Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development
Abstract
1. Introduction
2. Results
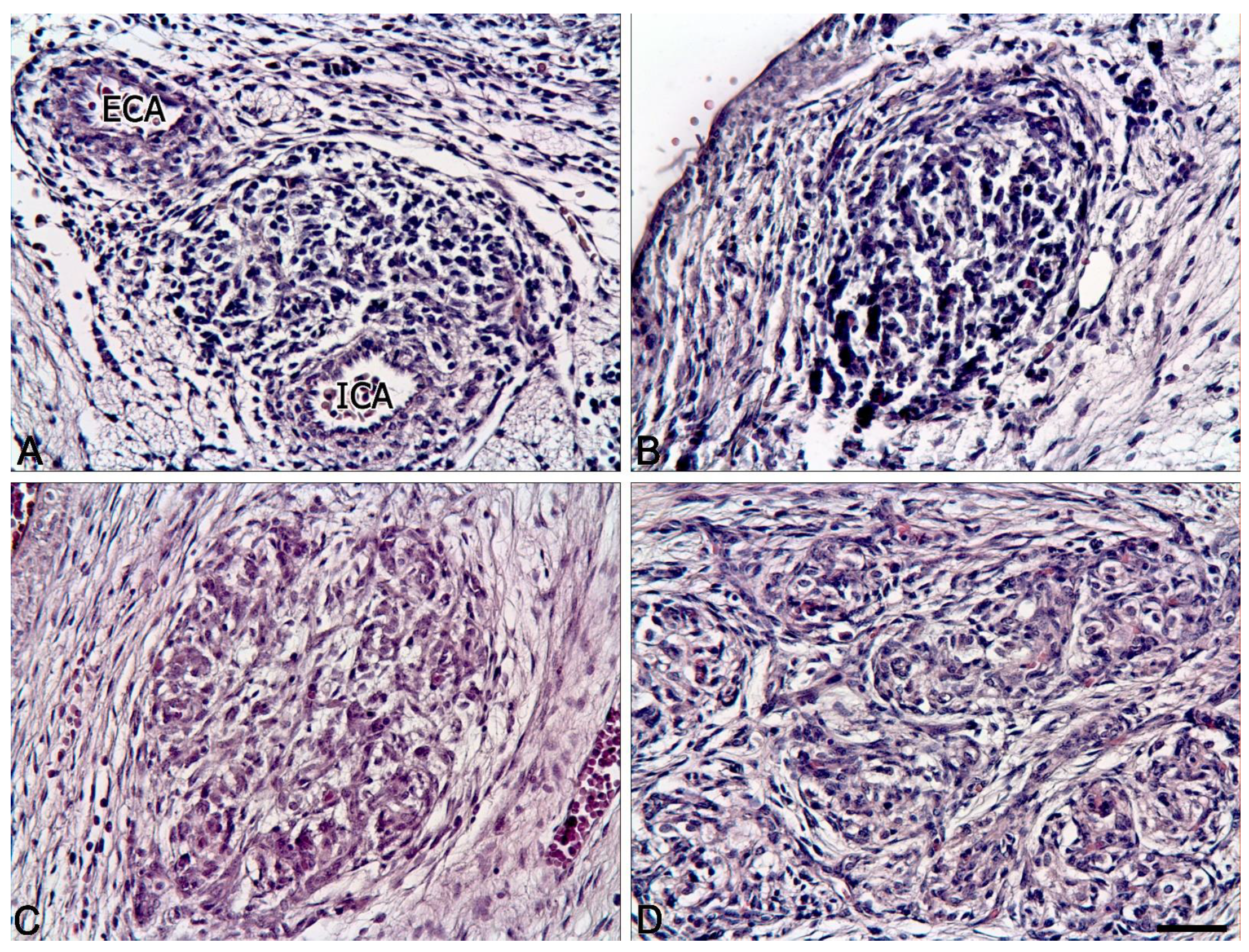
2.1. Comparison of the Human Carotid Body Structure in the Antenatal and Postnatal Periods
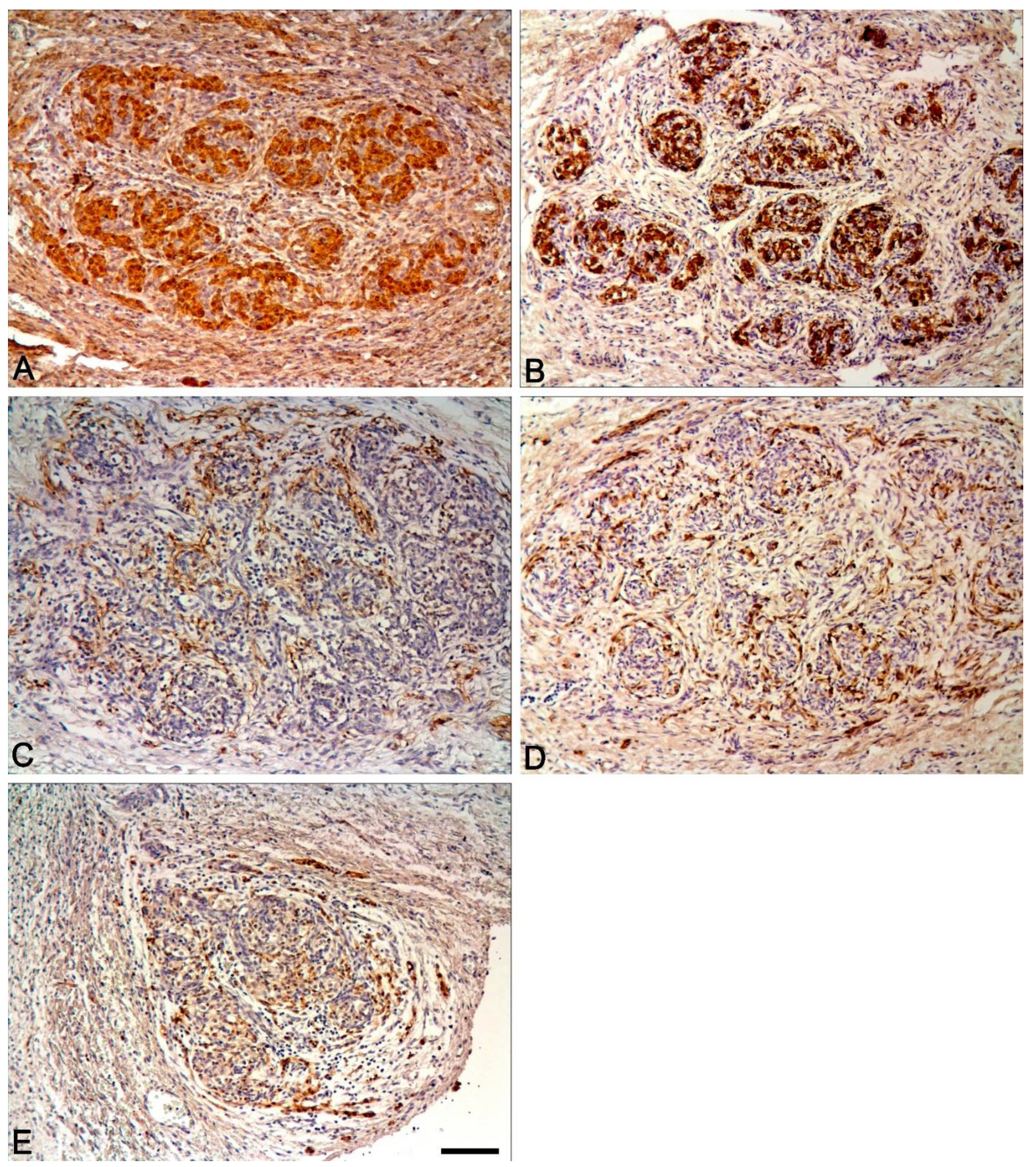
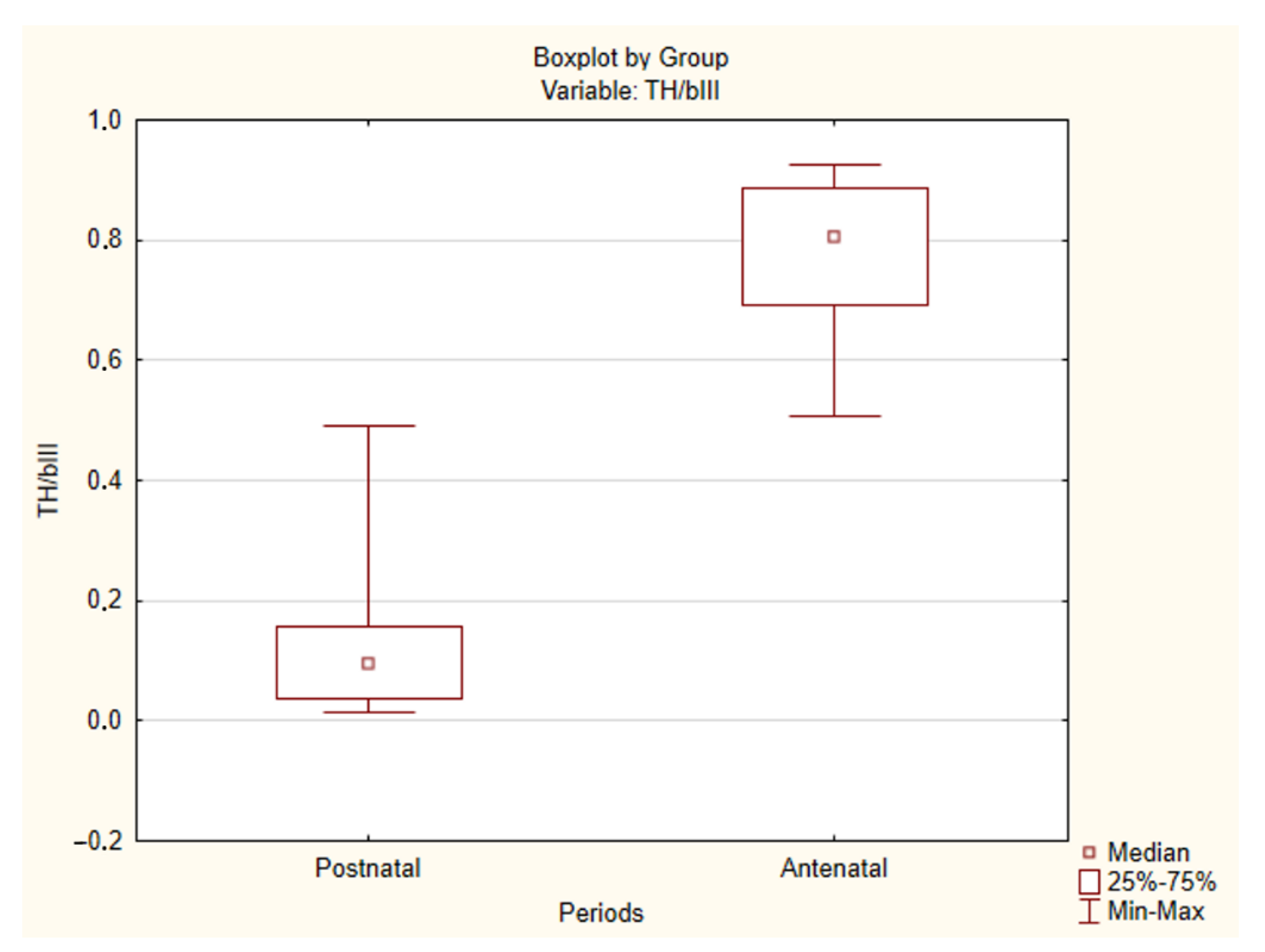
2.2. Immunohistochemical Study of the Human CAROTID Body
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohn, A. Die Paraganglien. Arch. Mikrosk. Anat. 1903, 62, 263–365. [Google Scholar] [CrossRef][Green Version]
- De Castro, F. Über die Struktur und Innervation des Glomus caroticum beim Menschen und bei den Säugetieren. Z. Anat. Entwicklungsgesch. 1929, 89, 250–265. (In German) [Google Scholar] [CrossRef]
- De Castro, F. Towards the sensory nature of the carotid body: Hering, De Castro and Heymans. Front. Neuroanat. 2009, 3, 23. [Google Scholar] [CrossRef]
- Kumar, P.; Prabhakar, N.R. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr. Physiol. 2011, 2, 141–219. [Google Scholar] [CrossRef]
- Biscoe, T.J. Carotid body: Structure and function. Physiol. Rev. 1971, 51, 437–495. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.P.; De Burgh Daly, M.; Howe, A. Early ultrastructural changes in the carotid body after degenerative section of the carotid sinus nerve in the cat. Cells Tissues Organs. 1972, 83, 161–185. [Google Scholar] [CrossRef]
- Grimley, P.M.; Glenner, G.G. Ultrastructure of the human carotid body. A perspective on the mode of chemoreception. Circulation 1968, 37, 648–665. [Google Scholar] [CrossRef]
- Hervonen, A.; Korkala, O. Fine structure of the carotid body of the midterm human fetus. Z. Anat. Entwicklungsgesch. 1972, 138, 135–144. [Google Scholar] [CrossRef]
- Kobayashi, S. Fine structure of the carotid body of the dog. Arch. Histol. Jpn. 1968, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Smitten, N.A. Sympatho-Adrenal System in Phylogenesis and Ontogenesis; Nauka: Moscow, Russia, 1972; 347p. (In Russian) [Google Scholar]
