Structure-Activity Relationships of the Imidazolium Compounds as Antibacterials of Staphylococcus aureus and Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results and Discussion
Comparison of DRSA with Alternative Methods
3. Materials and Methods
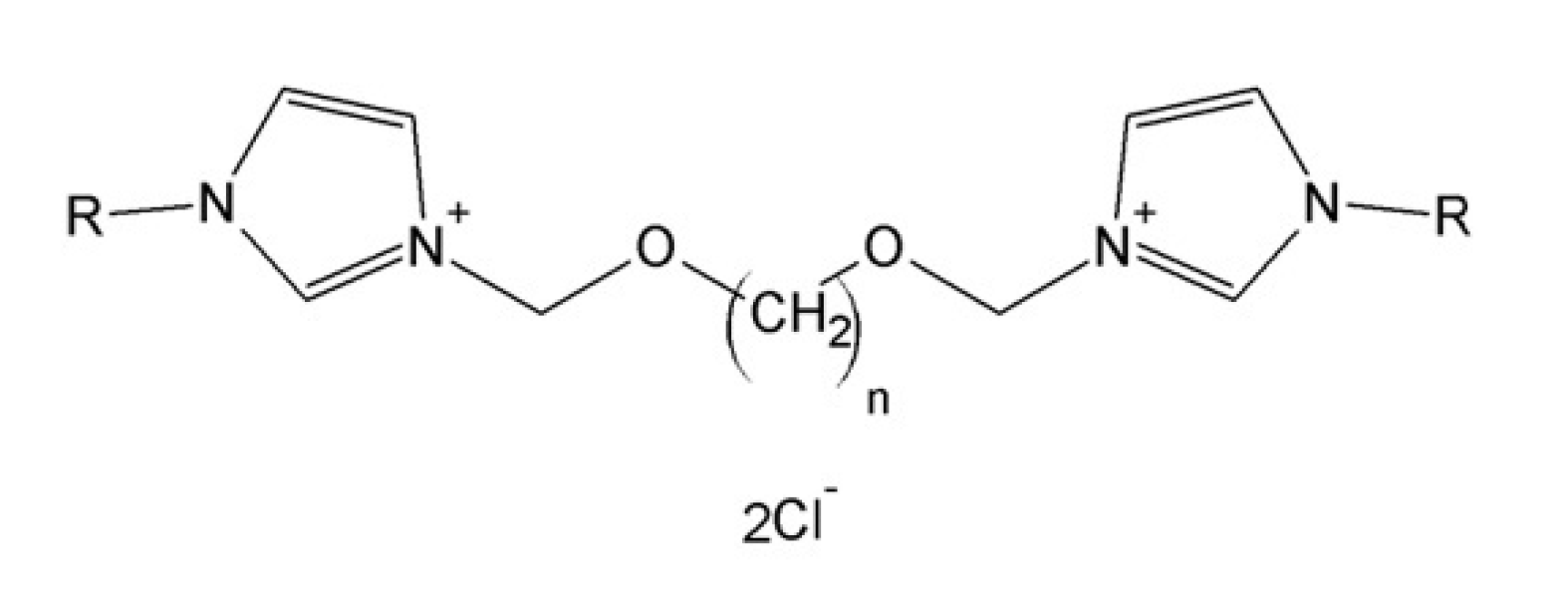
3.1. Chemical Compounds
3.2. Structural Parameters
3.3. Surface-Active Parameters
3.4. Molecular Descriptors
3.5. Antimicrobial Activity
3.6. Dominance-Based Rough Set Approach to SAR Study
3.6.1. Information System
3.6.2. Validation
3.6.3. Decision Rules
3.6.4. Attribute Relevance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, S.E.; Maillard, J.-Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef]
- Borrego, P.; Calado, R.; Marcelino, J.M.; Bartolo, I.; Rocha, C.; Cavaco-Silva, P.; Doroana, M.; Antunes, F.; Maltez, F.; Caixas, U.; et al. Baseline susceptibility of primary HIV-2 to entry inhibitors. Antivir. Ther. 2012, 17, 565–570. [Google Scholar] [CrossRef]
- Bharate, S.B.; Thompson, C.M. Antimicrobial, Antimalarial, and Antileishmanial Activities of Mono- and Bis-quaternary Pyridinium Compounds. Chem. Biol. Drug Des. 2010, 76, 546–551. [Google Scholar] [CrossRef]
- Lowy, F.D. Medical progress: Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbes Infect. 2000, 2, 1051–1060. [Google Scholar] [CrossRef]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Matarazzo, B.; Słowiński, R. The use of rough sets and fuzzy sets . In Advances in Multiple Criteria Decision Making; Gal, T., Stewart, T., Hanne, T., Eds.; Kluwer: Boston, MA, USA, 1999; Chapter 14; pp. 14.1–14.59. [Google Scholar]
- Greco, S.; Matarazzo, B.; Słowiński, R. Rough approximation of a preference relation by dominance relations. Eur. J. Oper. Res. 1999, 117, 63–83. [Google Scholar] [CrossRef]
- Pawlak, Z. Rough Sets: Theoretical Aspects of Reasoning about Data. In System Theory, Knowledge Engineering and Problem Solving; Kluwer Academic Publishers: Dordrecht, Germany, 1991; Volume 9. [Google Scholar]
- Słowiński, R. Rough Set Learning of Preferential Attitude in Multi-criteria Decision Making. In ISMIS 1993; LNCS (LNAI); Komorowski, J., Ras, Z.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 689, pp. 642–651. [Google Scholar]
