Hormonal Regulation and Crosstalk of Auxin/Cytokinin Signaling Pathways in Potatoes In Vitro and in Relation to Vegetation or Tuberization Stages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Auxin Regulatory System
2.1.1. Auxin Signaling Genes in Potato and Regularities of Their Expression
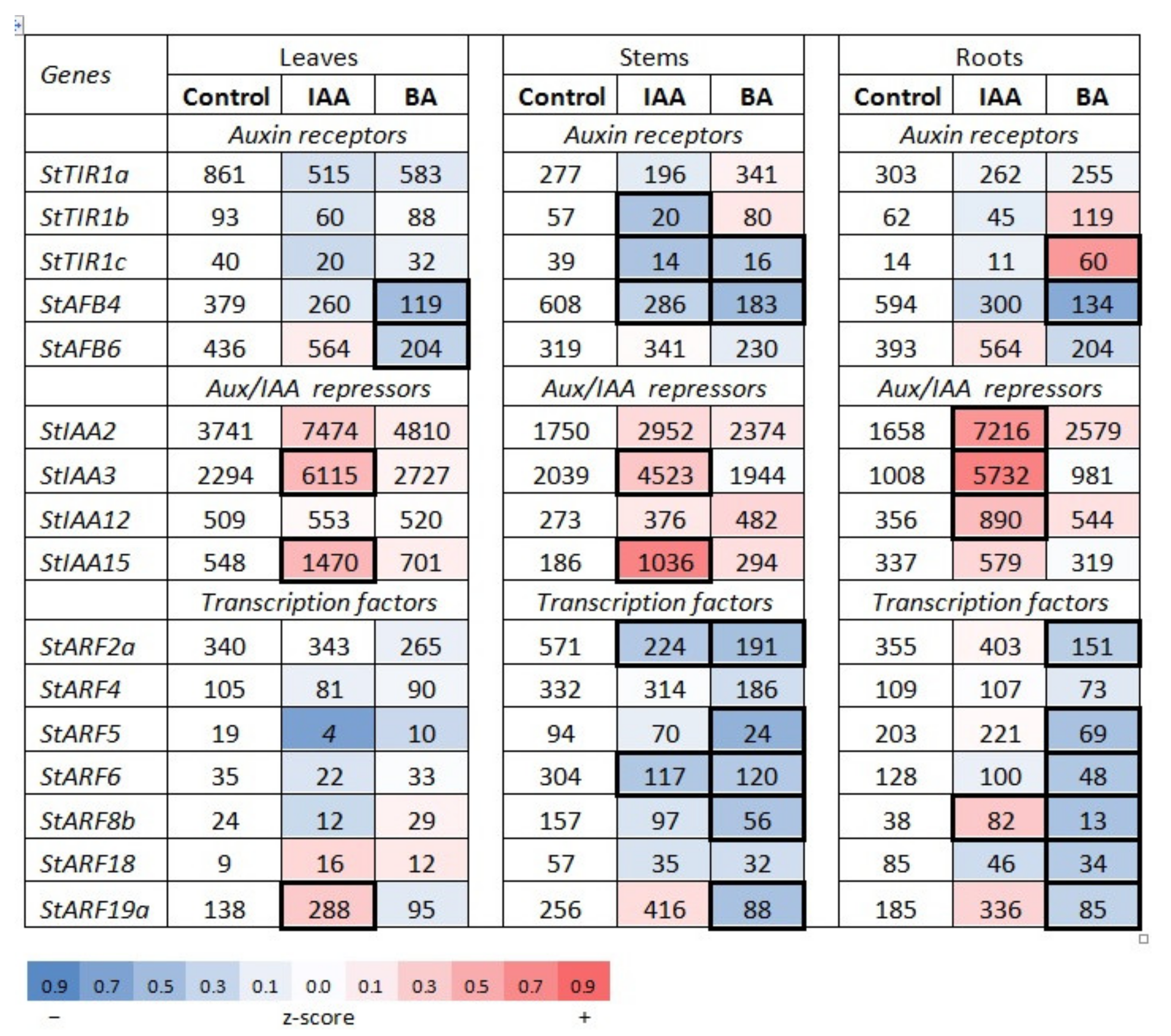
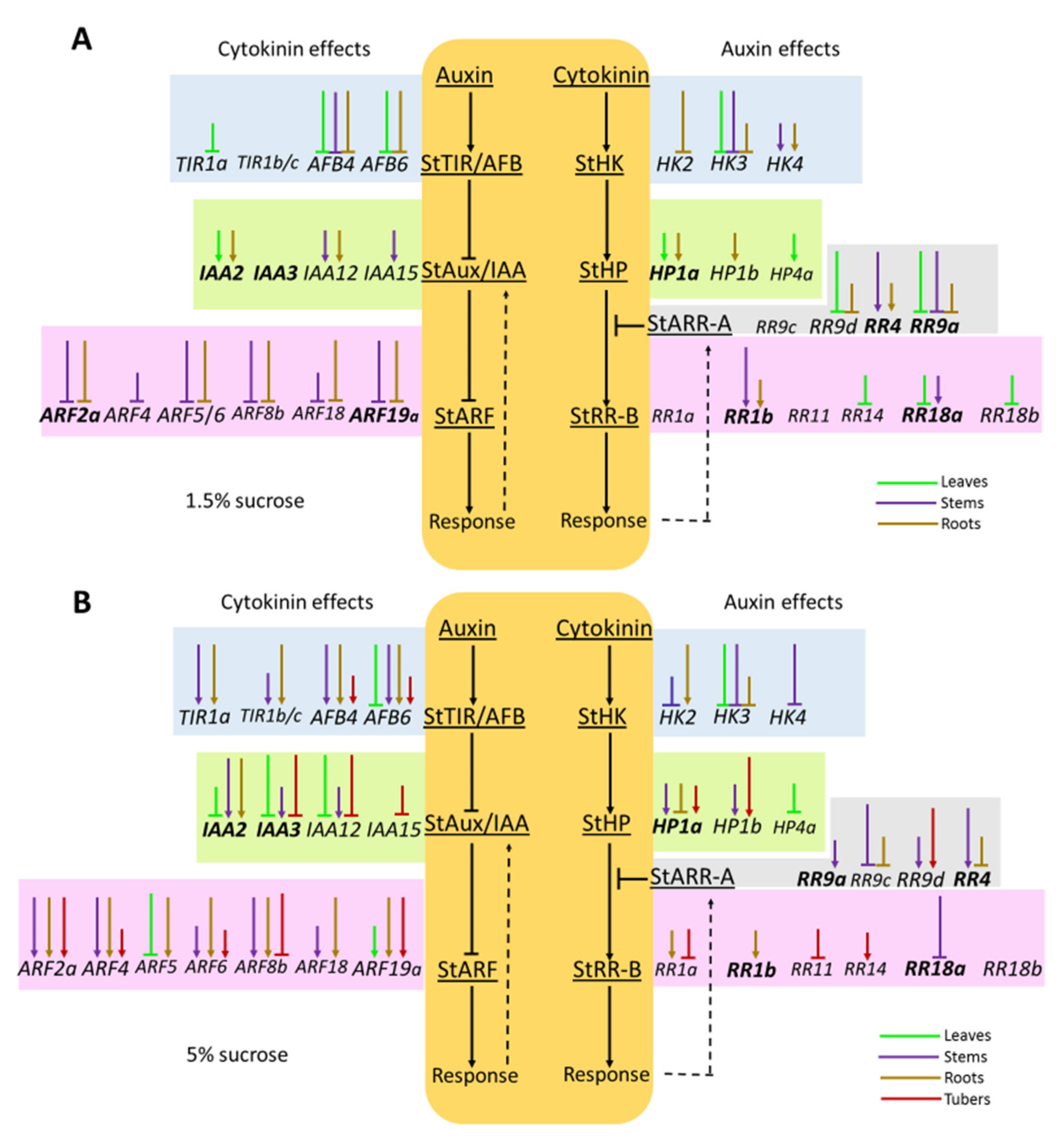
2.1.2. Hormonal Effects on the Expression Profile of Auxin Signaling Genes
Effect of Auxin
Effect of Cytokinin
2.2. Cytokinin Regulatory System
2.2.1. Cytokinin Signaling Genes in Potato and Regularities of Their Expression
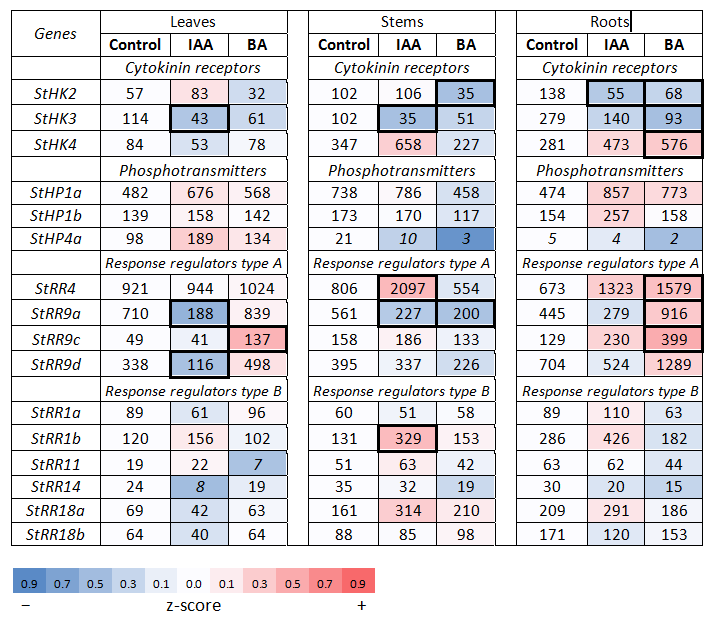
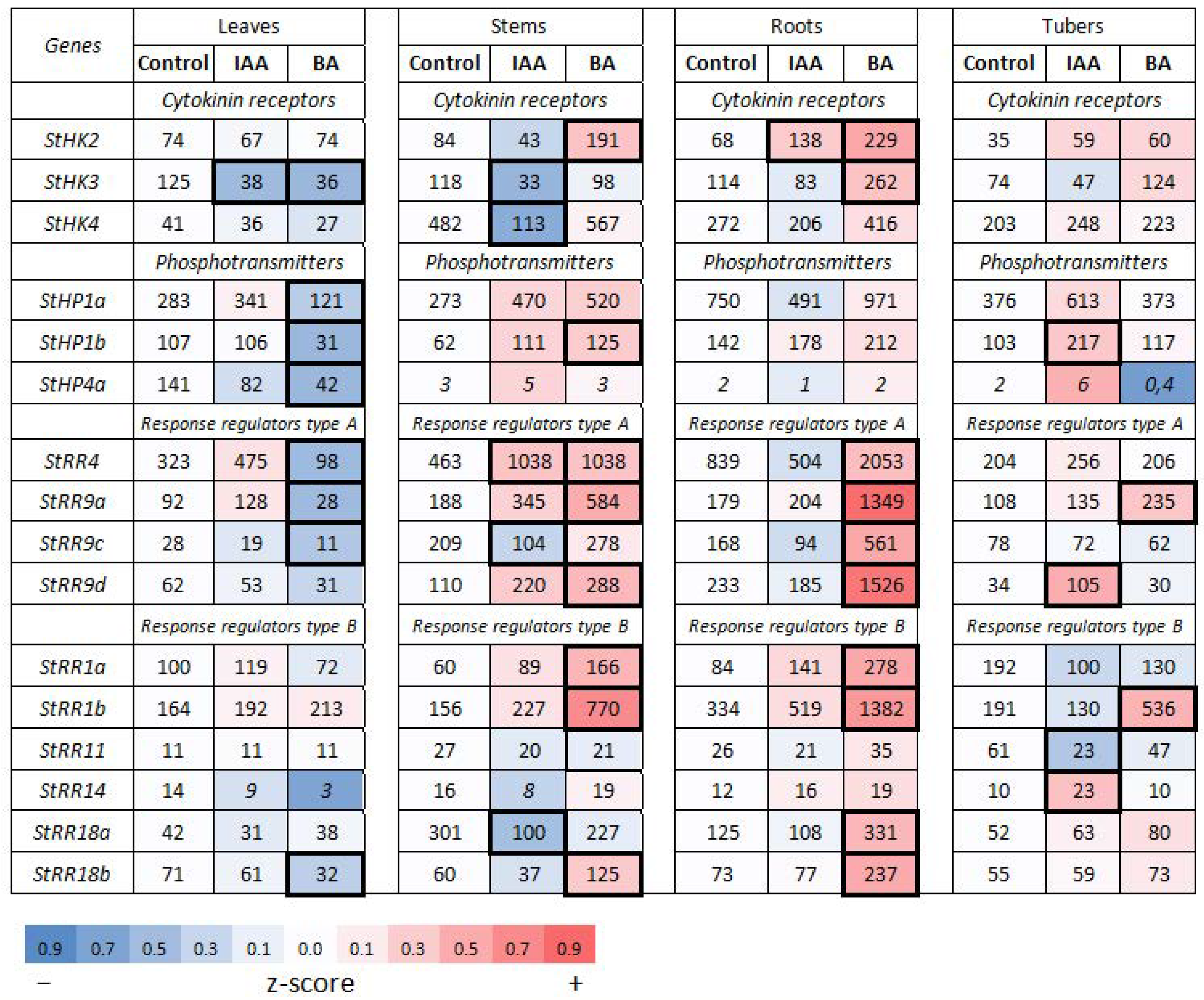
2.2.2. Hormonal Effects on the Expression Profile of CK Signaling Genes
Effect of Cytokinin
Effect of Auxin
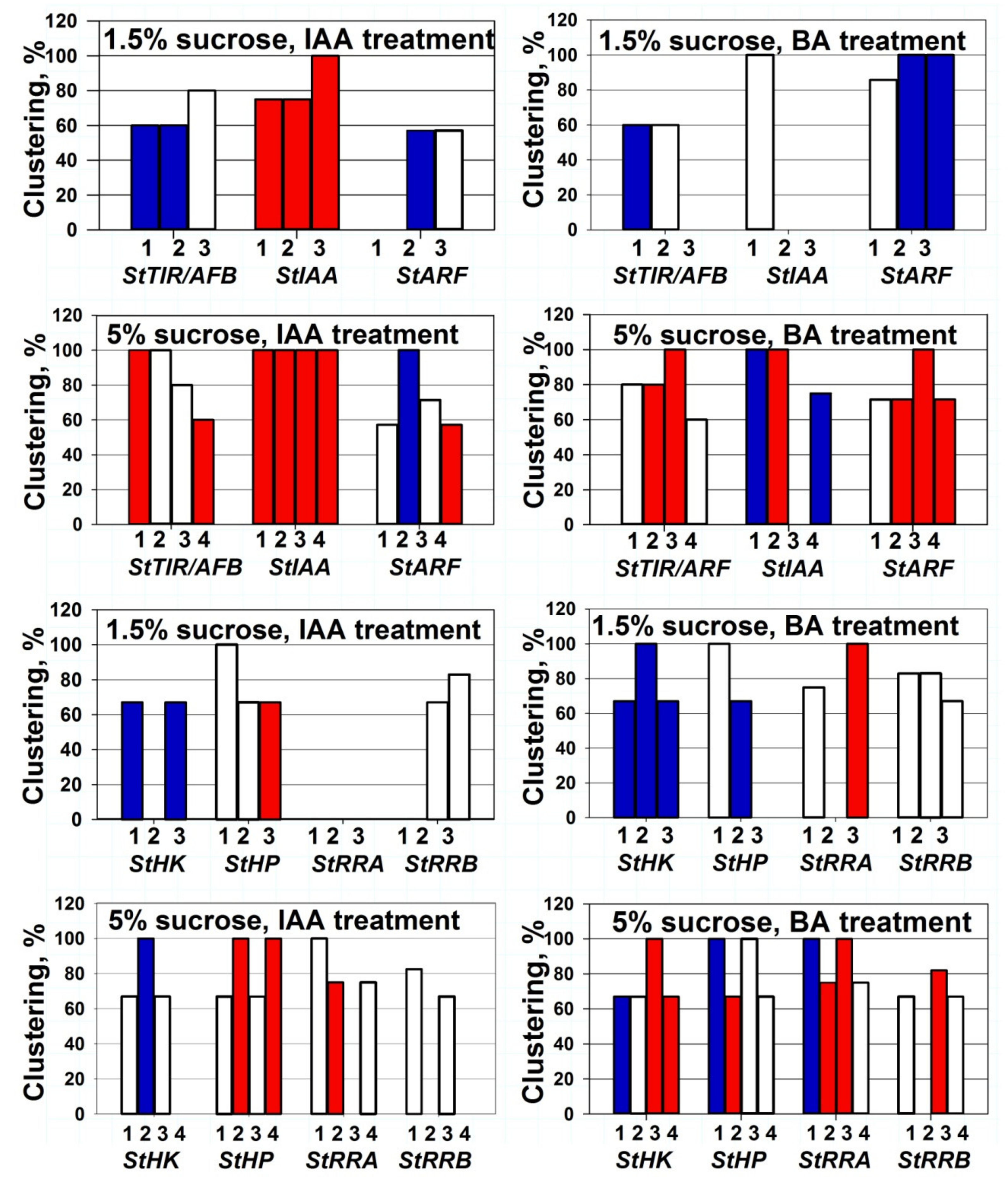
2.3. Clusterization of Gene Expression Changes and Effect of Sucrose
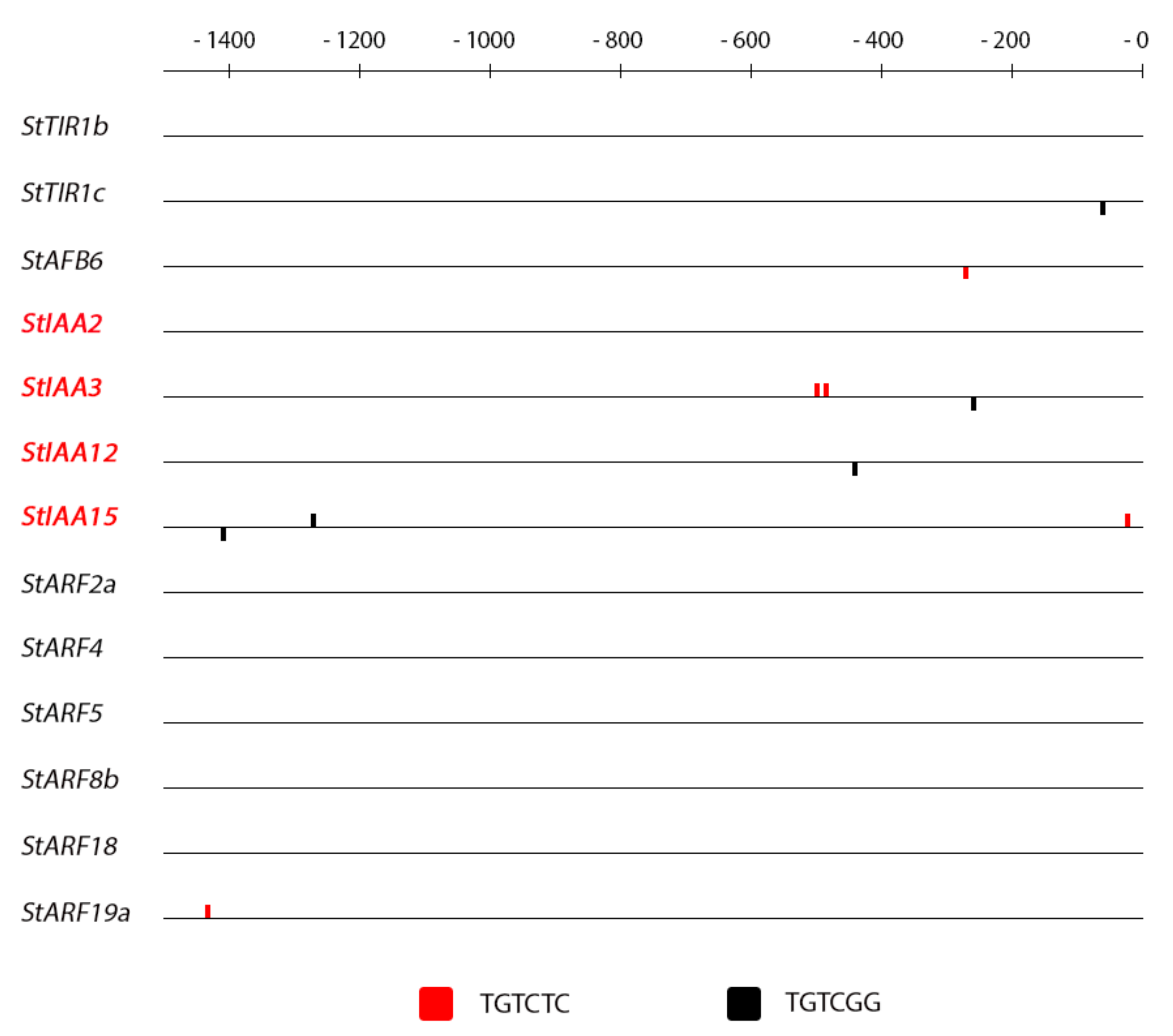
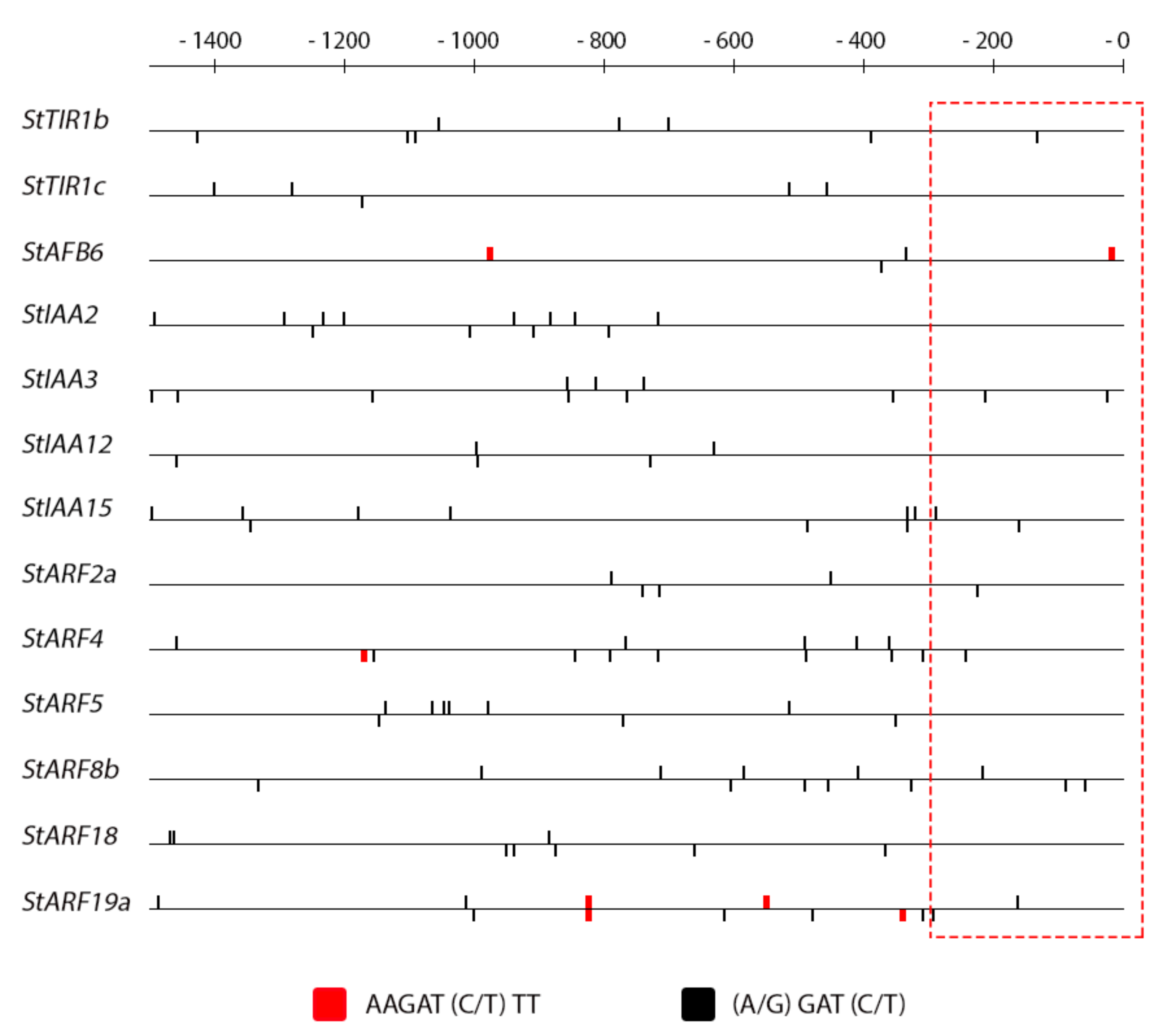
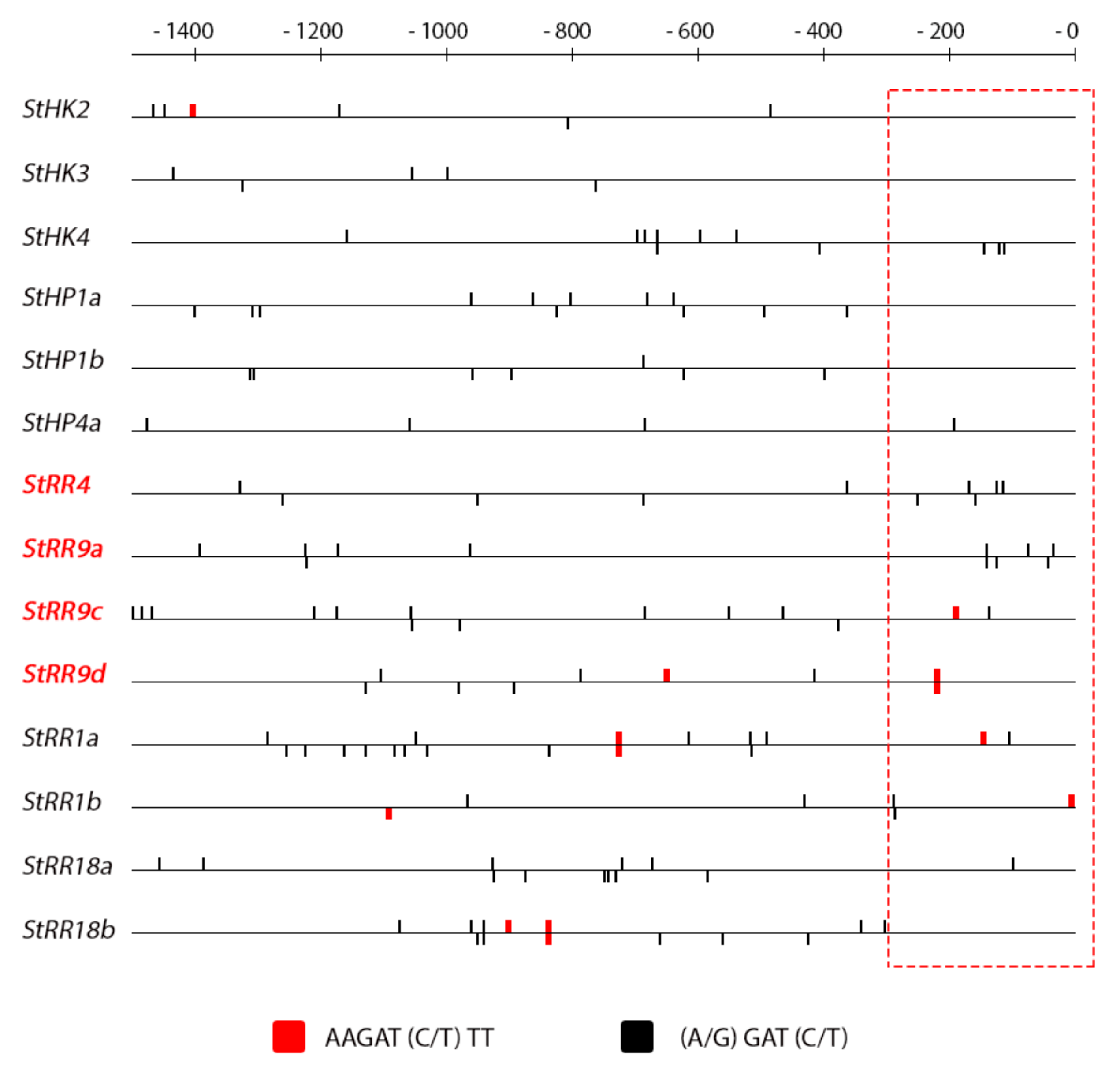
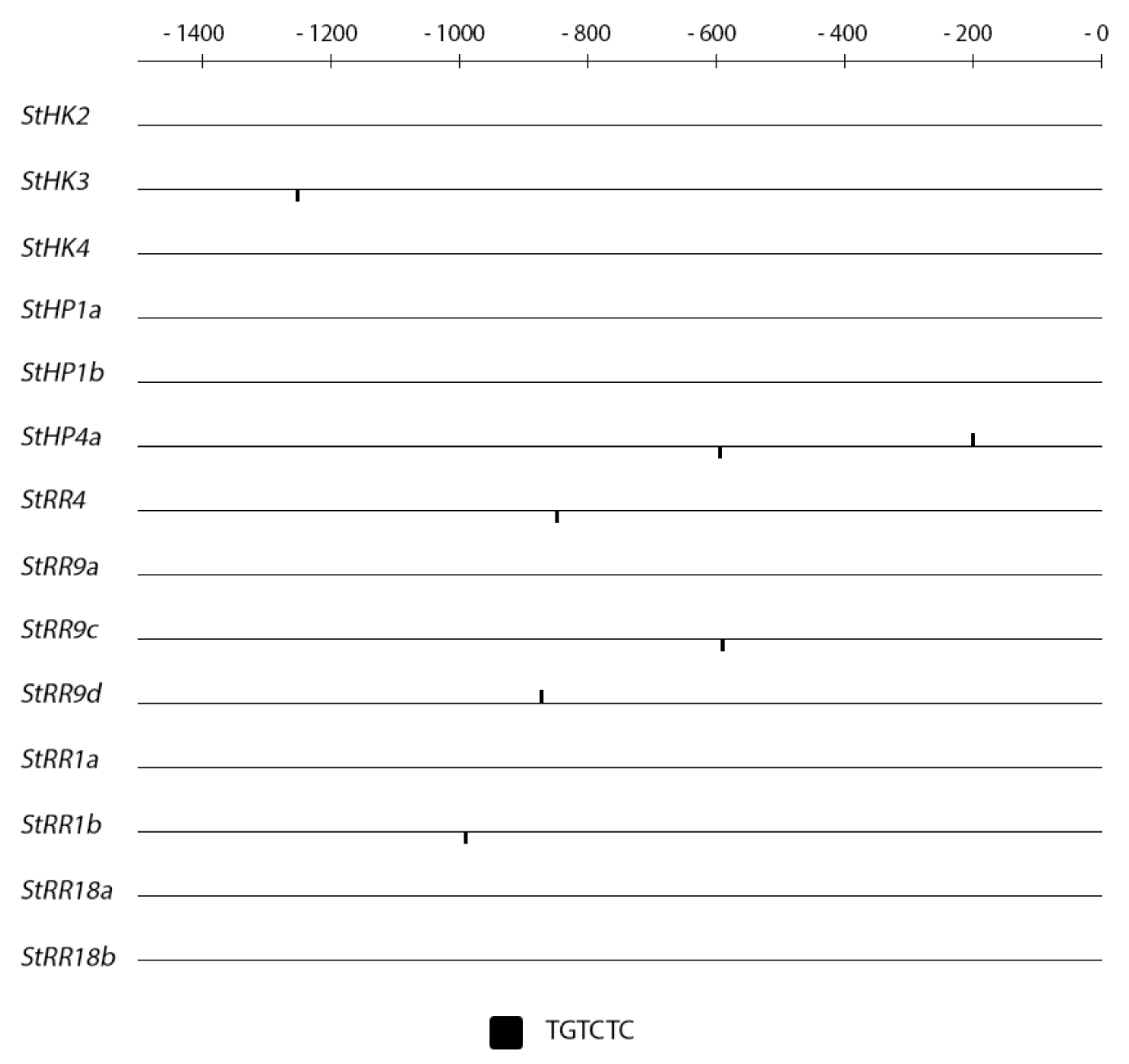
2.4. Promoter Analysis
2.5. Molecular Details of Crosstalk between Auxin- and CK Signaling Modules in Potatoes
3. Materials and Methods
3.1. Plant Growth Conditions
3.2. Quantitative Real-Time PCR
3.3. Functional Clusters Determination
3.4. Promoter Analysis
3.5. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandekar, T.; Naseem, M. Auxins and Cytokinins in Plant Biology, Methods and Protocols. In Part of the Methods in Molecular Biology Book Series; Springer: Cham, Switzerland, 2017; Volume 1569. [Google Scholar]
- Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969, 25, 1–47. [Google Scholar] [CrossRef]
- Barlow, P.W.; Carr, D.J. Positional Control in Plant Development; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Sukhoverov, V.S.; Romanov, G.A. Modeling hormone controlled bipolar growth in cell structures of plant type. Autom. Remote Control 2010, 71, 1184–1195. [Google Scholar] [CrossRef]