- Acker, H.; Pietruschka, F. Meaning of the Type I Cell for the Chemoreceptive Process–An Electrophysiological Study on Cultured Type I Cells of the Carotid Body. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrens, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 92–96. [Google Scholar]
- Verna, A.; Roumy, M.; Leitner, L.M. Loss of chemoreceptive properties of the rabbit carotid body after destruction of the glomus cells. Brain Res. 1975, 100, 13–23. [Google Scholar] [CrossRef]
- Monti-Bloch, L.; Stensaas, L.J.; Eyzaguirre, C. Carotid body grafts induced chemosensitivity in muscle nerve fibers of the cat. Brain Res. 1983, 270, 77–92. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, M.; Nurse, C.A. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J. Physiol. 1997, 503, 599–612. [Google Scholar] [CrossRef]
- Milsom, W.K.; Burleson, M.L. Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir. Physiol. Neurobiol. 2007, 157, 4–11. [Google Scholar] [CrossRef]
- Jonz, M.G.; Buck, L.T.; Perry, S.F.; Schwerte, T.; Zaccone, G. Sensing and surviving hypoxia in vertebrates. Ann. N. Y. Acad. Sci. 2016, 1365, 43–58. [Google Scholar] [CrossRef]
- Hockman, D.; Burns, A.J.; Schlosser, G.; Gates, K.P.; Jevans, B.; Mongera, A.; Fisher, S.; Unlu, G.; Knapik, E.W.; Kaufman, C.K.; et al. Evolution of the hypoxia-sensitive cells involved in amniote respiratory reflexes. Elife 2017, 6. [Google Scholar] [CrossRef]
- Lazarov, N.E.; Reindl, S.; Fischer, F.; Gratzl, M. Histaminergic and dopaminergic traits in the human carotid body. Respir. Physiol. Neurobiol. 2009, 165, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kåhlin, J.; Mkrtchian, S.; Ebberyd, A.; Hammarstedt-Nordenvall, L.; Nordlander, B.; Yoshitake, T.; Kehr, J.; Prabhakar, N.; Poellinger, L.; Fagerlund, M.J.; et al. The human carotid body releases acetylcholine, ATP and cytokines during hypoxia. Exp. Physiol. 2014, 99, 1089–1098. [Google Scholar] [CrossRef]
