Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy
Abstract
:1. Introduction
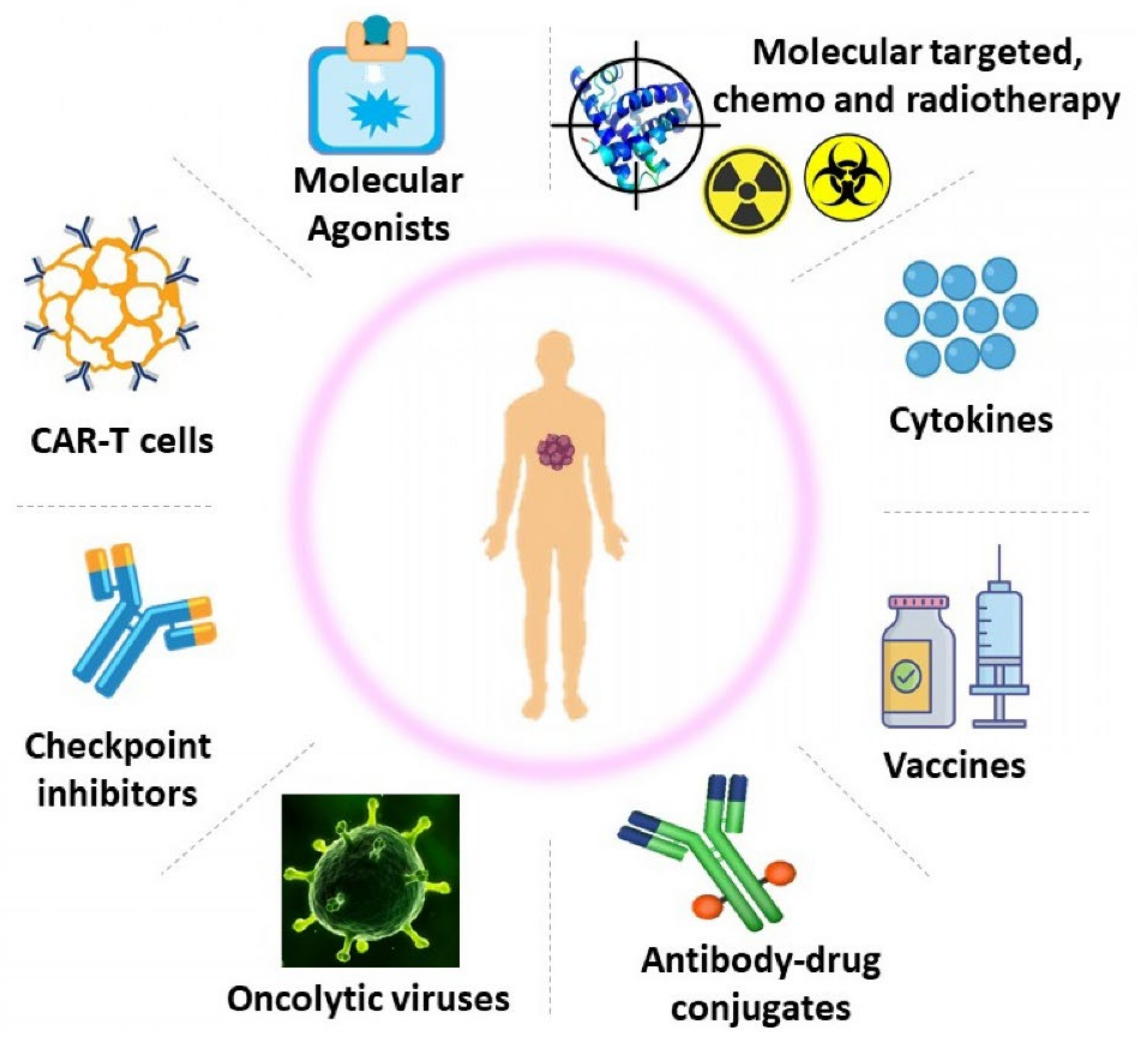
2. Strategies Used in Cancer Immunotherapy
2.1. Immune Checkpoint Inhibitors
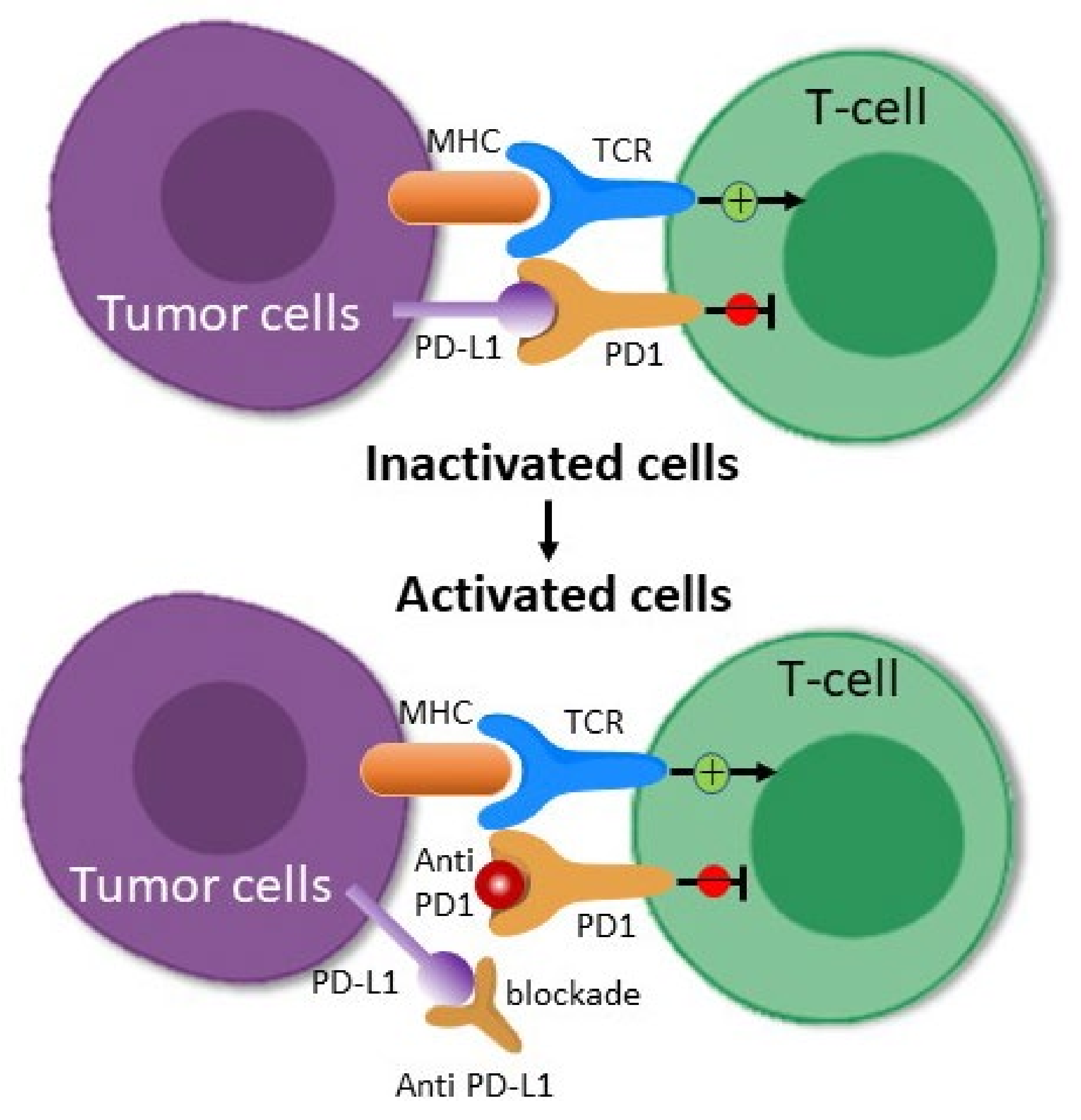
2.1.1. PD-1/PD-L1 Blockade
2.1.2. CTLA-4 Blockade
2.2. Adoptive T Cell Therapy
2.2.1. Cytotoxic T-Lymphocyte Therapy
2.2.2. Tumor Infiltrating Lymphocytes Therapy
2.2.3. Genetically Engineered T Cells
2.3. Immunological Adjuvants
2.4. Cancer Vaccine as Immunotherapy
3. Gold Nanoparticles
4. Biodistribution and Immune Response of GNPs
5. GNPs in Cancer Immunotherapy
6. Role of GNPs in Drug Delivery
6.1. GNPs Based Immune Adjuvants
6.2. GNPs Based Genetic Drugs
6.3. GNPs Based Tumor Vaccines
7. Combinatorial Effects of GNPs in Cancer Immunotherapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.B.; McFadden, G. Anti-Immunology: Evasion of the Host Immune System by Bacterial and Viral Pathogens. Cell 2006, 124, 767–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Pearson, R.; Casey, L.M.; Hughes, K.R.; Miller, S.D.; Shea, L.D. In vivo reprogramming of immune cells: Technologies for induction of antigen-specific tolerance. Adv. Drug Deliv. Rev. 2017, 114, 240–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. In Handbook of Immunological Properties of Engineered Nanomaterials; World Scientific Publishing Co., Inc.: Singapore, 2016; pp. 139–163. [Google Scholar] [CrossRef]
- Feng, X.; Xu, W.; Li, Z.; Song, W.; Ding, J.; Chen, X. Immunomodulatory Nanosystems. Adv. Sci. 2019, 6, 1900101. [Google Scholar] [CrossRef]
- Gordon, J.; Ma, Y.; Churchman, L.; Gordon, S.A.; Dawicki, W. Regulatory Dendritic Cells for Immunotherapy in Immunologic Diseases. Front. Immunol. 2014, 5, 7. [Google Scholar] [CrossRef]
- Shen, Y.; Hao, T.; Ou, S.; Hu, C.; Chen, L. Applications and perspectives of nanomaterials in novel vaccine development. MedChemComm 2018, 9, 226–238. [Google Scholar] [CrossRef]
- Tazaki, T.; Tabata, K.; Ainai, A.; Ohara, Y.; Kobayashi, S.; Ninomiya, T.; Orba, Y.; Mitomo, H.; Nakano, T.; Hasegawa, H.; et al. Shape-dependent adjuvanticity of nanoparticle-conjugated RNA adjuvants for intranasal inactivated influenza vaccines. RSC Adv. 2018, 8, 16527–16536. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Niu, L.; Larson, P.; Kucaba, T.A.; Murphy, K.A.; James, B.R.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials 2018, 164, 38–53. [Google Scholar] [CrossRef]
- Ding, J.; Xiao, C.; Li, Y.; Cheng, Y.; Wang, N.; He, C. Efficacious hepatoma-targeted nanomedicine self-assembled from galactopeptide and doxorubicin driven by two-stage physical interactions. J. Control. Release 2013, 169, 193–203. [Google Scholar] [CrossRef]
- Li, D.; Xu, W.; Li, P.; Ding, J.; Cheng, Z.; Chen, L.; Yan, L.; Chen, X. Self-Targeted Polysaccharide Prodrug Suppresses Orthotopic Hepatoma. Mol. Pharm. 2016, 13, 4231–4235. [Google Scholar] [CrossRef] [Green Version]
- Musetti, S.; Huang, L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano 2018, 12, 11740–11755. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ding, J.; Chen, X. Reduction-Responsive Polypeptide Micelles for Intracellular Delivery of Antineoplastic Agent. Biomacromolecules 2017, 18, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef] [PubMed]
- Mae, C.; Tulinao, S.; Jiang, Y.; Ding, J. Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J. Pharm. Sci. 2020, 15, 397–415. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Ye, L.; Guo, D.X.; Yang, C.; Compans, W.R.; Prausnitz, R.M. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/γ PGA Nanoparticles Administered Using a Microneedle Patch. Adv. Healthc. Mater. 2016, 6, 1600750. [Google Scholar] [CrossRef]
- Steenblock, E.R.; Fahmy, T.M. A Comprehensive Platform for Ex Vivo T-cell Expansion Based on Biodegradable Polymeric Artificial Antigen-presenting Cells. Mol. Ther. 2008, 16, 765–772. [Google Scholar] [CrossRef]