- Hartmann, F.P.; Rathgeber, C.B.K.; Badel, É.; Fournier, M.; Moulia, B. Modelling the spatial crosstalk between two biochemical signals explains wood formation dynamics and tree-ring structure. J. Exp. Bot. 2021, 72, 1727–1737. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin-cytokinin interaction regulates meristem development. Mol. Plant. 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Antagonistic activity of auxin and cytokinin in shoot and root organs. Plant Direct 2019, 3, e00121. [Google Scholar] [CrossRef] [Green Version]
- Jing, H.; Strader, L.C. Interplay of auxin and cytokinin in lateral root development. Int. J. Mol. Sci. 2019, 20, 486. [Google Scholar] [CrossRef] [Green Version]
- Kotov, A.A.; Kotova, L.M.; Romanov, G.A. Signaling network regulating plant branching: Recent advances and new challenges. Plant Sci. 2021, 307, 110880. [Google Scholar] [CrossRef]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benkova, E.; Mahonen, A.P.; Helariutta, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Naseem, M.; Dandekar, T. The role of auxin-cytokinin antagonism in plant-pathogen interactions. PLoS Pathol. 2012, 8, e1003026. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [Green Version]
- Chandler, J.W.; Werr, W. Cytokinin-auxin crosstalk in cell type specification. Trends Plant Sci. 2015, 20, 291–300. [Google Scholar] [CrossRef]
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How auxin and cytokinin phytohormones modulate root-microbe interactions. Front. Plant Sci. 2016, 7, 1240. [Google Scholar] [CrossRef] [Green Version]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under stress: Involvement of auxin and cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.J.; Larsson, E.; Spíchal, L.; Sundberg, E. Cytokinin-auxin crosstalk in the gynoecial primordium ensures correct domain patterning. Plant Physiol. 2017, 175, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Hurný, A.; Cuesta, C.; Cavallari, N.; Otvos, K.; Duclercq, J.; Dokladal, L.; Montesinos, J.C.; Gallemi, M.; Semeradova, H.; Rauter, T.; et al. Synergistic on Auxin and Cytokinin 1 positively regulates growth and attenuates soil pathogen resistance. Nat. Commun. 2020, 11, 2170. [Google Scholar] [CrossRef]