- Figueira, J.; Greco, S.; Ehrgott, M. (Eds.) Multiple Criteria Decision Analysis: State of the Art Surveys; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Błaszczyński, J.; Greco, S.; Słowiński, R. Inductive discovery of laws using monotonic rules. Eng. Appl. Artif. Intell. 2012, 25, 284–294. [Google Scholar] [CrossRef]
- Pałkowski, Ł.; Błaszczyński, J.; Skrzypczak, A.; Błaszczak, J.; Kozakowska, K.; Wróblewska, J.; Kożuszko, S.; Gospodarek, E.; Krysiński, J.; Słowiński, R. Antimicrobial activity and SAR study of new gemini imidazolium-based chlorides. Chem. Biol. Drug Des. 2014, 83, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Matarazzo, B.; Słowiński, R. Rough Sets Theory for Multicriteria Decision Analysis. Eur. J. Oper. Res. 2001, 129, 1–47. [Google Scholar] [CrossRef]
- Pałkowski, Ł.; Krysiński, J.; Błaszczyński, J.; Słowiński, R.; Skrzypczak, A.; Błaszczak, J.; Gospodarek, E.; Wróblewska, J. Application of Rough Set Theory to Prediction of Antimicrobial Activity of Bis-Quaternary Imidazolium Chlorides. Fundam. Inform. 2014, 132, 315–330. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. Cross-validation, The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer Series in Statistics; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Available online: http://www.cs.put.poznan.pl/jblaszczynski/Site/jRS.html (accessed on 10 May 2021).
- Błaszczyński, J.; Słowiński, R.; Stefanowski, J. Ordinal Classification with Monotonicity Constraints by Variable Consistency Bagging. In Rough Sets and Current Trends in Computing (RSCTC 2010), LNAI 6086; Szczuka, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 392–401. [Google Scholar]
- Greco, S.; Pawlak, Z.; Słowiński, R. Can Bayesian confirmation measures be useful for rough set decision rules? Eng. Appl. Artif. Intell. 2004, 17, 345–361. [Google Scholar] [CrossRef]
- Greco, S.; Słowiński, R.; Szczech, I. Properties of rule interestingness measures and alternative approaches to normalization of measures. Inf. Sci. 2012, 216, 1–16. [Google Scholar] [CrossRef]
- Tatsumi, T.; Imai, Y.; Kawaguchi, K.; Miyano, N.; Ikeda, I. Antimicrobial activity of cationic Gemini surfactant containing an oxycarbonyl group in the lipophilic portion against gram-positive and gram-negative microorganisms. J. Oleo Sci. 2014, 63, 137–140. [Google Scholar] [CrossRef][Green Version]