- Stead, S.O.; Kireta, S.; McInnes, S.J.P.; Kette, F.D.; Sivanathan, K.N.; Kim, J.; Cueto-Diaz, E.J.; Cunin, F.; Durand, J.O.; Drogemuller, C.J.; et al. Murine and Non-Human Primate Dendritic Cell Targeting Nanoparticles for in Vivo Generation of Regulatory T-Cells. ACS Nano 2018, 12, 6637–6647. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef]
- Heo, R.; Park, J.-S.; Jang, H.J.; Kim, S.-H.; Shin, J.M.; Suh, Y.D.; Jeong, J.H.; Jo, D.-G.; Park, J.H. Hyaluronan nanoparticles bearing γ-secretase inhibitor: In vivo therapeutic effects on rheumatoid arthritis. J. Control. Release 2014, 192, 295–300. [Google Scholar] [CrossRef]
- Yang, M.; Ding, J.; Zhang, Y.; Chang, F.; Wang, J.; Gao, Z.; Zhuang, X.; Chen, X. Activated macrophage-targeted dextran-methotrexate/folate conjugate prevents deterioration of collagen-induced arthritis in mice. J. Mater. Chem. B 2016, 4, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, J.; Feng, X.; Chang, F.; Wang, Y.; Gao, Z.; Zhuang, X.; Chen, X. Scavenger Receptor-Mediated Targeted Treatment of Collagen-Induced Arthritis by Dextran Sulfate-Methotrexate Prodrug. Theranostics 2017, 7, 97–105. [Google Scholar] [CrossRef]
- Ma, L.; Liu, T.-W.; Wallig, M.A.; Dobrucki, I.T.; Dobrucki, L.W.; Nelson, E.R.; Swanson, K.; Smith, A.M.; Cross, T.-W.; Dobrucki, W. Efficient Targeting of Adipose Tissue Macrophages in Obesity with Polysaccharide Nanocarriers. ACS Nano 2016, 10, 6952–6962. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Müllertz, O.O.; Styles, I.K.; Mörsdorf, A.; Quinn, J.F.; Whittaker, M.R.; Trevaskis, N.L. Lymphatic targeting by albumin-hitchhiking: Applications and optimisation. J. Control. Release 2020, 327, 117–128. [Google Scholar] [CrossRef]
- Klener, P.; Otahal, P.; Lateckova, L. Immunotherapy Approaches in Cancer Treatment. Curr. Pharm. Biotechnol. 2015, 16, 771–781. [Google Scholar] [CrossRef]
- Weiner, L.M. Cancer immunology for the clinician. Clin. Adv. Hematol. Oncol. 2015, 13, 299–306. [Google Scholar]
- Alatrash, G.; Jakher, H.; Stafford, P.D.; Mittendorf, E.A. Cancer immunotherapies, their safety and toxicity. Expert Opin. Drug Saf. 2013, 12, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D. Cancer and Immune System: Basic Concepts and Targets for Intervention. Semin. Oncol. 2015, 42, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Ye, Y.; Gu, Z. Local delivery of checkpoints antibodies. Hum. Vaccines Immunother. 2017, 13, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core–Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-R.; Bechstein, D.J.B.; Ooi, C.C.; Patel, A.; Gaster, R.S.; Ng, E.; Gonzalez, L.C.; Wang, S.X. Magneto-nanosensor platform for probing low-affinity protein–protein interactions and identification of a low-affinity PD-L1/PD-L2 interaction. Nat. Commun. 2016, 7, 12220. [Google Scholar] [CrossRef]
- Teo, P.Y.; Yang, C.; Whilding, L.M.; Parente-Pereira, A.C.; Maher, J.; George, A.; Hedrick, J.L.; Yang, Y.Y.; Ghaem-Maghami, S. Ovarian Cancer Immunotherapy Using PD-L1 siRNA Targeted Delivery from Folic Acid-Functionalized Polyethylenimine: Strategies to Enhance T Cell Killing. Adv. Healthc. Mater. 2015, 4, 1180–1189. [Google Scholar] [CrossRef]
- Zhao, P.; Atanackovic, D.; Dong, S.; Yagita, H.; He, X.; Chen, M. An Anti-Programmed Death-1 Antibody (αPD-1) Fusion Protein That Self-Assembles into a Multivalent and Functional αPD-1 Nanoparticle. Mol. Pharm. 2017, 14, 1494–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Lipson, E.J.; Lilo, M.T.; Ogurtsova, A.; Esandrio, J.; Xu, H.; Brothers, P.; Schollenberger, M.; Sharfman, W.H.; Taube, J.M. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J. Immunother. Cancer 2017, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Graff, J.N.; Alumkal, J.J.; Drake, C.G.; Thomas, G.V.; Redmond, W.L.; Farhad, M.; Cetnar, J.P.; Ey, F.S.; Bergan, R.C.; Slottke, R.; et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016, 7, 52810–52817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.M.; Gulley, J.L. Product review: Avelumab, an anti-PD-L1 antibody. Hum. Vaccines Immunother. 2019, 15, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Liu, Y.; Xu, C.F.; Shen, S.; Sun, R.; Du, X.J.; Xia, J.X.; Zhu, Y.H.; Wang, J. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Release 2016, 231, 17–28. [Google Scholar] [CrossRef]