- Fagerlund, M.J.; Kåhlin, J.; Ebberyd, A.; Schulte, G.; Mkrtchian, S.; Eriksson, L.I. The Human Carotid Body Expression of Oxygen Sensing and Signaling Genes of Relevance for Anesthesia. Anesthesiol. J. Am. Soc. Anesthesiol. 2010, 113, 1270–1279. [Google Scholar] [CrossRef]
- Ortega-Sáenz, P.; Pardal, R.; Levitsky, K.; Villadiego, J.; Muñoz-Manchado, A.B.; Durán, R.; Bonilla-Henao, V.; Arias-Mayenco, I.; Sobrino, V.; Ordóñez, A. Cellular properties and chemosensory responses of the human carotid body. J. Physiol. 2013, 591, 6157–6173. [Google Scholar] [CrossRef] [PubMed]
- Pallot, D.J.; Seker, M.; Abramovici, A. Post-mortem changes in the normal rat carotid body: Possible implications for human histopathology. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 31–35. [Google Scholar] [CrossRef]
- Seker, M.; Pallot, D.J.; Habeck, J.-O.; Abramovici, A. Postmortem changes in the human carotid body. In Arterial Chemoreceptors; O’Regan, R.G., Nolan, P., McQueen, D.S., Paterson, D.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 349–351. [Google Scholar]
- Otlyga, D.A.; Junemann, O.A.; Dzhalilova, D.S.; Tsvetkova, E.G.; Saveliev, S.V. Immunohistochemical Study of Dark and Progenitor Carotid Body Cells: Artefacts or Real Subtypes? Bull. Exp. Biol. Med. 2020, 168, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Otlyga, D.A.; Junemann, O.A.; Tsvetkova, E.G.; Kharlamova, A.S.; Besova, N.V.; Saveliev, S.V. Carotid body, adrenal medulla and Zuckerkandl organ as an integrated sympathoadrenal system in human prenatal development. Clin. Exp. Morphol. 2020, 9, 61–69. [Google Scholar] [CrossRef]
- Korkala, O.; Hervonen, A. Origin and development of the catecholamine-storing cells of the human fetal carotid body. Histochemie 1973, 37, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Scraggs, M.; Smith, P.; Heath, D. Glomic cells and their peptides in the carotid body of the human fetus. Fetal Pediatr. Pathol. 1992, 12, 823–834. [Google Scholar] [CrossRef]