- Mauri, A.; Consonni, V.; Todeschini, R. Molecular Descriptors. In Handbook of Computational Chemistry; Leszczynski, J., Kaczmarek-Kedziera, A., Puzyn, T., GPapadopoulos, M., Reis, H., KShukla, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Randić, M.; Zupan, J. On Interpretation of Well-Known Topological Indices. J. Chem. Inf. Comput. Sci. 2001, 41, 550–560. [Google Scholar] [CrossRef]
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA Workbench. Online Appendix for “Data Mining: Practical Machine Learning Tools and Techniques”, 4th ed.; Morgan Kaufmann: Burlington, MA, USA, 2016. [Google Scholar]
- Sheikh, M.S.; Khanam, A.J.; Matto, R.U.H. Comparative study of the micellar and antimicrobial activity of gemini-conventional surfactants in pure and mixed micelles. J. Surfactants Deterg. 2013, 16, 503–508. [Google Scholar] [CrossRef]
- Murguía, M.C.; Machuca, L.M.; Lurá, M.C.; Cabrera, M.I.; Grau, R.J. Synthesis and properties of novel antifungal gemini compounds derived from N-acetyl diethanolamines. J. Surfactants Deterg. 2008, 11, 223–230. [Google Scholar] [CrossRef]
- Pałkowski, Ł.; Błaszczyński, J.; Krysiński, J.; Słowiński, R.; Skrzypczak, A.; Błaszczak, J.; Gospodarek, E.; Wróblewska, J. Application of rough set theory to prediction of antimicrobial activity of bis-quaternary ammonium chlorides. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7414, pp. 107–116. [Google Scholar]
- Pałkowski, Ł.; Błaszczyński, J.; Skrzypczak, A.; Błaszczak, J.; Nowaczyk, A.; Wroblewska, J.; Kożuszko, S.; Gospodarek, E.; Słowiński, R.; Krysiński, J. Prediction of Antifungal Activity of Gemini Imidazolium Compounds. BioMed Res. Int. 2015, 2015, 392326. [Google Scholar] [CrossRef][Green Version]
- Mohareb, R.M.; Badawi, A.M.; Noor El-Din, M.R.; Fatthalah, N.A.; Mahrous, M.R. Synthesis and Characterization of Cationic Surfactants Based on N-Hexamethylenetetramine as Active Microfouling Agents. J. Surfactants Deterg. 2015, 18, 529–535. [Google Scholar] [CrossRef]
- Santhakumar, K.; Kumaraguru, N.; Arumugham, M.N.; Arunachalam, S. Metallomicelles of Co(III) coordination complexes-Synthesis, characterization and determination of CMC values. Polyhedron 2006, 25, 1507–1513. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable cationic antibacterial amphiphiles: Synthesis, mechanism of action, and cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef] [PubMed]