- Lei, C.; Liu, P.; Chen, B.; Mao, Y.; Engelmann, H.; Shin, Y.; Jaffar, J.; Hellstrom, I.; Liu, J.; Hellstrom, K.E. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J. Am. Chem. Soc. 2011, 132, 6906–6907. [Google Scholar] [CrossRef] [PubMed]
- Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; Akerley, W.; van den Eertwegh, A.J.M.; Lutzky, D.; Lorigan, P. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar]
- Walker, L.S.; Sansom, D.M. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol. 2015, 36, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Staley, C.; Kooby, D.; El-Rays, B.; Mao, H.; Yang, L. Current status of biomarker and targeted nanoparticle development: The precision oncology approach for pancreatic cancer therapy. Cancer Lett. 2017, 388, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Chae, Y.K.; Arya, A.; Iams, W.; Cruz, M.R.; Chandra, S.; Choi, J.; Giles, F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2018, 6, 39. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Cebon, J.S.; Jameson, M.B.; Fitzharris, B.M.; McNeil, C.M.; Hill, A.G.; Ribas, A.; Atkins, M.B.; Thompson, J.A.; et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): An open-label, phase 1b trial. Lancet Oncol. 2017, 18, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Youn, W.; Ko, E.H.; Kim, M.-H.; Park, M.; Hong, D.; Seisenbaeva, G.A.; Kessler, V.G.; Choi, I.S. Cytoprotective Encapsulation of Individual Jurkat T Cells within Durable TiO2 Shells for T-Cell Therapy. Angew. Chem. Int. Ed. 2017, 56, 10702–10706. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Powell, D.J.; Rosenberg, S.A.; Restifo, N.P. Adoptive immunotherapy for cancer: Building on success. Nat. Rev. Immunol. 2006, 6, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Spiess, P.; LaFreniere, R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986, 233, 1318–1321. [Google Scholar] [CrossRef]
- Jackson, H.J.; Rafiq, S.; Brentjens, R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016, 13, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Hombach, A.A.; Abken, H. Of CARs and TRUCKs: Chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 2014, 257, 83–90. [Google Scholar] [CrossRef]
- Kalaitsidou, M.; Kueberuwa, G.; Schütt, A.; Gilham, D.E. CAR T-cell therapy: Toxicity and the relevance of preclinical models. Immunotherapy 2015, 7, 487–497. [Google Scholar] [CrossRef]
- Crowe, J.E. Prevention of Fetal and Early Life Infections Through Maternal–Neonatal Immunization. Infect. Dis. Fetus Newborn 2011, 1212–1230. [Google Scholar] [CrossRef]
- Copper, C.H.; Yan, B.; Lu, W. Combinatorial Photothermal and Immuno Cancer Therapy Using Sulfide Nanoparticles. ACS Nano 2015, 8, 5670–5681. [Google Scholar]
- Zhang, Y.; Wang, F.; Ju, E.; Liu, Z.; Chen, Z.; Ren, J.; Qu, X. Metal-Organic-Framework-Based Vaccine Platforms for Enhanced Systemic Immune and Memory Response. Adv. Funct. Mater. 2016, 26, 6454–6461. [Google Scholar] [CrossRef]
- Kruit, W.H.J.; Suciu, S.; Dreno, B.; Mortier, L.; Robert, C.; Chiarion-Sileni, V.; Maio, M.; Testori, A.; Dorval, T.; Grob, J.J.; et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: Results of a randomized phase II study of the European organisation for research and treatment of cancer melanoma group in metastatic melanoma. J. Clin. Oncol. 2013, 31, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, P.J.; Bilusic, M. Cancer Vaccines. Hematol. Oncol. Clin. N. Am. 2019, 33, 199–214. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.; Ni, Q.; Niu, G.; Chen, X. Efficient Nanovaccine Delivery in Cancer Immunotherapy. ACS Nano 2017, 11, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Paulis, L.; Mandal, S.; Kreutz, M.; Figdor, C. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr. Opin. Immunol. 2013, 25, 389–395. [Google Scholar] [CrossRef]