- El-Showk, S.; Ruonala, R.; Helariutta, Y. Crossing paths: Cytokinin signalling and crosstalk. Development 2013, 140, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Pernisová, M.; Kuderová, A.; Hejátko, J. Cytokinin and auxin interactions in plant development: Metabolism, signalling, transport and gene expression. Curr. Protein Pept. Sci. 2011, 12, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinins. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2014; Volume 12, p. e0168. [Google Scholar]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanstraelen, M.; Benková, E. Hormonal interactions in the regulation of plant development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Skalický, V.; Kubeš, M.; Napier, R.; Novák, O. Auxins and cytokinins-the role of subcellular organization on homeostasis. Int. J. Mol. Sci. 2018, 19, 3115. [Google Scholar] [CrossRef] [Green Version]
- Waldie, T.; Leyser, O. Cytokinin targets auxin transport to promote shoot branching. Plant Physiol. 2018, 177, 803–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utsumi, Y.; Tanaka, M.; Utsumi, C.; Takahashi, S.; Matsui, A.; Fukushima, A.; Kobayashi, M.; Sasaki, R.; Oikawa, A.; Kusano, M.; et al. Integrative omics approaches revealed a crosstalk among phytohormones during tuberous root development in cassava. Plant Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Saidi, A.; Hajibarat, Z. Phytohormones: Plant switchers in developmental and growth stages in potato. J. Genet. Eng. Biotechnol. 2021, 19, 89. [Google Scholar] [CrossRef]
- Ortiz, O.; Mares, V. The Historical, Social, and Economic Importance of the Potato Crop. In The Potato Genome. Compendium of Plant Genomes; Kumar Chakrabarti, S., Xie, C., Kumar Tiwari, J., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Prat, S. Hormonal and Daylength Control of Potato Tuberization. In Plant Hormones; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Sergeeva, L.I.; Romanov, G.A. Hormonal regulation of tuber formation in potato plants. Russ. J. Plant Physiol. 2012, 59, 451–466. [Google Scholar] [CrossRef]