- Patial, P.; Shaheen, A.; Ahmad, I. Synthesis, characterization and evaluation of the surface active properties of novel cationic imidazolium gemini surfactants. J. Surfactants Deterg. 2014, 17, 253–260. [Google Scholar] [CrossRef]

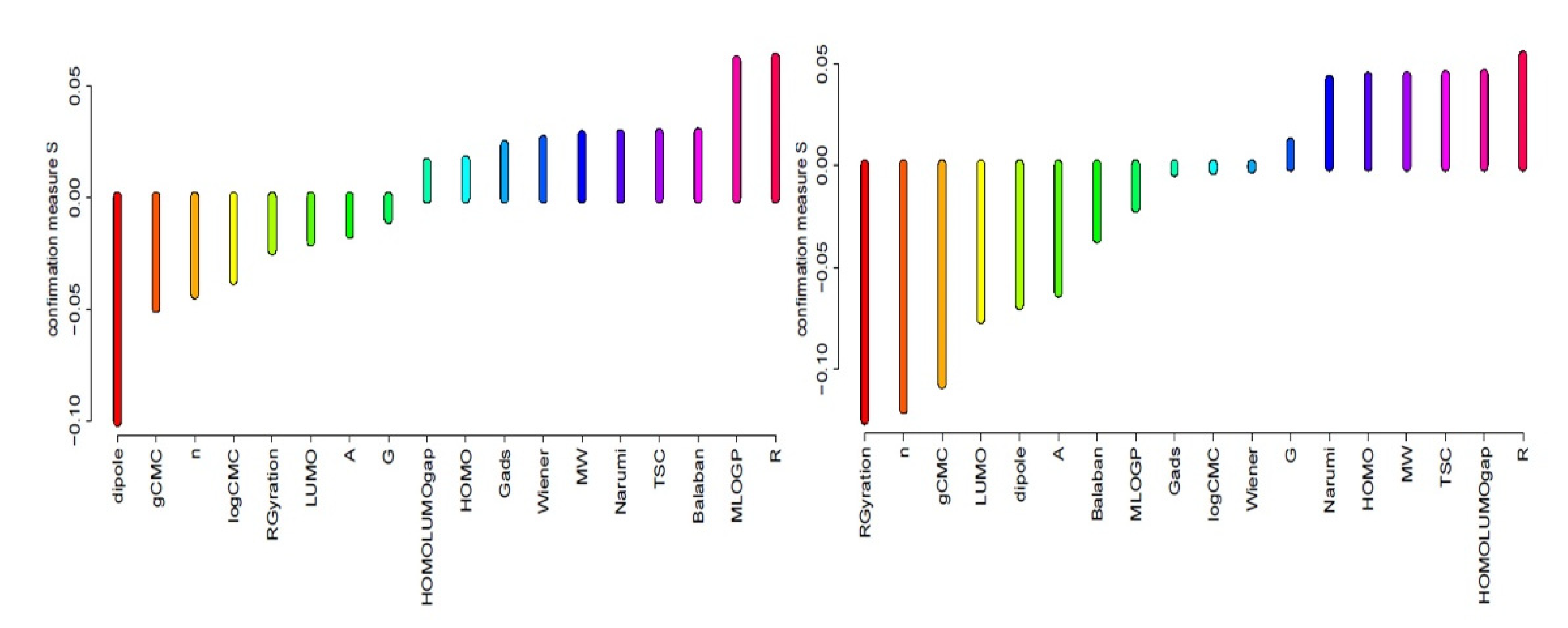
| No. | Condition Attributes | Support | Strength | Confirmation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | R | γCMC | Γ 106 | ∆Gads | HLgap | WI | ||||
| Decision Class “Weak” | ||||||||||
| 1 | ≥50.3 | ≥2.57 | ≤8.56 | 18 | 12.85 | 0.9230 | ||||
| 2 | ≥2.57 | ≤23 | ≤−0.15 | ≤8.56 | 17 | 12.14 | 0.9583 | |||
| 3 | ≥52.1 | ≥2.57 | ≤−0.16 | ≤8.56 | 17 | 12.14 | 0.9230 | |||
| 4 | ≥53.4 | ≥2.57 | ≤7.46 | 17 | 12.14 | 0.9200 | ||||
| 5 | ≤−0.16 | ≤8.43 | 17 | 12.14 | 0.8888 | |||||
| Decision Class “Good” | ||||||||||
| 6 | ≥7 | ≤2.47 | ≥10.49 | 41 | 29.28 | 0.7894 | ||||
| 7 | [6,7,8,9] | ≤2.47 | ≥10.49 | 33 | 23.57 | 0.7500 | ||||
| 8 | ≥7 | ≤2.47 | ≥9.67 | 25 | 17.85 | 0.6956 | ||||
| 9 | ≤11 | ≥2.71 | ≥10.49 | 24 | 17.14 | 0.7500 | ||||
| 10 | ≥7 | ≤2.46 | ≥10.49 | 24 | 17.14 | 0.6956 | ||||
| No. | Condition Attributes | Support | Strength | Confirmation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | R | γCMC | A × 1020 | ∆Gads | MlogP | MW | HLgap | BI | TSC | ||||
| Decision Class “Weak” | |||||||||||||
| 1 | ≥2 | ≥0.21 | 32 | 0.2285 | 0.8947 | ||||||||
| 2 | ≤7 | ≤3.025 | 32 | 0.2285 | 0.9000 | ||||||||
| 3 | ≥22 | ≤3.025 | 27 | 0.1928 | 0.8181 | ||||||||
| 4 | ≥51.1 | ≥1.33 | 19 | 0.1357 | 0.8571 | ||||||||
| 5 | ≤3 | ≤−0.1746 | 19 | 0.1357 | 0.8571 | ||||||||
| Decision Class “Good” | |||||||||||||
| 6 | ≥6 | ≤83 | 45 | 0.3214 | 0.8333 | ||||||||
| 7 | ≥6 | ≥−0.1797 | ≥0.18 | 41 | 0.2928 | 0.7500 | |||||||
| 8 | ≥6 | ≥6 | ≤83 | 31 | 0.2214 | 0.7500 | |||||||
| 9 | ≥6 | ≤83 | ≤1.277 | 30 | 0.2142 | 0.7500 | |||||||
| 10 | ≥7 | ≤603 | 27 | 0.1928 | 0.7894 | ||||||||
| VC-Bagging (DRSA) | Random Forest | Logistic Regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Validation Parameter | SAU | PAE | SAU | PAE | SAU | PAE | ||||||
| Cl. 1 | Cl. 3 | Cl. 1 | Cl. 3 | Cl. 1 | Cl. 3 | Cl. 1 | Cl. 3 | Cl. 1 | Cl. 3 | Cl. 1 | Cl. 3 | |
| Correctly Classified Instances (%) | 78.92 | 94.93 | 81.17 | 87.22 | 77.43 | 95.16 | 80.90 | 87.37 | 72.26 | 92.42 | 76.96 | 84.22 |