- Li, W.A.; Mooney, D.J. Materials based tumor immunotherapy vaccines. Curr. Opin. Immunol. 2013, 25, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi-Nik, H.; Corwin, W.L.; Shcheglova, T.; Das Mohapatra, A.; Mandoiu, I.; Srivastava, P.K. CD11c+ MHCIIlo GM-CSF-bone marrow-derived dendritic cells act as antigen donor cells and as antigen presenting cells in neoepitope-elicited tumor immunity against a mouse fibrosarcoma. Cancer Immunol. Immunother. 2018, 67, 1449–1459. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, P.; Wang, X.; Shi, L.; Fan, Z.; Zhang, G.; Yang, D.; Bahavar, C.F.; Zhou, F.; Chen, W.R.; et al. Antitumor effects of DC vaccine with ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma in mice. Technol. Cancer Res. Treat. 2018, 17, 1533033818785275. [Google Scholar] [CrossRef] [Green Version]
- Chondronasiou, D.; Eisden, T.-J.T.H.D.; Stam, A.G.M.; Matthews, Q.L.; Icyuz, M.; Hooijberg, E.; Dmitriev, I.; Curiel, D.T.; de Gruijl, T.D.; van de Ven, R. Improved induction of anti-melanoma T cells by adenovirus-5/3 fiber modification to target human DCs. Vaccines 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, O.J. Cancer vaccines: Between the idea and the reality. Nat. Rev. Immunol. 2003, 3, 630–641. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759. [Google Scholar] [CrossRef]
- Personick, M.; Langille, M.R.; Zhang, J.; Mirkin, C.A. Shape Control of Gold Nanoparticles by Silver Underpotential Deposition. Nano Lett. 2011, 11, 3394–3398. [Google Scholar] [CrossRef] [PubMed]
- Rescignano, N.; Kenny, J.M. Stimuli-Responsive Core-Shell Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Jingyue, Z. Synthesis of Gold Nanoparticles via Chemical Reduction Method. 2015. Available online: https://www.researchgate.net/publication/306360987_Synthesis_of_Gold_Nanoparticles_via_Chemical_Reduction_Method (accessed on 23 May 2021).
- Chithrani, D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Mason, M.M.; Wise, J.P. Genotoxicity of metal nanoparticles. Rev. Environ. Healthc. 2011, 26, 251–268. [Google Scholar] [CrossRef]
- De Moura, M.; van Houten, B. Effects of silver nanoparticles on oxidative DNA damage–repair as a function of p38 MAPK status: A comparative approach using human Jurkat T cells and the nematode Caenorhabditis elegans. Environ. Mol. Mutagen. 2010, 405, 391–405. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, M.; Barabadi, H.; Ramachandran, B.; Venkatraman, G.; Ponmurugan, K. Emerging Plant-Based Anti-Cancer Green Nanomaterials in Present Scenario, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Havaki, S.; Kotsinas, A.; Chronopoulos, E.; Kletsas, D.; Georgakilas, A.; Gorgoulis, V.G. The role of oxidative DNA damage in radiation induced bystander effect. Cancer Lett. 2015, 356, 43–51. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2010, 28, 237–259. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Anshup, J.; Venkataraman, S.; Subramaniam, C.; Kumar, R.R.; Priya, S.; Kumar, T.R.S.; Omkumar, R.V.; John, A.; Pradeep, T. Growth of gold nanoparticles in human cells. Langmuir 2005, 21, 11562–11567. [Google Scholar] [CrossRef] [PubMed]
- Focsan, M.; Ardelean, I.I.; Craciun, C.; Astilean, S. Interplay between gold nanoparticle biosynthesis and metabolic activity of cyanobacterium Synechocystis sp. PCC 6803. Nanotechnology 2011, 22, 485101. [Google Scholar] [CrossRef]