- Izal-Azcárate, A.; Belzunegui, S.; Sebastián, W.S.; Garrido-Gil, P.; Vázquez-Claverie, M.; López, B.; Marcilla, I.; Luquin, M.R. Immunohistochemical characterization of the rat carotid body. Respir. Physiol. Neurobiol. 2008, 161, 95–99. [Google Scholar] [CrossRef]
- Ortega-Sáenz, P.; Pascual, A.; Gómez-Díaz, R.; López-Barneo, J. Acute oxygen sensing in heme oxygenase-2 null mice. J. Gen. Physiol. 2006, 128, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Gauda, E.B.; Bamford, O.; Gerfen, C.R. Developmental expression of tyrosine hydroxylase, D2-dopamine receptor and substance P genes in the carotid body of the rat. Neuroscience 1996, 75, 969–977. [Google Scholar] [CrossRef]
- Blanco, C.E.; Dawes, G.S.; Hanson, M.A.; McCooke, H.B. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J. Physiol. 1984, 351, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.L.; Bamford, O.S.; Fitzgerald, R.S. Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J. Appl. Physiol. 1993, 75, 2383–2391. [Google Scholar] [CrossRef]
- Wasicko, M.J.; Sterni, L.M.; Bamford, O.S.; Montrose, M.H.; Carroll, J.L. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J. Physiol. 1999, 514, 493–503. [Google Scholar] [CrossRef]
- Subramanian, A.; Maker, V.K. Organs of Zuckerkandl: Their surgical significance and a review of a century of literature. Am. J. Surg. 2006, 192, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.; Edwards, C.; Harris, P. Post-mortem size and structure of the human carotid body. Thorax 1970, 25, 129–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gosney, J.R. Adrenal corticomedullary hyperplasia in hypobaric hypoxia. J. Pathol. 1985, 146, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Stensaas, L.J. The ultrastructure and source of nerve endings in the carotid body. Cell Tiss. Res. 1974, 154, 303–319. [Google Scholar] [CrossRef]
- Kondo, H. Innervation of the carotid body of the adult rat. Cell Tiss. Res. 1976, 173, 1–15. [Google Scholar] [CrossRef]
