianamonothiol glutaredoxin 17 | AT4G04950 | Encodes a monothiol glutaredoxin that is a critical component involved in ROS accumulation, auxin signaling, and temperature-dependent postembryonic growth in plants. |
| HIS1-3 | histone H1-3 | AT2G18050 | encodes a structurally divergent linker histone whose gene expression is induced by dehydration and ABA. The mRNA is cell-to-cell mobile. |
| PER52 | peroxidase 52 | AT5G05340 | Encodes a protein with sequence similarity to peroxidases that is involved in lignin biosynthesis. Loss of function mutations show abnormal development of xylem fibers and reduced levels of lignin biosynthetic enxymes. |
| ARP6 | actin-related protein 6 | AT3G33520 | Encodes ACTIN-RELATED PROTEIN6 (ARP6), a putative component of a chromatin-remodeling complex. |
| GID1A | GA insensitive dwarf1a | AT3G05120 | Encodes a gibberellin (GA) receptor ortholog of the rice GA receptor gene (OsGID1). |
| PIF3 | phytochrome interacting factor 3 | AT1G09530 | Transcription factor interacting with photoreceptors phyA and phyB. |
| RGA1 | repressor of GAL-3 1 | AT2G01570 | Putative transcriptional regulator repressing the gibberellin response and integration of phytohormone signalling. |
| ATGA2OX8 | Arabidopsis thalianagibberellin 2-oxidase 8 | AT4G21200 | Encodes a protein with gibberellin 2-oxidase activity which acts specifically on C-20 gibberellins. |
| SPY | spindly | AT3G11540 | Encodes a N-acetyl glucosamine transferase that may glycosylate other molecules involved in GA signaling. |
| SPT | spatula | AT4G36930 | Encodes a transcription factor of the bHLH protein family. Mutants have abnormal, unfused carpels and reduced seed dormancy. |
| GASA4 | GAST1 protein homolog 4 | AT5G15230 | Encodes gibberellin-regulated protein GASA4. Promotes GA responses and exhibits redox activity. |
| CBF4 | C-repeat-binding factor 4 | AT5G51990 | This gene is involved in response to drought stress and abscisic acid treatment, but not to low temperature. |
| SPL9 | squamosa promoter binding protein-like 9 | AT2G42200 | Encodes a putative transcriptional regulator that is involved in the vegetative to reproductive phase transition. |
| SVP | short vegetative phase | AT2G22540 | Encodes a nuclear protein that acts as a floral repressor and that functions within the thermosensory pathway. |
| AGL20 | agamous-like 20 | AT2G45660 | Controls flowering and is required for CO to promote flowering. |
| AP1 | apetala1 | AT1G69120 | Floral homeotic gene encoding a MADS domain protein homologous to SRF transcription factors. |
| GRF2 | general regulatory factor 2, | AT1G78300 | G-box binding factor GF14 omega encoding a 14-3-3 protein The mRNA is cell-to-cell mobile. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, X.; Shi, X.; Shao, L.; Xu, T.; Xia, Y.; Li, D.; Zhang, J. Chilling Requirement Validation and Physiological and Molecular Responses of the Bud Endodormancy Release in Paeonia lactiflora ‘Meiju’. Int. J. Mol. Sci. 2021, 22, 8382. https://doi.org/10.3390/ijms22168382
Zhang R, Wang X, Shi X, Shao L, Xu T, Xia Y, Li D, Zhang J. Chilling Requirement Validation and Physiological and Molecular Responses of the Bud Endodormancy Release in Paeonia lactiflora ‘Meiju’. International Journal of Molecular Sciences. 2021; 22(16):8382. https://doi.org/10.3390/ijms22168382
Chicago/Turabian StyleZhang, Runlong, Xiaobin Wang, Xiaohua Shi, Lingmei Shao, Tong Xu, Yiping Xia, Danqing Li, and Jiaping Zhang. 2021. "Chilling Requirement Validation and Physiological and Molecular Responses of the Bud Endodormancy Release in Paeonia lactiflora ‘Meiju’" International Journal of Molecular Sciences 22, no. 16: 8382. https://doi.org/10.3390/ijms22168382
APA StyleZhang, R., Wang, X., Shi, X., Shao, L., Xu, T., Xia, Y., Li, D., & Zhang, J. (2021). Chilling Requirement Validation and Physiological and Molecular Responses of the Bud Endodormancy Release in Paeonia lactiflora ‘Meiju’. International Journal of Molecular Sciences, 22(16), 8382. https://doi.org/10.3390/ijms22168382