- Aksenova, N.P.; Sergeeva, L.I.; Kolachevskaya, O.O.; Romanov, G.A. Hormonal Regulation of Tuber Formation in Potato. In Bulbous Plants: Biotechnology; Ramavat, K., Mérillon, J., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 3–36. [Google Scholar]
- The Potato Genome Sequencing Consortium. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Kolachevskaya, O.O.; Sergeeva, L.I.; Floková, K.; Getman, I.A.; Lomin, S.N.; Alekseeva, V.V.; Rukavtsova, E.B.; Buryanov, Y.I.; Romanov, G.A. Auxin synthesis gene tms1 driven by tuber-specific promoter alters hormonal status of transgenic potato plants and their responses to exogenous phytohormones. Plant Cell Rep. 2017, 36, 419–435. [Google Scholar] [CrossRef]
- Kolachevskaya, O.O.; Lomin, S.N.; Arkhipov, D.V.; Romanov, G.A. Auxins in potato: Molecular aspects and emerging roles in tuber formation and stress resistance. Plant Cell Rep. 2019, 38, 681–698. [Google Scholar] [CrossRef]
- Lomin, S.N.; Myakushina, Y.A.; Kolachevskaya, O.O.; Getman, I.A.; Arkhipov, D.V.; Savelieva, E.M.; Osolodkin, D.I.; Romanov, G.A. Cytokinin perception in potato: New features of canonical players. J. Exp. Bot. 2018, 69, 3839–3853. [Google Scholar] [CrossRef]
- Lomin, S.N.; Myakushina, Y.A.; Kolachevskaya, O.O.; Getman, I.A.; Savelieva, E.M.; Arkhipov, D.V.; Deigraf, S.V.; Romanov, G.A. Global view on the cytokinin regulatory system in potato. Front. Plant Sci. 2020, 11, 613624. [Google Scholar] [CrossRef]
- Arkhipov, D.V.; Lomin, S.N.; Myakushina, Y.A.; Savelieva, E.M.; Osolodkin, D.I.; Romanov, G.A. Modeling of protein-protein interactions in cytokinin signal transduction. Int. J. Mol. Sci. 2019, 20, 2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; van Lammeren, A.A.; Vermeer, E.; Vreugdenhil, D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998, 117, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kossmann, J.; Willmitzer, L.; Romanov, G.A. Transformed potato plants as a model for studying the hormonal and carbohydrate regulation of tuberization. Russ. J. Plant Physiol. 2000, 47, 370–379. [Google Scholar]