- King, A.S.; King, D.Z.; Hodges, R.D.; Henry, J. Synaptic morphology of the carotid body of the domestic fowl. Cell Tiss. Res. 1975, 162, 459–473. [Google Scholar] [CrossRef]
- McDonald, D.M.; Mitchell, R.A. The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: A quantitative ultrastructural analysis. J. Neurocytol. 1975, 4, 177–230. [Google Scholar] [CrossRef]
- McDonald, D.M.; Mitchell, R.A. The neural pathway involved in “efferent inhibition” of chemoreceptors in the cat carotid body. J. Comp. Neurol. 1981, 201, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Zavarzin, A.A. Essays on the evolutionary histology of the nervous system. In Zavarzin, A.A. Selected Works; Nevmyvaka, G.A., Tsvileneva, V.A., Eds.; Izdatel’stvo AN SSSR: Moscow-Leningrad, Russia, 1950; Volume 3, pp. 271–281. (In Russian) [Google Scholar]
- Hellström, S. Effects of hypoxia on carotid body type I cells and their catecholamines. A biochemical and morphologic study. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrens, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 122–129. [Google Scholar]
- Zapata, P. Effects of dopamine on carotid chemo- and baroreceptors in vitro. J. Physiol. 1975, 244, 235–251. [Google Scholar] [CrossRef]
- Milovanov, A.P.; Saveliev, S.V. Rational periodization of prenatal human development and methodical aspects of embryology. In Prenatal Human Development; Milovanov, A.P., Saveliev, S.V., Eds.; MDV: Moscow, Russia, 2006; pp. 21–32. (In Russian) [Google Scholar]



| Case No | βIII-Tubulin | PGP9.5 | NF 200 kd | TH | Syn | GFAP Ab-4 | S100 |
|---|---|---|---|---|---|---|---|
| Antenatal | |||||||
| 1 | + | + | |||||
| 2 | + | + | |||||
| 3 | + | + | |||||
| 4 | + | + | + | + | |||
| 5 | + | + | + | + | |||
| 6 | + | + | + | + | + | ||
| 7 | + | + | + | + | + | ||
| 8 | + | + | + | + | + | + | |
| 9 | + | + | + | + | + | + | |
| 10 | + | + | + | + | |||
| 11 | + | + | + | + | |||
| 12 | + | + | + | + | |||