- Li, J.J.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Li, W.; Jiang, X.; Ji, Y.; Wu, X.; Xu, L.; Qiu, Y.; Zhao, K.; Wei, T.; et al. Selective Targeting of Gold Nanorods at the Mitochondria of Cancer Cells: Implications for Cancer Therapy. Nano Lett. 2011, 11, 772–780. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Shiau, A.-L.; Chen, Y.-H.; Chang, C.-J.; Chen, H.H.-W.; Wu, C.-L. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008, 99, 1479–1484. [Google Scholar] [CrossRef]
- Wang, B.; Chen, N.; Wei, Y.; Li, J.; Sun, L.; Wu, J.; Huang, Q.; Liu, C.; Fan, C.; Song, H. Akt signaling-associated metabolic effects of dietary gold nanoparticles in Drosophila. Sci. Rep. 2012, 2, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; . Figueroa, E.R.; Drezek, R.A. Gold Nanoparticle Mediated Cancer Immunotherapy. Nanomedicine 2014, 10, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.; Hsu, S.; Tsai, C. Cytotoxicity and Immunological Response of Gold and Silver Nanoparticles of Different Sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Sumbayev, V.V.; Yasinska, I.; Garcia, C.P.; Gilliland, D.; Lall, G.; Gibbs, B.F.; Bonsall, D.; Varani, L.; Rossi, F.; Calzolai, L. Gold Nanoparticles Downregulate Interleukin-1β-Induced Pro-Inflammatory Responses. Small 2012, 9, 472–477. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Lu, S.-L.; Hu, C.-W.; Yeh, C.-S.; Lee, G.-B.; Lei, H.-Y. Size-Dependent Attenuation of TLR9 Signaling by Gold Nanoparticles in Macrophages. J. Immunol. 2012, 188, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-S.; Chen, Y.-H.; Cheng, P.-C.; Tsai, H.-T.; Shiau, A.-L.; Tzai, T.-S.; Wu, C.-L. TGF-β1 Conjugated to Gold Nanoparticles Results in Protein Conformational Changes and Attenuates the Biological Function. Small 2013, 9, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Qiu, H.; Min, Y.; Rodgers, Z.; Zhang, L.; Wang, A. Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1456. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Incoronato, M.; Salvatore, M.; Soricelli, A. Nanoparticle-based strategies for cancer immunotherapy and immunodiagnostics. Nanomedicine 2017, 12, 2349–2365. [Google Scholar] [CrossRef]
- Hu, X.; Wu, T.; Bao, Y.; Zhang, Z. Nanotechnology based therapeutic modality to boost anti-tumor immunity and collapse tumor defense. J. Control. Release 2017, 256, 26–45. [Google Scholar] [CrossRef]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, E.R.; Bugga, P.; Asthana, V.; Drezek, R. Metallic nanoparticles for cancer immunotherapy. Mater. Today 2018, 21, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Selvan, S.T.; Yang, Y.; Kim, M.J.; Yi, D.K.; Kwon, I.C.; Kim, K. Engineering nanoparticle strategies for effective cancer immunotherapy. Biomaterials 2018, 178, 597–607. [Google Scholar] [CrossRef]
- Mahjub, R.; Jatana, S.; Lee, S.E.; Qin, Z.; Pauli, G.; Soleimani, M.; Madadi, S.; Li, S.D. Recent advances in applying nanotechnologies for cancer immunotherapy. J. Control. Release 2018, 288, 239–263. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Xin, Y.; Jiang, C.; Yan, B.; Zhai, S. Interactions Between Nanoparticles and Dendritic Cells: From the Perspective of Cancer Immunotherapy. Front. Oncol. 2018, 8, 404. [Google Scholar] [CrossRef]
- Englinger, B.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B.K.; Berger, W. Metal Drugs and the Anticancer Immune Response. Chem. Rev. 2019, 119, 1519–1624. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, F.; Candini, D.; Carrasco, E.; Francés, M.A.B.; Candini, D. Nanoparticles applied to cancer immunoregulation. Rep. Pract. Oncol. Radiother. 2019, 24, 47–55. [Google Scholar] [CrossRef]
- Le, Q.-V.; Yang, G.; Wu, Y.; Jang, H.W.; Shokouhimehr, M.; Oh, Y.-K. Nanomaterials for modulating innate immune cells in cancer immunotherapy. Asian J. Pharm. Sci. 2019, 14, 16–29. [Google Scholar] [CrossRef]
- Ykman, L.A.D.; Hlebtsov, N.G.K. Gold nanoparticles in chemo-, immuno- and combined therapy. Biomed. Opt. Express 2019, 10, 3152–3182. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, S.; Zhang, Y.; Chu, X.; Lin, Z.; Zhao, Z.; Qiu, S.; Guo, Y.; Ding, H.; Pan, Y.; et al. The Application of and Strategy for Gold Nanoparticles in Cancer Immunotherapy. Front. Pharmacol. 2021, 12, 1430. [Google Scholar] [CrossRef]