- Romanov, G.A.; Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kossmann, J.; Willmitzer, L. Effect of indole-3-acetic acid and kinetin on tuberization parameters of different cultivars and transgenic lines of potato in vitro. Plant Growth Regul. 2000, 32, 245–251. [Google Scholar] [CrossRef]
- Kolachevskaya, O.O.; Lomin, S.N.; Kojima, M.; Getman, I.A.; Sergeeva, L.I.; Sakakibara, H.; Romanov, G.A. Tuber-specific expression of two gibberellins oxidase transgenes from Arabidopsis regulates over wide ranges the potato tuber formation. Russ. J. Plant Physiol. 2019, 66, 984–991. [Google Scholar] [CrossRef]
- Boer, D.R.; Freire-Rios, A.; van den Berg, W.A.M.; Saaki, T.; Manfield, I.W.; Kepinski, S.; López-Vidrieo, I.; Franco-Zorrilla, J.M.; de Vries, S.C.; Solano, R.; et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 2014, 156, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Lanctot, A.; Taylor-Teeples, M.; Oki, E.A.; Nemhauser, J.L. Specificity in auxin responses is not explained by the promoter preferences of activator ARFs. Plant Physiol. 2020, 182, 1533–1536. [Google Scholar] [CrossRef] [Green Version]
- Abel, S.; Nguyen, M.D.; Theologis, A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J. Mol. Biol. 1995, 251, 533. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakimoto, T. Perception and signal transduction of cytokinins. Annu. Rev. Plant Biol. 2003, 54, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Heyl, A.; Schmülling, T. Cytokinin signal perception and transduction. Curr. Opin. Plant Biol. 2003, 6, 480–486. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Ueguchi, C.; Sato, S.; Kato, T.; Tabata, S. The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 751–755. [Google Scholar] [CrossRef]
- Higuchi, M.; Pischke, M.S.; Mähönen, A.P.; Miyawaki, K.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Shinozaki, K.; Kato, T.; Tabata, S.; et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 2004, 101, 8821–8826. [Google Scholar] [CrossRef] [Green Version]