| 13 | + | + | + | + | |||
| 14 | + | + | |||||
| 15 | + | + | |||||
| 16 | + | + | |||||
| 17 | + | + | |||||
| 18 | + | + | |||||
| 19 | + | + | + | + | + | + | |
| 20 | + | + | + | + | + | + | |
| 21 | + | + | + | + | |||
| Postnatal | |||||||
| 22 | + | + | + | + | + | + | + |
| 23 | + | + | + | + | + | + | + |
| 24 | + | + | + | + | + | + | + |
| 25 | + | + | + | + | + | + | + |
| 26 | + | + | + | + | + | + | + |
| 27 | + | + | + | + | + | + | + |
| 28 | + | + | + | + | + | + | + |
| 29 | + | + | + | + | + | + | + |
| 30 | + | + | + | + | + | + | + |
| 31 | + | + | + | + | + | + | + |
| 32 | + | + | + | + | + | + | + |
| 33 | + | + | + | + | + | + | + |
| 34 | + | + | + | + | + | + | + |
| Case No. | Type I Cells | Type II Cells | Schwann Cells | Nerve Fibers |
|---|---|---|---|---|
| βIII-tubulin | + | + | ||
| PGP9.5 | + | + | ||
| NF 200 kd | + | |||
| TH | + | + | ||
| Syn | + | |||
| GFAP Ab-4 | + | + | ||
| S100 | + | + |
| Case No | TH/βIII Ratio | Case No | TH/βIII Ratio | Case No | TH/βIII Ratio |
|---|---|---|---|---|---|
| Antenatal period | Postnatal period | ||||
| 1 L | 0.900731 | 12 | 0.70254 | 22 | 0.145537 |
| 1 R | 0.924528 | 13 | 0.854295 | 23 | 0.035849 |
| 2. | 0.885407 | 14 | 0.624743 | 24 | 0.226393 |
| 3. | 0.807854 | 15 | 0.535936 | 25 | 0.491257 |
| 4. | 0.881466 | 16 | 0.646983 | 26 | 0.026529 |
| 5. | 0.92536 | 17 | 0.507709 | 27 | 0.095764 |
| 6. | 0.732308 | 18 | 0.691227 | 28 | 0.156351 |
| 7. | 0.804473 | 19 | 0.892741 | 29 | 0.130611 |
| 8. | 0.771863 | 20 | 0.845743 | 30 | 0.467732 |
| 9. | 0.833736 | 21 | 0.910662 | 31 | 0.082004 |
| 10. | 0.518871 | 32 | 0.058105 | ||
| 11 L | 0.70143 | 33 | 0.023186 | ||
| 11 R | 0.727811 | 34 | 0.014426 | ||
| Antenatal Period | Postnatal Period | ||
|---|---|---|---|
| Case No. | Gestational Age | Case No. | Age, Years |
| 1 | 8 PCW 1 | 22 | 24 |
| 2 | 10 PCW | 23 | 87 |
| 3 | 13–14 GW 2 | 24 | 63 |
| 4 | 18–19 GW | 25 | 69 |
| 5 | 19–20 GW | 26 | 62 |