- Cheng, Y.; Samia, A.C.; Li, J.; Kenney, M.E.; Resnick, A.; Burda, C. Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir 2010, 26, 2248–2255. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, J.-W. Application of Plasmonic Gold Nanoparticle for Drug Delivery System. Curr. Drug Targets 2017, 19, 271–278. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, L.; Chen, W.; Weng, W.; Song, J.; Ji, J. Delivery strategies of cancer immunotherapy: Recent advances and future perspectives. J. Hematol. Oncol. 2019, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Emami, F.; Banstola, A.; Vatanara, A.; Lee, S.; Kim, J.O.; Jeong, J.-H.; Yook, S. Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy. Mol. Pharm. 2019, 16, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Emami, F.; Jeong, J.-H.; Yook, S. Current Applications of Gold Nanoparticles for Medical Imaging and as Treatment Agents for Managing Pancreatic Cancer. Macromol. Res. 2018, 26, 955–964. [Google Scholar] [CrossRef]
- Meir, R.; Shamalov, K.; Sadan, T.; Motiei, M.; Yaari, G.; Cohen, C.J.; Popovtzer, R. Fast Image-Guided Stratification Using Anti-Programmed Death Ligand 1 Gold Nanoparticles for Cancer Immunotherapy. ACS Nano 2017, 11, 11127–11134. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cao, W.; Qiao, G.; Yao, S.; Pan, S.; Wang, L.; Yue, C.; Ma, L.; Liu, Y.; Cui, D. Effects of gold nanoprism-assisted human PD-L1 siRNA on both gene down-regulation and photothermal therapy on lung cancer. Acta Biomater. 2019, 99, 307–319. [Google Scholar] [CrossRef]
- Merino, M.; Lozano, T.; Casares, N.; Lana, H.; Troconiz, I.F.; Hagen, T.L.M.t.; Kochan, G.; Berraondo, P.; Zalba, S.; Garrido, M.J. Dual activity of PD-L1 targeted Doxorubicin immunoliposomes promoted an enhanced efficacy of the antitumor immune response in melanoma murine model. J. Nanobiotechnol. 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chalbatani, G.M.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Partially PEGylated Dendrimer-Entrapped Gold Nanoparticles: A Promising Nanoplatform for Highly Efficient DNA and siRNA Delivery. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Labala, S.; Jose, A.; Chawla, R.S.; Khan, S.M.; Bhatnagar, S.; Kulkarni, P.O.; Venuganti, K.V.V. Effective Melanoma Cancer Suppression by Iontophoretic Co-Delivery of STAT3 siRNA and Imatinib Using Gold Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Xue, X.; Li, J.; Fan, Y.; Shen, M.; Shi, X. Gene silencing-mediated immune checkpoint blockade for tumor therapy boosted by dendrimer-entrapped gold nanoparticles. Sci. China Mater. 2021, 64, 2045–2055. [Google Scholar] [CrossRef]
- Dykman, L.A.; Staroverov, S.; Fomin, A.S.; Khanadeev, V.A.; Khlebtsov, B.; Bogatyrev, V.A. Gold nanoparticles as an adjuvant: Influence of size, shape, and technique of combination with CpG on antibody production. Int. Immunopharmacol. 2018, 54, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Yan, M.; Liu, Y.; Liu, L.; Ma, G. Photothermally Controlled MHC Class I Restricted CD8+T-Cell Responses Elicited by Hyaluronic Acid Decorated Gold Nanoparticles as a Vaccine for Cancer Immunotherapy. Adv. Healthc. Mater. 2018, 7, e1701439. [Google Scholar] [CrossRef]
- Shinchi, H.; Yamaguchi, T.; Moroishi, T.; Yuki, M.; Wakao, M.; Cottam, H.B.; Hayashi, T.; Carson, D.A.; Suda, Y. Gold Nanoparticles Coimmobilized with Small Molecule Toll-Like Receptor 7 Ligand and α-Mannose as Adjuvants. Bioconjug. Chem. 2019, 30, 2811–2821. [Google Scholar] [CrossRef]
- Wei, M.; Chen, N.; Li, J.; Yin, M.; Liang, L.; He, Y.; Song, H.; Fan, C.; Huang, Q. Polyvalent Immunostimulatory Nanoagents with Self-Assembled CpG Oligonucleotide-Conjugated Gold Nanoparticles. Angew. Chem. 2012, 124, 1228–1232. [Google Scholar] [CrossRef]