| Incorrectly Classified Instances (%) | 21.07 | 5.06 | 18.82 | 12.77 | 22.56 | 4.83 | 19.09 | 12.62 | 27.73 | 7.57 | 23.03 | 15.77 |
| Average Classification Accuracy (%) | 78.91 | 89.23 | 81.34 | 85.97 | 77.57 | 89.50 | 81.03 | 86.24 | 72.40 | 88.48 | 77.07 | 83.22 |
| Average Precision (%) | 78.82 | 90.67 | 81.15 | 86.88 | 77.42 | 91.30 | 80.87 | 86.98 | 72.27 | 84.26 | 76.94 | 83.44 |
| Code | Condition Attributes | |
|---|---|---|
| n-Spacer | R-Substituent | |
| 1 | CH3 | |
| 2 | C2H5 | C2H5 |
| 3 | C3H7 | C3H7 |
| 4 | C4H9 | C4H9 |
| 5 | C5H11 | C5H11 |
| 6 | C6H13 | C6H13 |
| 7 | C7H15 | C7H15 |
| 8 | C8H17 | C8H17 |
| 9 | C9H19 | C9H19 |
| 10 | C10H21 | C10H21 |
| 11 | C11H23 | |
| 12 | C12H25 | C12H25 |
| 14 | C14H29 | |
| 16 | C16H33 | |
| No. | n | R | lgCMC | γCMC | G | A | Gads | MLOGP | BI | NTI | WI | MW | HOMO | LUMO | HL Gap | dip | ROG | TSC | MIC SAU (mM/L) | MIC PAE (mM/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2.15 | 61.9 | 2.75 | 52 | 20.2 | 0.175 | 1.397 | 12.712 | 5.275 | 252.36 | −0.38777 | −0.19852 | −0.18925 | 1.646 | 4.908 | 0.28 | 16.937 | 33.875 |
| 2 | 2 | 2 | 2.23 | 60.1 | 2.71 | 54 | 20.8 | 0.711 | 1.407 | 14.099 | 5.768 | 280.42 | −0.38416 | −0.19108 | −0.19308 | 0.103 | 5.294 | 0.266 | 3.558 | 28.468 |
| 3 | 2 | 3 | 2.38 | 59.8 | 2.69 | 56 | 21.3 | 1.216 | 1.405 | 15.485 | 6.307 | 308.48 | −0.38269 | −0.18871 | −0.19398 | 2.314 | 5.804 | 0.254 | 3.295 | 13.181 |
| 4 | 2 | 4 | 2.41 | 57.4 | 2.65 | 58 | 21.7 | 1.697 | 1.397 | 16.871 | 6.877 | 336.54 | −0.38194 | −0.18751 | −0.19443 | 5.474 | 6.246 | 0.243 | 1.634 | 13.180 |
| 5 | 2 | 5 | 2.49 | 55.5 | 2.61 | 60 | 22.3 | 2.157 | 1.386 | 18.257 | 7.468 | 364.6 | −0.38150 | −0.18679 | −0.19471 | 8.628 | 6.777 | 0.234 | 0.712 | 11.483 |
| 6 | 2 | 6 | 2.58 | 53.4 | 2.57 | 62 | 22.7 | 2.599 | 1.373 | 19.644 | 8.074 | 392.66 | −0.38544 | −0.18641 | −0.19903 | 12.501 | 7.25 | 0.226 | 0.086 | 10.788 |
| 7 | 2 | 7 | 2.65 | 51.2 | 2.53 | 64 | 23.5 | 3.025 | 1.359 | 21.03 | 8.692 | 420.72 | −0.38109 | −0.18618 | −0.19491 | 16.201 | 7.791 | 0.218 | 0.020 | 5.086 |
| 8 | 2 | 8 | 2.72 | 48.9 | 2.49 | 66 | 23.9 | 4.349 | 1.346 | 22.416 | 9.319 | 448.78 | −0.36560 | −0.18605 | −0.17955 | 20.472 | 8.282 | 0.211 | 0.005 | 1.193 |
| 9 | 2 | 9 | 2.81 | 47.5 | 2.45 | 68 | 24.3 | 4.748 | 1.333 | 23.803 | 9.952 | 476.84 | −0.35218 | −0.18590 | −0.16628 | 24.499 | 8.831 | 0.205 | 0.002 | 1.132 |