- Stolz, A.; Riefler, M.; Lomin, S.N.; Achazi, K.; Romanov, G.A.; Schmülling, T. The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J. 2011, 67, 157–168. [Google Scholar] [CrossRef]
- Romanov, G.A. How do cytokinins affect the cell? Russ. J. Plant Physiol. 2009, 56, 268–290. [Google Scholar] [CrossRef]
- Gordon, S.P.; Chickarmane, V.S.; Ohno, C.; Meyerowitz, E.M. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 2009, 106, 16529–16534. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, R.J.; Michno, J.M.; Myers, C.L. Unraveling gene function in agricultural species using gene co-expression networks. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, C.; Pereira, A. Recent advances in gene function prediction using context-specific coexpression networks in plants. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Kushwah, S.; Laxmi, A. The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana. Plant Cell Environ. 2014, 37, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An update on the signals controlling shoot branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef]
- Brandstatter, I.; Kieber, J.J. Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell 1998, 10, 1009–1019. [Google Scholar] [CrossRef] [Green Version]
- Brenner, W.G.; Romanov, G.A.; Köllmer, I.; Bürkle, L.; Schmülling, T. Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J. 2005, 44, 314–333. [Google Scholar] [CrossRef]
- Muller, B.; Sheen, J. Cytokinin and auxin interaction in root stem cell specification during early embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishopp, A.; Benkova, E.; Helariutta, Y. Sending mixed messages: Auxin-cytokinin crosstalk in roots. Curr. Opin. Plant Biol. 2011, 14, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Andersen, S.U.; Ljung, K.; Dolezal, K.; Miotk, A.; Schultheiss, S.J.; Lohmann, J.U. Hormonal control of the shoot stem-cell niche. Nature 2010, 465, 1089–1092. [Google Scholar] [CrossRef]