- Lee, I.-H.; Kwon, H.; An, S.; Kim, D.; Kim, S.; Yu, M.K.; Lee, J.-H.; Lee, T.S.; Im, S.-H.; Jon, S. Imageable Antigen-Presenting Gold Nanoparticle Vaccines for Effective Cancer Immunotherapy In Vivo. Angew. Chem. Int. Ed. 2012, 51, 8800–8805. [Google Scholar] [CrossRef]
- Lin, A.Y.; Almeida, J.P.M.; Bear, A.; Liu, N.; Luo, L.; Foster, A.E.; Drezek, R.A. Gold Nanoparticle Delivery of Modified CpG Stimulates Macrophages and Inhibits Tumor Growth for Enhanced Immunotherapy. PLoS ONE 2013, 8, e63550. [Google Scholar] [CrossRef] [Green Version]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal Gold: A Novel Nanoparticle Vector for Tumor Directed Drug Delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Visaria, R.K.; Griffin, R.; Williams, B.W.; Ebbini, E.; Paciotti, G.F.; Song, C.W.; Bischof, J.C. Enhancement of tumor thermal therapy using gold nanoparticle–assisted tumor necrosis factor-α delivery. Mol. Cancer Ther. 2006, 5, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Sousa, F.; Castro, P.; Fonte, P.; Kennedy, P.J.; Neves-Petersen, M.T.; Sarmento, B. Nanoparticles for the delivery of therapeutic antibodies: Dogma or promising strategy? Expert Opin. Drug Deliv. 2016, 14, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.S.; Moynihan, K.D.; Bekdemir, A.; Dichwalkar, T.M.; Noh, M.M.; Watson, N.; Melo, M.; Ingram, J.; Suh, H.; Ploegh, H.; et al. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles. Biomater. Sci. 2019, 7, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottas, I.; Bekdemir, A.; Cereghetti, A.; Spagnuolo, L.; Yang, Y.-S.S.; Müller, M.; Irvine, D.J.; Stellacci, F.; Bourquin, C. Amphiphilic nanoparticle delivery enhances the anticancer efficacy of a TLR7 ligand via local immune activation. Biomaterials 2019, 190–191, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.P.M.; Lin, A.Y.; Langsner, R.J.; Eckels, P.; Foster, A.E.; Drezek, R.A. In vivo immune cell distribution of gold nanoparticles in Naïve and tumor bearing mice. Small 2014, 10, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.A.; Khlebtsov, N.G. Immunological properties of gold nanoparticles. Chem. Sci. 2017, 8, 1719–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Xiong, X.; Chakraborty, P.K.; Shameer, K.; Arvizo, R.R.; Kudgus, R.A.; Dwivedi, S.K.D.; Hossen, M.N.; Gillies, E.M.; Robertson, J.D.; et al. Gold Nanoparticle Reprograms Pancreatic Tumor Microenvironment and Inhibits Tumor Growth. ACS Nano 2016, 10, 10636–10651. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Lin, A.Y.; Figueroa, E.R.; Foster, A.E.; Drezek, R.A. In vivo Gold Nanoparticle Delivery of Peptide Vaccine Induces Anti-Tumor Immune Response in Prophylactic and Therapeutic Tumor Models. Small 2015, 11, 1453–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.Y.; Lunsford, J.; Bear, A.S.; Young, J.K.; Eckels, P.; Luo, L.; Foster, A.E.; Drezek, R.A. High-density sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Res. Lett. 2013, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Zhang, Y.; Du, J.; Li, Y.; Zhou, Y.; Fu, Q.; Zhang, J.; Wang, X.; Zhan, L. Different-Sized Gold Nanoparticle Activator/Antigen Increases Dendritic Cells Accumulation in Liver-Draining Lymph Nodes and CD8+ T Cell Responses. ACS Nano 2016, 10, 2678–2692. [Google Scholar] [CrossRef]
- Ma, X.; Hui, H.; Jin, Y.; Dong, D.; Liang, X.; Yang, X.; Tan, K.; Dai, Z.; Cheng, Z.; Tian, J. Enhanced immunotherapy of SM5-1 in hepatocellular carcinoma by conjugating with gold nanoparticles and its in vivo bioluminescence tomographic evaluation. Biomaterials 2016, 87, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.; Lee, I.-H.; Kang, S.; Kim, D.; Choi, M.; Saw, P.E.; Shin, E.-C.; Jon, S. Gold Nanoparticles Displaying Tumor-Associated Self-Antigens as a Potential Vaccine for Cancer Immunotherapy. Adv. Healthc. Mater. 2014, 3, 1194–1199. [Google Scholar] [CrossRef]