| 6 | 21–22 GW | 27 | 78 |
| 7 | 17–18 GW | 28 | 67 |
| 8 | 30 GW | 29 | 86 |
| 9 | 30 GW | 30 | 80 |
| 10 | 19–20 GW | 31 | 79 |
| 11 | 19–20 GW | 32 | 95 |
| 12 | 19–20 GW | 33 | 68 |
| 13 | 16–17 GW | 34 | 56 |
| 14 | 14–15 GW | ||
| 15 | 15–16 GW | ||
| 16 | 15–16 GW | ||
| 17 | 18–19 GW | ||
| 18 | 15–16 GW | ||
| 19 | 16 GW | ||
| 20 | 16 GW | ||
| 21 | 21 GW | ||
| No. | Antigen, Host Species, Supplier | Working Dilution |
|---|---|---|
| 1 | βIII-tubulin, rabbit polyclonal. Abcam (Cambridge, UK) | 1:500 |
| 2 | PGP9.5, murine monoclonal. Thermo Fisher Scientific (Waltham, MA, USA) | 1:300 |
| 3 | Neurofilaments 200 kDa, murine monoclonal. Merck (Darmstadt, Germany) | 1:160 |
| 4 | S100, rabbit polyclonal. Thermo Fisher Scientific (Waltham, MA, USA) | 1:1200 |
| 5 | Tyrosine hydroxylase, rabbit polyclonal. Abcam (Cambridge, UK) | 1:160 |
| 6 | GFAP Ab-4, rabbit polyclonal. Thermo Fisher Scientific (Waltham, MA, USA) | 1:200–1:1000 |
| 7 | Synaptophysin, murine monoclonal. Abcam (Cambridge, UK) | 1:100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otlyga, D.; Tsvetkova, E.; Junemann, O.; Saveliev, S. Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development. Int. J. Mol. Sci. 2021, 22, 8222. https://doi.org/10.3390/ijms22158222
Otlyga D, Tsvetkova E, Junemann O, Saveliev S. Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development. International Journal of Molecular Sciences. 2021; 22(15):8222. https://doi.org/10.3390/ijms22158222
Chicago/Turabian StyleOtlyga, Dmitry, Ekaterina Tsvetkova, Olga Junemann, and Sergey Saveliev. 2021. "Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development" International Journal of Molecular Sciences 22, no. 15: 8222. https://doi.org/10.3390/ijms22158222
APA StyleOtlyga, D., Tsvetkova, E., Junemann, O., & Saveliev, S. (2021). Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development. International Journal of Molecular Sciences, 22(15), 8222. https://doi.org/10.3390/ijms22158222