- Perilli, S.; Perez-Perez, J.M.; Di Mambro, R.; Peris, C.L.; Díaz-Triviño, S.; Del Bianco, M.; Pierdonati, E.; Moubayidin, L.; Cruz-Ramírez, A.; Costantino, P.; et al. RETINOBLASTOMA-RELATED protein stimulates cell differentiation in the Arabidopsis root meristem by interacting with cytokinin signaling. Plant Cell 2013, 25, 4469–4478. [Google Scholar] [CrossRef] [Green Version]
- Dello Ioio, R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S.A. Genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: From experiments to systems modeling, and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilova, M.N.; Doroshenko, A.S.; Kudryakova, N.V.; Klepikova, A.V.; Shtratnikova, V.Y.; Kusnetsov, V.V. The crosstalk between cytokinin and auxin signaling pathways in the control of natural senescence of Arabidopsis thaliana leaves. Russ. J. Plant Physiol. 2020, 67, 1028–1035. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, R.; Zi, H.; Li, Y.; Cao, X.; Li, D.; Guo, L.; Tong, J.; Pan, Y.; Jiao, Y.; et al. AUXIN RESPONSE FACTOR3 regulates floral meristem determinacy by repressing cytokinin biosynthesis and signaling. Plant Cell 2018, 30, 324–346. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.; Schneider, H.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeldon, C.D.; Bennett, T. There and back again: An evolutionary perspective on long-distance coordination of plant growth and development. Semin. Cell Devel. Biol. 2020, 109, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Honma, T.; Aoyama, T.; Sato, S.; Kato, T.; Tabata, S.; Oka, A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 2001, 294, 1519–1521. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, M.; Sasaki, N.; Tsuge, T.; Aoyama, T.; Oka, A. ARR1 directly activates cytokinin response genes that encode proteins with diverse regulatory functions. Plant Cell Physiol. 2007, 48, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Aoyama, T.; Oka, A. Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. Plant J. 2000, 24, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Imamura, A.; Katoh, E.; Hatta, T.; Tachiki, M.; Yamada, H.; Mizuno, T.; Yamazaki, T. Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 2002, 14, 2015–2029. [Google Scholar] [CrossRef] [Green Version]