- Tomić, S.; Dokić, J.; Vasilijić, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vučević, D.; Milosavljević, P.; Janković, S.; Anžel, I.; et al. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS ONE 2014, 9, e96584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.-C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 2017, 256, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, H.; Wu, X.; Cong, X.; Wang, L.; Wang, Y.; Yang, Y.; Li, W.; Sun, T. The influence of tumor-induced immune dysfunction on the immune cell distribution of gold nanoparticles: In vivo. Biomater. Sci. 2017, 5, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.A.; Kim, Y.S.; O’Neill, B.E.; Shi, Z.Z.; Serda, R.E. HSP70 promoter-driven activation of gene expression for immunotherapy using gold nanorods and near infrared light. Vaccines 2014, 2, 216–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, R.; Xie, J.; Li, J.; Wang, K.; Liu, L.; Gao, Y.; Hussain, M.; Shen, G.; Zhu, J.; Tao, J. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Ahmad, S.; Zamry, A.A.; Tan, H.-T.T.; Wong, K.K.; Lim, J.; Mohamud, R. Targeting dendritic cells through gold nanoparticles: A review on the cellular uptake and subsequent immunological properties. Mol. Immunol. 2017, 91, 123–133. [Google Scholar] [CrossRef]
- Fogli, S.; Montis, C.; Paccosi, S.; Silvano, A.; Michelucci, E.; Berti, D.; Bosi, A.; Parenti, A.; Romagnoli, P. Inorganic nanoparticles as potential regulators of immune response in dendritic cells. Nanomedicine 2017, 12, 1647–1660. [Google Scholar] [CrossRef]
- Dings, R.; Cannon, M.; Vang, K.B. Design of Gold Nanoparticles in Dendritic Cell-Based Vaccines. Part. Part. Syst. Charact. 2018, 35, 1800109. [Google Scholar] [CrossRef]
- Cruz, L.J.; Rueda, F.; Cordobilla, B.; Simoón, L.; Hosta, L.; Albericio, F.; Domingo, J.C.; Rigau, L.H. Targeting Nanosystems to Human DCs via Fc Receptor as an Effective Strategy to Deliver Antigen for Immunotherapy. Mol. Pharm. 2010, 8, 104–116. [Google Scholar] [CrossRef]
- Dreaden, E.; Mwakwari, S.C.; Austin, L.; Kieffer, M.J.; Oyelere, A.K.; El-Sayed, M.A. Small Molecule-Gold Nanorod Conjugates Selectively Target and Induce Macrophage Cytotoxicity towards Breast Cancer Cells. Small 2012, 8, 2819–2822. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, J.; Kawazoe, N.; Chen, G. Photothermal ablation of cancer cells by albumin-modified gold nanorods and activation of dendritic cells. Materials 2018, 12, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.R.; Stanton-Maxey, K.J.; Stanley, J.K.; Levin, C.S.; Bardhan, R.; Akin, D.; Badve, S.; Sturgis, J.; Robinson, J.P.; Bashir, R.; et al. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007, 7, 3759–3765. [Google Scholar] [CrossRef] [PubMed]

| FDA-Approved Drugs | Diseases |
|---|---|
| Ipilimumab (anti-CTLA-4) | Melanoma |
| Nivolumab (anti-PD1) | Multiple cancers |
| Pembolizumab (anti-PD1) | Multiple cancers |
| Atezolizumab (PD-L1 inhibitor) | Non-small cell lung cancer |
| Avelumab (anti-PDL1) | Merkel cell carcinoma |
| Durvalumab (anti-PDL1) | Bladder cancer |
| Encapsulated Compounds | Non-Encapsulated Compounds |
|---|---|
|
|
| Advantages | Limitations |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, A.; Khan, T.; Omri, A. Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 8037. https://doi.org/10.3390/ijms22158037
Chauhan A, Khan T, Omri A. Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy. International Journal of Molecular Sciences. 2021; 22(15):8037. https://doi.org/10.3390/ijms22158037
Chicago/Turabian StyleChauhan, Akshita, Tabassum Khan, and Abdelwahab Omri. 2021. "Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy" International Journal of Molecular Sciences 22, no. 15: 8037. https://doi.org/10.3390/ijms22158037
APA StyleChauhan, A., Khan, T., & Omri, A. (2021). Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy. International Journal of Molecular Sciences, 22(15), 8037. https://doi.org/10.3390/ijms22158037