| 10 | 2 | 10 | 2.92 | 45.3 | 2.41 | 70 | 24.8 | 5.136 | 1.32 | 25.189 | 10.59 | 504.9 | −0.34105 | −0.18584 | −0.15521 | 29.011 | 9.333 | 0.199 | 0.017 | 0.538 |
| 11 | 2 | 11 | 3.04 | 42.5 | 2.37 | 72 | 25.6 | 5.514 | 1.308 | 26.575 | 11.233 | 532.96 | −0.33153 | −0.18577 | −0.14576 | 33.258 | 9.883 | 0.194 | 0.017 | 0.513 |
| 12 | 2 | 12 | 3.15 | 41.4 | 2.33 | 74 | 26.3 | 5.883 | 1.297 | 27.961 | 11.879 | 561.02 | −0.32343 | −0.18573 | −0.13770 | 37.935 | 10.392 | 0.189 | 0.016 | 0.491 |
| 13 | 2 | 14 | 3.34 | 37.5 | 2.25 | 78 | 27.5 | 6.595 | 1.277 | 30.734 | 13.18 | 617.14 | −0.31025 | −0.18566 | −0.12459 | 47.138 | 11.454 | 0.18 | 0.116 | 1.817 |
| 14 | 2 | 16 | 3.52 | 33.9 | 2.17 | 82 | 28.8 | 7.278 | 1.26 | 33.507 | 14.488 | 673.26 | −0.30006 | −0.18563 | −0.11443 | 56.553 | 12.515 | 0.173 | 0.108 | 1.680 |
| 15 | 3 | 1 | 2.18 | 60.8 | 2.74 | 54 | 20.5 | 0.447 | 1.363 | 13.405 | 5.649 | 266.39 | −0.37891 | −0.19219 | −0.18672 | 1.941 | 5.192 | 0.273 | 29.652 | 29.652 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pałkowski, Ł.; Karolak, M.; Błaszczyński, J.; Krysiński, J.; Słowiński, R. Structure-Activity Relationships of the Imidazolium Compounds as Antibacterials of Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 7997. https://doi.org/10.3390/ijms22157997
Pałkowski Ł, Karolak M, Błaszczyński J, Krysiński J, Słowiński R. Structure-Activity Relationships of the Imidazolium Compounds as Antibacterials of Staphylococcus aureus and Pseudomonas aeruginosa. International Journal of Molecular Sciences. 2021; 22(15):7997. https://doi.org/10.3390/ijms22157997
Chicago/Turabian StylePałkowski, Łukasz, Maciej Karolak, Jerzy Błaszczyński, Jerzy Krysiński, and Roman Słowiński. 2021. "Structure-Activity Relationships of the Imidazolium Compounds as Antibacterials of Staphylococcus aureus and Pseudomonas aeruginosa" International Journal of Molecular Sciences 22, no. 15: 7997. https://doi.org/10.3390/ijms22157997
APA StylePałkowski, Ł., Karolak, M., Błaszczyński, J., Krysiński, J., & Słowiński, R. (2021). Structure-Activity Relationships of the Imidazolium Compounds as Antibacterials of Staphylococcus aureus and Pseudomonas aeruginosa. International Journal of Molecular Sciences, 22(15), 7997. https://doi.org/10.3390/ijms22157997