- Imamura, A.; Kiba, T.; Tajima, Y.; Yamashino, T.; Mizuno, T. In vivo and in vitro characterization of the ARR11 response regulator implicated in the His-to-Asp phosphorelay signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. ARF1, a transcription factor that binds to auxin response elements. Science 1997, 276, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Aerts, S.; Van Loo, P.; Thijs, G.; Mayer, H.; de Martin, R.; Moreau, Y.; De Moor, B. TOUCAN 2: The all-inclusive open source workbench for regulatory sequence analysis. Nucl. Acids Res. 2005, 33, W393–W396. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolachevskaya, O.O.; Myakushina, Y.A.; Getman, I.A.; Lomin, S.N.; Deyneko, I.V.; Deigraf, S.V.; Romanov, G.A. Hormonal Regulation and Crosstalk of Auxin/Cytokinin Signaling Pathways in Potatoes In Vitro and in Relation to Vegetation or Tuberization Stages. Int. J. Mol. Sci. 2021, 22, 8207. https://doi.org/10.3390/ijms22158207
Kolachevskaya OO, Myakushina YA, Getman IA, Lomin SN, Deyneko IV, Deigraf SV, Romanov GA. Hormonal Regulation and Crosstalk of Auxin/Cytokinin Signaling Pathways in Potatoes In Vitro and in Relation to Vegetation or Tuberization Stages. International Journal of Molecular Sciences. 2021; 22(15):8207. https://doi.org/10.3390/ijms22158207
Chicago/Turabian StyleKolachevskaya, Oksana O., Yulia A. Myakushina, Irina A. Getman, Sergey N. Lomin, Igor V. Deyneko, Svetlana V. Deigraf, and Georgy A. Romanov. 2021. "Hormonal Regulation and Crosstalk of Auxin/Cytokinin Signaling Pathways in Potatoes In Vitro and in Relation to Vegetation or Tuberization Stages" International Journal of Molecular Sciences 22, no. 15: 8207. https://doi.org/10.3390/ijms22158207
APA StyleKolachevskaya, O. O., Myakushina, Y. A., Getman, I. A., Lomin, S. N., Deyneko, I. V., Deigraf, S. V., & Romanov, G. A. (2021). Hormonal Regulation and Crosstalk of Auxin/Cytokinin Signaling Pathways in Potatoes In Vitro and in Relation to Vegetation or Tuberization Stages. International Journal of Molecular Sciences, 22(15), 8207. https://doi.org/10.3390/ijms22158207





